Abstract
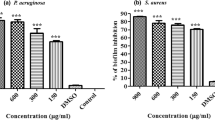
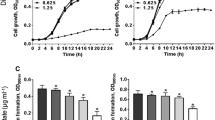
Plant extracts have been used to treat microbiological diseases for centuries. This study examined plant triterpenoids tormentic acid (TA) and 23-hydroxycorosolic acid (HCA) for their antibiofilm effects on Staphylococcus aureus strains (MTCC-96 and MTCC-7405). Biofilms are bacterial colonies bound by a matrix of polysaccharides, proteins, and DNA, primarily impacting healthcare. As a result, ongoing research is being conducted worldwide to control and prevent biofilm formation. Our research showed that TA and HCA inhibit S. aureus planktonic growth by depolarizing the bacterial membrane. In addition, zone of inhibition studies confirmed their effectiveness, and crystal violet staining and biofilm protein quantification confirmed their ability to prevent biofilm formation. TA and HCA exhibited substantial reductions in biofilm formation for S. aureus (MTCC-96) by 54.85% and 48.6% and for S. aureus (MTCC-7405) by 47.07% and 56.01%, respectively. Exopolysaccharide levels in S. aureus biofilm reduced significantly by TA (25 μg/mL) and HCA (20 μg/mL). Microscopy, bacterial motility, and protease quantification studies revealed their ability to reduce motility and pathogenicity. Furthermore, TA and HCA treatment reduced the mRNA expression of S. aureus virulence genes. In silico analysis depicted a high binding affinity of triterpenoids for biofilm and quorum-sensing associated proteins in S. aureus, with TA having the strongest affinity for TarO (– 7.8 kcal/mol) and HCA for AgrA (– 7.6 kcal/mol). TA and HCA treatment reduced bacterial load in S. aureus-infected peritoneal macrophages and RAW264.7 cells. Our research indicates that TA and HCA can effectively combat S. aureus by inhibiting its growth and suppressing biofilm formation.






Similar content being viewed by others
Data availability
The authors have stated that the datasets produced and analyzed in the course of the present study are accessible upon reasonable request.
Abbreviations
- TA:
-
Tormentic acid
- HCA:
-
23-Hydroxycorosolic acid
- S. aureus :
-
Staphylococcus aureus
- LB:
-
Luria broth
- MIC:
-
Minimum inhibitory concentration
- CV:
-
Crystal violet
- Str:
-
Streptomycin
- AO:
-
Acridine orange
- DiSC3(5):
-
3,3′-Dipropylthiadicarbocyanine iodide
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- FBS:
-
Fetal bovine serum
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
- DNA:
-
Deoxyribonucleic acid
- cDNA:
-
Complementary DNA
- mRNA:
-
Messenger RNA
- QS:
-
Quorum sensing
- CFU:
-
Colony-forming unit
- EPS:
-
Extra polymeric substances
- ADT:
-
AutoDock Tools
- MOI:
-
Multiplicity of infection
References
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. https://doi.org/10.4161/viru.2.5.17724
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496. https://doi.org/10.1093/ajcp/45.4_ts.493
Bhattacharjee S, Gupta G, Bhattacharya P, Mukherjee A, Mujumdar SB, Pal A, Majumdar S (2009) Quassin alters the immunological patterns of murine macrophages through generation of nitric oxide to exert antileishmanial activity. J Antimicrob Chemother 63:317–324. https://doi.org/10.1093/jac/dkn479
Bishayee A, Ahmed S, Brankov N, Perloff M (2011) Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci 16:980. https://doi.org/10.2741/3730
Bogino PC, Oliva Mde L, Sorroche FG, Giordano W (2013) The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci 14:15838–15859. https://doi.org/10.3390/ijms140815838
Bolton EE, Wang Y, Thiessen PA, Bryant SH (2008) PubChem: integrated platform of small molecules and biological activities. Annu Rep Comput Chem 4:217–241. https://doi.org/10.1016/S1574-1400(08)00012-1
Das MC, Paul S, Gupta P, Tribedi P, Sarkar S, Manna D, Bhattacharjee S (2016a) 3-Amino-4-aminoximidofurazan derivatives: small molecules possessing antimicrobial and antibiofilm activity against Staphylococcus aureus and Pseudomonas aeruginosa. J Appl Microbiol 120:842–859. https://doi.org/10.1111/jam.13063
Das MC, Sandhu P, Gupta P, Rudrapaul P, De UC, Tribedi P, Akhter Y, Bhattacharjee S (2016b) Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci Rep 6:23347. https://doi.org/10.1038/srep23347
Das A, Das MC, Das N, Bhattacharjee S (2017) Evaluation of the antileishmanial potency, toxicity and phytochemical constituents of methanol bark extract of Sterculia villosa. Pharm Biol 55:998–1009. https://doi.org/10.1080/13880209.2017.1285946
Das MC, Samaddar S, Jawed JJ, Ghosh C, Acharjee S, Sandhu P, Das A, Daware AV, De UC, Majumdar S, Gupta SKD, Akhter Y, Bhattacharjee S (2022) Vitexin alters Staphylococcus aureus surface hydrophobicity to obstruct biofilm formation. Microbiol Res 263:127126. https://doi.org/10.1016/j.micres.2022.127126
DeLano WL (2002) The PyMOL Molecular Graphic System San Carlos, CA: DeLano Scientific. http://www.pymol.org. Accessed 6 Nov 2023
Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL (2016) Botanicals and their bioactive phytochemicals for women’s health. Pharmacol Rev 68:1026–1073. https://doi.org/10.1124/pr.115.010843
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, Markova L, Urban M, Sarek J (2006) Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep. https://doi.org/10.1039/b515312n
Fiser A, Šali A (2003) Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol 374:461–491. https://doi.org/10.1016/S0076-6879(03)74020-8
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. https://doi.org/10.1038/nrmicro.2016.94
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity- a rapid access to atomic charges. Tetrahedron 36:3219–3228. https://doi.org/10.1016/0040-4020(80)80168-2
Ghosh C, Sarkar A, Anuja K, Das MC, Chakraborty A, Jawed JJ, Gupta P, Majumdar S, Banerjee B, Bhattacharjee S (2019) Free radical stress induces DNA damage response in RAW264. 7 macrophages during Mycobacterium smegmatis infection. Arch Microbiol 201:487–498. https://doi.org/10.1007/s00203-018-1587-y
Ghosh C, Bhowmik J, Ghosh R, Das MC, Sandhu P, Kumari M, Acharjee S, Daware AV, Akhter Y, Banerjee B, De UC, Bhattacharjee S (2020) The anti-biofilm potential of triterpenoids isolated from Sarcochlamys pulcherrima (Roxb.) Gaud. Microb Pathog 139:103901. https://doi.org/10.1016/j.micpath.2019.103901
Gupta P, Sarkar B, Bhattacharjee S, Tribedi P (2016) Biofilm, pathogenesis and prevention-a journey to break the wall: a review. Arch Microbiol 198:1–15. https://doi.org/10.1007/s00203-015-1148-6
Gupta P, Sarkar A, Sandhu P, DawareA DMC, Akhter Y, Bhattacharjee S (2017) Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. J Appl Microbiol 123:246–261. https://doi.org/10.1111/jam.13476
Harborne JB (1998) Phytochemical methods A guide to modern techniques of plant analysis, 3rd edn. Thompson Science, London, pp 1–317
Howden BP, Giulieri SG, Wong Fok Lung T, Baines SL, Sharkey LK, Lee JY, Hachani A, Monk IR, Stinear TP (2023) Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol 27:1–6. https://doi.org/10.1038/s41579-023-00852-y
Jakobsen TH, Tolker-Nielsen T, Givskov M (2017) Bacterial biofilm control by perturbation of bacterial signaling processes. Int J Mol Sci 18:1970. https://doi.org/10.3390/ijms18091970
Kaito C, Sekimizu K (2007) Colony spreading in Staphylococcus aureus. J Bacteriol 189:2553–2557. https://doi.org/10.1128/JB.01635-06
Karygianni L, Ren Z, Koo H, Thurnheer T (2020) Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681. https://doi.org/10.1016/j.tim.2020.03.016
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306. https://doi.org/10.1101/cshperspect.a010306
Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V (2005) D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 187:6719–6725. https://doi.org/10.1128/JB.187.19.6719-6725.2005
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786. https://doi.org/10.1021/ci200227u
Li YH, Tian X (2012) Quorum sensing and bacterial social interactions in biofilms. Sensors (basel) 12:2519–2538. https://doi.org/10.3390/s120302519
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3:0011–2014. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Mukherjee K, Tribedi P, Mukhopadhyay B, Sil AK (2013) Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol Lett 338:177–183. https://doi.org/10.1111/1574-6968.12043
Namvar AE, Asghari B, Ezzatifar F, Azizi G, Lari AR (2013) Detection of the intercellular adhesion gene cluster (ica) in clinical Staphylococcus aureus isolates. GMS Hyg Infect Control 8:1. https://doi.org/10.3205/dgkh000203
National Committee for Clinical Laboratory Standards (2003) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Sixth Edition: Approved Standard M7A6. Wayne, PA, USA: NCCLS
Nüsslein K, Arnt L, Rennie J, Owens C, Tew GN (2006) Broad-spectrum antibacterial activity by a novel abiogenic peptide mimic. Microbiology (reading, England) 152:1913–1918. https://doi.org/10.1099/mic.0.28812-0
Palma V, Gutiérrez MS, Vargas O, Parthasarathy R, Navarrete P (2022) Methods to evaluate bacterial motility and its role in bacterial–host interactions. Microorganisms 10:563. https://doi.org/10.3390/microorganisms10030563
Preda VG, Săndulescu O (2019) Communication is the key: biofilms, quorum sensing, formation and prevention. Discoveries 7:e100. https://doi.org/10.15190/d.2019.13
Pronk S, Pall S, Schuiz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) Gromacs 4.5: a high throughput and highly parallel open source molecular simulations toolkit. Bioinformatics 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61. https://doi.org/10.1016/S1093-3263(99)99999-0
Schilcher K, Horswill AR (2020) Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev 84:10–128. https://doi.org/10.1128/MMBR.00026-19
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Sharma AK, Dhasmana N, Dubey N, Kumar N, Gangwal A, Gupta M, Singh Y (2017) Bacterial virulence factors: secreted for survival. Indian J Microbiol 57:1–10. https://doi.org/10.1007/s12088-016-0625-1
Thomer L, Schneewind O, Missiakas D (2016) Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu Rev Pathol 11:343–364. https://doi.org/10.1146/annurev-pathol-012615-044351
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 9:59. https://doi.org/10.3390/antibiotics9020059
Wang B, Muir TW (2016) Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem Biol 23:214–224. https://doi.org/10.1016/j.chembiol.2016.01.004
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. https://doi.org/10.1038/nprot.2007.521
Acknowledgements
The authors express their sincere gratitude to the State Biotech Hub at Tripura University and Dr. Syed Arshad Hussain from the Department of Physics for generously providing access to the instrumental facility. The research conducted in Dr. Bhattacharjee's laboratory was supported by grants received from the Science and Engineering Research Board (DST-SERB) India, the Department of Biotechnology (DBT) India, and the Indian Council of Medical Research (ICMR) India.
Funding
No external funding was utilized for this study.
Author information
Authors and Affiliations
Contributions
CG—Designed and performed experiments analyzed data and co- wrote the paper; SB, MCD, SA, JB, and RG—Performed experiments; PS, MK and YA—Performed bioinformatics analysis; BB—performed microscopy analysis; UCD—Designed experiments; and SB—Designed experiments, co-ordinate and supervised overall research and co-wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
One of the authors is the Editor-in-Chief of the journal, however, he has no role in editorial or peer review of this submission.
Additional information
Communicated by Ran Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, C., Das, M.C., Acharjee, S. et al. Combating Staphylococcus aureus biofilm formation: the inhibitory potential of tormentic acid and 23-hydroxycorosolic acid. Arch Microbiol 206, 25 (2024). https://doi.org/10.1007/s00203-023-03762-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03762-y