Abstract
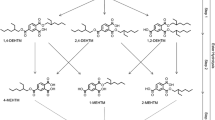
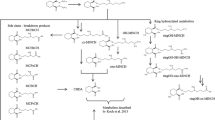
Di(2-ethylhexyl) terephthalate (DEHTP) is used as a substitute for di(2-ethylhexyl) phthalate (DEHP), an ortho-phthalate-based plasticizer that is classified and labeled due to its toxicity to reproduction. In this study the metabolism and urinary excretion kinetics of DEHTP were investigated by single oral dosage of 50 mg DEHTP to three male volunteers (resulting in individual dosages between 0.55 and 0.59 mg/kg body weight). Separate urine samples were consecutively collected for 48 h. In analogy to DEHP, we quantified specific side-chain-oxidized monoester metabolites of DEHTP (5OH-MEHTP, 5oxo-MEHTP, 5cx-MEPTP and 2cx-MMHTP) by HPLC–MS/MS with online sample clean-up and isotope dilution. All postulated metabolites were detectable in all samples after dosage. The predominant, specific urinary metabolite was 5cx-MEPTP representing about 13.0 % of the applied dose as mean of the three volunteers (range 7.0–20.4 %) in urine, followed by 5OH-MEHTP (mean: 1.8 %; range 1.3–2.4 %) and 5oxo MEHTP (mean: 1.0 %; range 0.6–1.6 %). 2cx-MMHTP was a minor metabolite representing only 0.3 % (range 0.2–0.4 %). In total, about 16.1 % of the dose was recovered in urine as the above investigated specific metabolites within 48 h with the major share (95 %) being excreted within the first 24 h. Investigation of the glucuronidation patterns revealed that the carboxy-metabolites are excreted almost completely in their free form (>90 %), whereas for 5OH-MEHTP and 5oxo-MEHTP, glucuronidation is preferred (>70 %). With this study we provide reliable urinary excretion factors to calculate DEHTP intakes based on metabolite concentrations in environmental and occupational studies.



Similar content being viewed by others
References
Anderson WAC, Castle L, Hird S, Jeffery J, Scotter MJ (2011) A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional axcretion of primary and secondary urinary metabolites of di-2-ethylhexylphthtalate and di-iso-nonylphthalate. Food Chem Toxicol 49(9):2022–2029
Axelrod J (1956) The enzymatic cleavage of aromatic ethers. Biochem J 63(4):634–639
Barber ED, Topping DC (1995) Subchronic 90-day oral toxicology of di(2-ethylhexyl) terephthalate in the rat. Fd Chem Toxic 33(11):971–978
Barber ED, Fox JA, Giordano CJ (1994) Hydrolysis, absorption and metabolism of di(2-ethylhexy1) terephthalate in the rat. Xenobiotica 24(5):441–450
Bizzari SN, Blagoev M, Kishi A (2013) Chemical economics handbook plasticizers. IHS Global Inc., Douglas County
Boberg J, Christiansen S, Axelstad M, Seidler Kledal T, Vinggaard AM, Dalgaard M, Nellemann C, Hass U (2011) Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 31(2):200–209
Bui TT, Giovanoulis G, Cousins AP, Magnér J, Cousins IT, de Wit CA (2016) Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 541:451–467
Clark B, Smith DA (eds) (1986) An introduction to pharmacokinetics, 2nd edn. Blackwell Scientific, Oxford
Deyo JA (2007) Carcinogenicity and chronic toxicity of di-2-ethylheyl terephthalate (DEHT) following a 2-year dietary exposure in Fischer 344 rats. Food Chem Toxicol 46(3):990–1005
Eastman Chemical Company (2014) Product Datasheet Eastmann 168TM non- phthalate plasticizers; http://ws.eastman.com/ProductCatalogApps/PageControllers/ProdDatasheet_PC.aspx?Product=71072819&sCategoryName=Generic. Accessed 25 June 2015
European Food Safety Authority (2005) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials Question No EFSA-Q-2003-191, Adopted on 23 June 2005 by written procedure. EFSA J 243:1–20
European Food Safety Authority (2008) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 18th list of substances for food contact materials. Question No EFSA-Q-2007-167, EFSA-Q-2006-177, EFSA-Q-2005-152, EFSA-Q-2007-022, EFSA-Q-2007-004, EFSA-Q-2007-024. EFSA J 628–633:1–19
Foster PMD (2006) Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29(1):140–147
Furr J, Lambright C, Wilson VS, Foster PM, Gray LE Jr (2014) A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci 140(2):403–424
Gray LE Jr, Ostby J, Furr J, Price M, Rao Veeramachaneni DN, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP but not DEP, DMP or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58(2):350–365
Jaffe MZ (1886) About the precipitation caused by pikrinic acid in normal urine and about a new reaction of creatinine. Physiol Chem 10:391–400
Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Brüning T, Wilhelm M (2014) Phthalate metabolites and bisphenol A in urines from German school-aged children: results of the Duisburg Birth Cohort and Bochum Cohort Studies. Int J Hyg Environ Health 217(8):830–838
Kato K, Silva MJ, Needham LL, Calafat AM (2005) Determination of 16 pthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem 77(9):2985–2991
Kessler W, Numtip W, Völkel W, Seckin E, Csanády GA, Pütz C, Klein D, Fromme H, Filser JG (2012) Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol 264(2):284–291
Koch HM, Angerer J (2007) Di-iso-nonylphthalate (DINP) metabolites in human urine after single oral dose of deuterium-labelled DINP. Int J Hyg Environ-Health 210(1):9–19
Koch HM, Drexler H, Angerer J (2003a) An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 206(2):77–83
Koch HM, Gonzalez-Reche LM, Angerer J (2003b) On-line clean-up by multidimensional liquid chromatography electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary pththalate metabolites in human urine. J Chromatogr B 784(1):169–182
Koch HM, Preuss R, Drexler H, Angerer J (2004) Exposure of nursery school children and their parents and teachers to di-n-butylphthalate and butylbenzylphthalate. Int Arch Occup Environ Health 78(3):223–229
Koch HM, Angerer J, Drexler H, Eckstein R, Weisbach V (2005) Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health 208(6):489–498
Koch HM, Preuss R, Angerer J (2006) Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure—an update and latest results. Int J Androl 29(1):155–165
Koch HM, Haller A, Weiß T, Käfferlein HU, Stork J, Brüning T (2012) Phthalate exposure during cold plastisol application—a human biomonitoring study. Toxicol Lett 213(1):100–106
Koch HM, Schütze A, Pälmke C, Angerer J, Brüning T (2013) Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH) in humans after single oral doses. Arch Toxicol 87(5):799–806
Leng G, Koch HM, Gries W, Schütze A, Langsch A, Brüning T, Otter R (2014) Urinary metabolite excretion after oral dosage of bis(2-propylheptyl) phthalate (DPHP) to five male volunteers—characterization of suitable biomarkers for human biomonitoring. Toxicol Lett 231(2):282–288
Lessmann F, Schütze A, Weiss T, Brüning T, Koch HM (2016) Determination of metabolites of Di(2-ethylhexyl) terephthalate (DEHTP) in human urine by HPLC-MS/MS with on-line clean-up. J Chromatogr B 1011:196–203
Schütze A, Kolossa-Gehring M, Apel P, Brüning T, Koch HM (2014) Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll® DINCH® in 24 h urine samples from the German Environmental Specimen Bank. Int J Hyg Environ Health 217(2–3):421–426
Schütze A, Gries W, Kolossa-Gehring M, Apel P, Schröter-Kermani C, Fiddicke U, Leng G, Brüning T, Koch HM (2015) Bis-(2-propylheptyl)phthalate (DPHP) metabolites emerging in 24 h urine samples from the German Environmental Specimen Bank (1999–2012). Int J Hyg Environ Health 218(6):559–563
Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D III, Calafat AM, Needham LL, Brock JW (2003) Glucuronidation patterns of common urinary and serum monoester pththalate metabolites. Arch Tox 77(10):561–567
Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM (2004) Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(3):331–338
Silva MJ, Samandar E, Preau JL Jr, Needham LL, Calafat AM (2006) Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219(1–3):22–32
Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM (2007) Quantification of 22 phthalate metabolites in human urine. J Chromatogr B 860(1):106–112
Silva MJ, Samandar E, Calafat AM, Ye X (2015) Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol In Vitro 29(4):716–721
The European Parliament (2006) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC., Official Journal L 396 30/12/2006, p. 1, Consolidated text of 5 September 2015
The European Parliament (2008) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal L 353, 31/12/2008 P. 0001-1355, Consolidated text of 06 December 2014
Topping DC, Ford GP, Evans JG, Lake BG, O´Donoghue JL, Lockhart HB (1987) Peroxisome induction studies on di(2-ethylhexyl)terephthalate. Toxicol Ind Health 3(2):63–78
United States Environmental Protection Agency (1987) Integrated Risk Information System, Chemical Assessment Summary Di(2-ethylhexyl)phthalate; CASRN 117-81-7. Last Revised 31 January 1987. http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0014_summary.pdf. Accessed 03 Feb 2016
Acknowledgments
The development of the analytical method and its first application in a human metabolism and population study are part of a large-scale 10-year project on the advancement of human biomonitoring in Germany. This project is a cooperation agreed in 2010 between the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) and the Verband der Chemischen Industrie e.V. (German Chemical Industry Association—VCI) and is managed by the German Environment Agency (UBA). In this cooperation project the analytical method development and the human metabolism study are financed by the Chemie Wirtschaftsförderungsgesellschaft mbH while the first application of the novel methodology in a population study is financed by the German Environment Agency. Experts from governmental scientific authorities, industry and science closely accompany and advise the project in selecting substances and developing methods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study design was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval for the study protocol was obtained from the Ethics Commission of the Faculty of Medicine of the Ruhr-Universität Bochum, Germany (IRB Reg. No.: 4757-13). Written informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lessmann, F., Schütze, A., Weiss, T. et al. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch Toxicol 90, 1659–1667 (2016). https://doi.org/10.1007/s00204-016-1715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1715-x