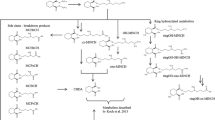
Abstract
2,2,4-Trimethyl-1,3-pentanediol monoisobutyrate (TMPD-MB) and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TMPD-DB) are widely used primarily as surface stabilizers for water-based paints and plasticizers, respectively. Exposure to these compounds has been suspected as being associated with sick building syndrome and allergic diseases such as asthma in general populations. Therefore, it is very important to be able to know the amounts of these compounds absorbed into the body in order to evaluate its adverse effects on humans in living environments. In the present study, the urinary excretion kinetics of TMPD-MB and TMPD-DB were studied in animals to establish for urinary metabolites suitable as biomarkers for monitoring exposure. A single dose (48–750 mg/kg body weight) of TMPD-MB or TMPD-DB was administered intraperitoneally to male Sprague–Dawley rats, and their urine was collected periodically for a week. Two major metabolites, 2,2,4-trimethyl-1,3-pentanediol (TMPD) and 3-hydroxy-2,2,4-trimethylvaleric acid (HTMV), were measured in the urine samples. Their kinetics were evaluated by moment analysis of the urinary excretion rates of the metabolites versus time curves. The urinary excretion amounts of HTMV were suggested to be proportional to the absorption amounts over a wide exposure range of both TMPD-MB and TMPD-DB. The amounts of HTMV accounted for almost the same level, i.e., 4–5% of the dose at the lowest dosage, in rats tested for both TMPD-MB and TMPD-DB. Urinary HTMV was considered to be an optimal biomarker for monitoring exposure to mixtures of these compounds.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Abe Y, Sugita T, Wakui C, Niino T, Yomota C, Ishiwata H, Tanamoto K, Maitani T (2003) Material labeling of soft plastic toys and plasticizers in polyvinyl chloride products. Food Hyg Saf Sci 44:168–174
Abe Y, Yamaguchi M, Mutsuga M, Hirahara Y, Kawamura Y (2012) Survey of plasticizers in polyvinyl chloride toys. Food Hyg Saf Sci 53:19–27
Adachi A, Ibusuki C, Fukuda K, Sasaki Y, Mise M, Mori A, Sasaki K (2014) Allergic contact dermatitis due to 2,2,4-trimethyl-1,3-pentanediol-diisobutyrate in vinyl chloride gloves. J Environ Dermatol Cutan Allergol 8:271–280
Astill BD, Terhaar CJ, Fassett DW (1972) The toxicology and fate of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate. Toxicol Appl Pharmacol 22:387–399. https://doi.org/10.1016/0041-008x(72)90244-x
Babich MA, Bevington C, Dreyfus MA (2020) Plasticizer migration from children’s toys, child care articles, art materials, and school supplies. Regul Toxicol Pharmacol 111:104574. https://doi.org/10.1016/j.yrtph.2019.104574
Bönisch U, Böhme A, Kohajda T, Mögel I, Schütze N, von Bergen M, Simon JC, Lehmann I, Polte T (2012) Volatile organic compounds enhance allergic airway inflammation in an experimental mouse model. PLoS ONE 7:e39817. https://doi.org/10.1371/journal.pone.0039817
Cain WS, de Wijk RA, Jalowayski AA, Pilla Caminha G, Schmidt R (2005) Odor and chemesthesis from brief exposures to TXIB. Indoor Air 15:445–457. https://doi.org/10.1111/j.1600-0668.2005.00390.x
Chemical Daily (2019) 17019 no kagaku shohin. The Chemical Daily, Tokyo, Japan
Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag C-G (2010) Common household chemicals and the allergy risks in pre-school age children. PLoS ONE 5:e13423. https://doi.org/10.1371/journal.pone.0013423
Cosmetic Ingredient Review (CIR) (2017) Safety assessment of monoalkylglycol dialkyl acid esters as used in cosmetics. Washington, DC. http://www.cir-safety.org/sites/default/files/Monoalkylglycol%20Dialkyl%20Acid%20Esters_0.pdf. Accessed 16 June 2023
Eastman (2007) Toxicity summary for Eastman® TXIB formulation additive. Eastman Chemical Company, Kingsport, TN. https://www.cpsc.gov/s3fs-public/EastmanTXIB11282007.pdf. Accessed 16 June 2023
Ishizaka T, Makino T, Yoshida R, Horio I, Kawashima A (2022) Indoor air survey of school environment in Ehime Prefecture using passive sampling. Indoor Environ 25:267–274
Kempf M, Ramm S, Feuerbach T, Schreier P (2009) Occurrence of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (Texanol®) in foods packed in polystyrene and polypropylene cups. Food Addit Contam 26:563–567. https://doi.org/10.1080/02652030802562920
Kim JL, Elfman L, Mi Y, Wieslander G, Smedje G, Norbäck D (2007) Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools—associations with asthma and respiratory symptoms in pupils. Indoor Air 17:153–163. https://doi.org/10.1111/j.1600-0668.2006.00466.x
Kobayashi S, Takeuchi S, Kojima H, Takahashi T, Jin K, Akitsu H, Isaji S (2010) Indoor air pollution in a newly constructed elementary school caused by 1-methyl-2-pyrrolidone and Texanol emitted from water-based paints. Indoor Environ 13:39–54
Kostiainen R (1995) Volatile organic compounds in the indoor air of normal and sick houses. Atmos Environ 29:693–702. https://doi.org/10.1016/1352-2310(94)00309-9
Krasavage WJ, Tischer KS, Roudabush RL (1972) The reversibility of increased rat liver weights and microsomal processing enzymes after feeding high levels of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate. Toxicol Appl Pharmacol 22:400–408. https://doi.org/10.1016/0041-008x(72)90245-1
Maag J, Lassen C, Brandt UK, Kjølholt J, Molander L, Mikkelsen SH (2010) Identification and assessment of alternatives to selected phthalates. Environmental Project No. 1341 2010, Danish Ministry of the Environment, Environmental Protection Agency, COWI A/S, Denmark. https://www2.mst.dk/udgiv/publications/2010/978-87-92708-00-7/pdf/978-87-92708-01-4.pdf. Accessed 16 June 2023
Maddalena R, Russell M, Sullivan DP, Apte MG (2009) Formaldehyde and other volatile organic chemical emissions in four FEMA temporary housing units. Environ Sci Technol 43:5626–5632. https://doi.org/10.1021/es9011178
Metiäinen P, Mussalo-Rauhamaa H, Viinikka M (2002) TXIB-emission from floor structure as a marker of increased risk for some specific symptoms. In: Proceedings of the 9th international conference on indoor air quality and climate, vol 2, pp 108–113
Mizouchi S, Ichiba M, Miyajima T, Kodama H, Takamuku T, Someya T, Ueno D (2014) Current status of unregulated volatile organic compounds (VOCs) in indoor air from elementary schools, Japan. Indoor Environ 17:69–79
Mori Y, Tanaka-Kagawa T, Tahara M, Kawakami T, Aoki A, Okamoto Y, Isobe T, Ohkawara S, Hanioka N, Azuma K, Sakai S, Jinno H (2023) Species differences in activation of TRPA1 by resin additive-related chemicals relevant to indoor air quality. J Toxicol Sci 48:37–45. https://doi.org/10.2131/jts.48.37
Nakashima H, Onji Y (2005) Status of the use of plasticizers used for commercially available polyvinyl chloride products. Proc Osaka Pref Inst Pub Health 43:31–37
Nielsen GD, Hansen LF, Wolkoff P (1997) Chemical and biological evaluation of building material emissions. II. Approaches for setting indoor air standards or guidelines for chemicals. Indoor Air 7:17–32. https://doi.org/10.1111/j.1600-0668.1997.t01-3-00004.x
Nishioka K, Koizumi A, Takita Y, Sasaki K, Numata M (2020) Contact dermatitis due to 2,2,4-trimethyl 1,3-pentanediol diisobutyrate contained in latex-free, accelerator-free nitrile rubber gloves. Contact Dermatitis 82:255–257. https://doi.org/10.1111/cod.13455
Onuki A, Saito I, Tada T, Fukuda M, Kurita M, Ogata A, Todaka E, Nakaoka H, Mori C (2009) Trends in indoor air chemicals detected at high concentrations in newly built houses. Ann Rep Tokyo Metr Inst Pub Health 60:245–251
Organisation for Economic Co-operation and Development (OECD) (1995) SIDS initial assessment report for SIAM 3, 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate, CAS N°: 6846-50-0. Williamsburg, VA. https://hpvchemicals.oecd.org/ui/handler.axd?id=eaa03221-5936-4827-893f-437d73335ab6. Accessed 16 June 2023
Organisation for Economic Co-operation and Development (OECD) (2001) TEXANOL, CAS N°: 25265-77-4, Williamsburg, VA. https://hpvchemicals.oecd.org/UI/handler.axd?id=E59DBE55-B2D4-4929-86CC-4A6B7647E1C3. Accessed 16 June 2023
Pharmaceutical and Food Safety Bureau (PFSB) of the Ministry of Health, Labour and Welfare (2001) A Notice (IYAKU-828), Japanese Government, Tokyo, Japan
Raw GJ, Coward SKD, Brown VM, Crump DR (2004) Exposure to air pollutants in English homes. J Exp Anal Environ Epidemiol 14:S85–S94. https://doi.org/10.1038/sj.jea.7500363
Risk Science Center (RSC) (2018) Toxicity review for 2,2,4-trimethyl-1,3-pentanediol-diisobutyrate (TPIB), Risk Science Center, University of Cincinnati, Cincinnati, OH. https://www.cpsc.gov/s3fs-public/Toxicity%20Review%20of%20TPIB.pdf. Accessed 16 June 2023
Saarinen A, Saarinen L, Viinikka M (2005) Concentration of VOCs in dwellings where residents reported symptoms. In: Proceedings of the 10th international conference on indoor air quality and climate, vol 1–5, pp 926–931
Sahlberg B, Gunnbjörnsdottir M, Soon A, Jogi R, Gislason T, Wieslander G, Janson C, Norback D (2013) Airborne molds and bacteria, microbial volatile organic compounds (MVOC), plasticizers and formaldehyde in dwellings in three North European cities in relation to sick building syndrome (SBS). Sci Total Environ 444:433–440. https://doi.org/10.1016/j.scitotenv.2012.10.114
Saito I, Onuki A, Todaka E, Nakaoka H, Hosaka M, Ogata A (2011) Recent trends in indoor air pollution: health risks from unregulated chemicals. Jpn J Risk Anal 21:91–100
Spjøtvoll E, Stoline MR (1973) An extension of the T-method of multiple comparison to include the cases with unequal sample sizes. J Am Stat Assoc 68:976–978. https://doi.org/10.1080/01621459.1973.10481458
Takeuchi S, Kojima H, Saito I, Jin K, Kobayashi S, Tanaka-Kagawa T, Jinno H (2014) Detection of 34 plasticizers and 25 flame retardants in indoor air from houses in Sapporo, Japan. Sci Total Environ 491–492:28–33. https://doi.org/10.1016/j.scitotenv.2014.04.011
Tao L, Tan H, Qiao X, Li L, Yu Y, Xie J, Chen D (2022) Emerging plasticizers in South China house dust and hand wipes: calling for potential concern? Environ. Sci Technol 56:12190–12199. https://doi.org/10.1021/acs.est.2c02106
Ueta I, Takenaka R, Fujimura K, Narukami S, Sasaki T, Maeda T (2019) Quantitative determination of 2-ethyl-1-hexanol, Texanol and TXIB in in-door air using a solid-phase extraction-type collection device followed by gas chromatography-mass spectrometry. Anal Sci 35:855–859. https://doi.org/10.2116/analsci.19P033
Villberg K, Mussalo-Rauhamaa H, Haahtela T, Saarela K (2008) Prevalence of plastic additives in indoor air related to newly diagnosed asthma. Indoor Built Environ 17:455–459. https://doi.org/10.1177/1420326X08096413
Wells SB (2005) The identification of Isopar H in vinyl flooring. J Forensic Sci 50:865–872
Wieslander G, Norbäck D, Björnsson E, Janson C, Boman G (1997) Asthma and the indoor environment: the significance of emission of formaldehyde and volatile organic compounds from newly painted indoor surfaces. Int Arch Occup Environ Health 69:115–124. https://doi.org/10.1007/s004200050125
Yamaoka K, Nakagawa T, Uno T (1978) Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm 6:547–558
Yoshida T (2021) Analytical method for urinary metabolites of 2,2,4-trimethyl-1,3-pentanediolmonoisobutyrate and 2,2,4-trimethyl-1,3-pentanedioldiisobutyrate in the general population. Ann Rep Osaka Inst Pub Health 5:80–89
Acknowledgements
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI under Grant [number 21K10431].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interests to declare that are relevant to the content of this article.
Ethical approval
All experiments with animals were carried out according to the Guidelines of the Animal Use and Care Committee of the Osaka Institute of Public Health (approval number: D-R4-1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshida, T. A urinary biomarker for monitoring exposures to 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate and 2,2,4-trimethyl-1,3-pentanediol diisobutyrate in rats. Arch Toxicol 97, 2687–2695 (2023). https://doi.org/10.1007/s00204-023-03570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03570-9