Abstract
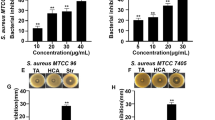
Human pathogens have readily been converted into multidrug-resistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), because of the long-term use of conventional antibiotics. In addition, the biofilms formed by S. aureus cells are especially problematic and are related to the persistence of chronic infections because they constitute a major mechanism of promoting tolerance to diverse antimicrobial agents. Hence, the inhibitions of biofilm formation and/or toxin production are accepted as alternative means of controlling S. aureus infections. The present study was aimed at identifying novel anti-biofilm and/or anti-virulence compounds in friedelane-based pentacyclic triterpenoids present in many edible and medicinal plants—and investigating them against MRSA strains. As a result, dihydrocelastrol and dihydrocelastryl diacetate were found to both inhibit the biofilm formation of, and to disrupt the preformed biofilms of, MRSA strains to an increasingly greater degree with increasing concentrations of each compound. Furthermore, these two triterpenoids also clearly inhibited the hemolytic activity of MRSA—and in-line with their anti-biofilm activities, rendered the cell more hydrophilic. Additionally, corroborating phenotypic results, transcriptional analyses showed that both dihydrocelastrol and dihydrocelastryl diacetate disturbed the expression of gene related to α-hemolysin (hla) and down-regulated the expressions of the crucial biofilm-associated genes (agrA, sarA, ica, RNAIII, and rbf) in MRSA. The findings of this study suggest that friedelane-based pentacyclic triterpenoids—especially dihydrocelastrol and dihydrocelastryl diacetate—have the potential to be candidates both for use in controlling biofilm-related infections and for use as important components of anti-virulence strategies for fighting against MRSA infection.







Similar content being viewed by others
References
An YH, Friedman RJ (1998) Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43(3):338–348
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33(26):5967–5982
Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS (2004) Global Gene Expression in Staphylococcus aureus Biofilms. J Bacteriol 186(14):4665–4684
Beenken KE, Mrak LN, Griffin LM, Zielinska AK, Shaw LN, Rice KC, Horswill AR, Bayles KW, Smeltzer MS (2010) Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS One 5(5):e10790
Boles BR, Horswill AR (2011) Staphylococcal biofilm disassembly. Trends Microbiol 19(9):449–455
Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O (2007) Poring over pores: α-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13(12):1405–1406
Caiazza NC, O’Toole GA (2003) Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol 185(10):3214–3217
Chen Y, Liu T, Wang K, Hou C, Cai S, Huang Y, Du Z, Huang H, Kong J, Chen Y (2016) Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One 11(4):e0153468
Cheung AL, Ying P (1994) Regulation of α- and β-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol 176(3):580–585
Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA (1992) Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA 89(14):6462–6466
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22(6):996–1006
Clinical and Laboratory Standards Institute (2007) Performance standards for antimicrobial susceptibility testing; 17th informational supplement, M100-S17. Clinical and Laboratory Standards Institute, Wayne
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67(10):5427–5433
Deora R, Tseng T, Misra TK (1997) Alternative transcription factor σSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol 179(20):6355–6359
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13(1):16–34
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Dunne WM Jr (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15(2):155–166
Field D, O’Connor R, Cotter PD, Ross RP, Hill C (2016) In vitro activities of nisin and nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front Microbiol 7:508
Fitzpatrick F, Humphreys H, O’Gara JP (2005) Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J Clin Microbiol 43(4):1973–1976
Gil C, Solano C, Burgui S, Latasa C, Garcia B, Toledo-Arana A, Lasa I, Valle J (2014) Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect Immun 82(3):1017–1029
Gilabert M, Marcinkevicius K, Andujar S, Schiavone M, Arena ME, Bardón A (2015) Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine 22(1):77–85
Gustafsson E, Nilsson P, Karlsson S, Arvidson S (2004) Characterizing the dynamics of the quorum-sensing system in Staphylococcus aureus. J Mol Microbiol Biotechnol 8(4):232–242
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112(9):1300–1307
Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI (2005) Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436(7054):1171–1175
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4):322–332
Jusko M, Potempa J, Kantyka T, Bielecka E, Miller HK, Kalinska M, Dubin G, Garred P, Shaw LN, Blom AM (2014) Staphylococcal proteases aid in evasion of the human complement system. J Innate Immun 6(1):31–46
Kiran MD, Adikesavan NV, Cirioni O, Giacometti A, Silvestri C, Scalise G, Ghiselli R, Saba V, Orlando F, Shoham M, Balaban N (2008) Discovery of a quorum sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol Pharmacol 73(5):1578–1586
Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM (1997) Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol 24(5):895–904
Lee JH, Kim YG, Yong Ryu S, Lee J (2016) Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci Rep 6:19267
Li JW, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325(5937):161–165
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339(8):520–532
Luong TT, Lei MG, Lee CY (2009) Staphylococcus aureus Rbf activates biofilm formation in vitro and promotes virulence in a murine foreign body infection model. Infect Immun 77(1):335–340
Manna AC, Bayer MG, Cheung AL (1998) Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol 180(15):3828–3836
McAdow M, DeDent AC, Emolo C, Cheng AG, Kreiswirth BN, Missiakas DM, Schneewind O (2012) Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect immune 80(10):3389–3398
Nosyk O, ter Haseborg E, Metzger U, Frimmel FH (2008) A standardized pre-treatment method of biofilm flocs for fluorescence microscopic characterization. J Microbiol Methods 75(3):449–456
Ohlsen K, Koller KP, Hacker J (1997) Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla:lacZ gene fusion. Infect Immun 65(9):3606–3614
Oscarsson J, Kanth A, Tegmark-Wisell K, Arvidson S (2006) SarA is a repressor of hla (α-hemolysin) transcription in Staphylococcus aureus: its apparent role as an activator of hla in the prototype strain NCTC 8325 depends on reduced expression of sarS. J Bacteriol 188(24):8526–8533
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188
Parsek MR, Greenberg EP (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13(1):27–33
Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Otto M (2012) How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA 109(4):1281–1286
Potera C (1999) Forging a link between biofilms and disease. Science 283(5409):1837–1839
Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I (2007) Revisiting the ancient concept of botanical therapeutics. Nat Chem Boil 3(7):360–366
Singh R, Ray P, Das A, Sharma M (2010) Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65(9):1955–1958
Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE (1996) Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274(5294):1859–1866
Sperandio V (2007) Novel approaches to bacterial infection therapy by interfering with bacteria-to-bacteria signaling. Expert Rev Anti Infect Ther 5(2):271–276
Stefani S, Varaldo PE (2003) Epidemiology of methicillin-resistant staphylococci in Europe. Clin Microbiol Infect 9(12):1179–1186
Ta CA, Arnason JT (2015) Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules 21(1):E29
Tamber S, Cheung AL (2009) SarZ promotes the expression of virulence factors and represses biofilm formation by modulating SarA and agr in Staphylococcus aureus. Infect Immun 77(1):419–428
Tu Quoc PH, Genevaux P, Pajunen M, Savilahti H, Georgopoulos C, Schrenzel J, Kelley WL (2007) Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect Immun 75(3):1079–1088
Zhang XS, García-Contreras R, Wood TK (2007) YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J Bacteriol 189(8):3051–3062
Acknowledgements
This study was supported by Soonchunhyang University research fund and by Laboratory Safety Management Program, through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (NRF- 2016H1D7A2020904).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest relevant to this article.
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Woo, SG., Lee, SM., Lee, SY. et al. The effectiveness of anti-biofilm and anti-virulence properties of dihydrocelastrol and dihydrocelastryl diacetate in fighting against methicillin-resistant Staphylococcus aureus . Arch Microbiol 199, 1151–1163 (2017). https://doi.org/10.1007/s00203-017-1386-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1386-x