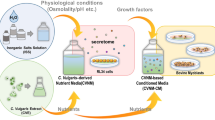
Abstract
For sustainable production of cultured meat, we propose a novel circular cell culture (CCC) system in which microalgae are used as nutrient supply for the mammalian cell culture and as a waste-medium recycler. Chlorococcum littorale, RL34 hepatocytes, and C2C12 myoblasts were used as cell sources for microalgae, growth factor-producing cells, and muscle cells, respectively. In the first cycle, C2C12 cells were amplified 4.0-fold after 48 h of culture in an RL34 cell-conditioned medium. In the second cycle, C2C12 cells were cultured in the C. littorale culture waste medium to which the C. littorale-derived nutrients were added. The proliferation rates of C. littorale and C2C12 and the nutrient extraction efficiency from C. littorale were the same in the first and second cycles. Therefore, this CCC system, which works without additional grain-derived nutrients and animal sera, will help drastically reduce environmental load, resource/energy consumption, and costs in future cultured meat production.
Similar content being viewed by others
Introduction
The agricultural sector is responsible for 9% of the global carbon dioxide emissions, 37% of the methane emissions, which is a greenhouse gas (GHG) potentially 20–28 times more harmful than carbon dioxide, and 65% of the nitrous oxide emissions, which is potentially 300 times more harmful than carbon dioxide (Miranda et al. 2015; Pandurangan and Kim 2015; Cassia et al. 2018; EPA 2021; Simdi and Seker 2022). Livestock farming contributes to 14.5–18% of the global GHG emissions, which is more than the contribution of the transport sector (Tuomisto and Teixeira de Mattos 2011; Tuomisto 2019; FAO 2021). Presently, 30% of global ice-free land and 25% of the available fresh water are used for livestock (Tuomisto and Teixeira de Mattos 2011; Tuomisto 2019). Furthermore, 33% of the global arable land is used to produce livestock feed. Climate change, environmental contamination, and livestock diseases have threatened the stability, safety, and sustainability of livestock-dependent meat production. To solve the problems associated with the conventional meat production system, “cultured meat” has attracted attention worldwide as a sustainable alternative to meat (Post 2014).
The high demand for cultured meat requires large-scale production of mammalian cells, which consume large quantities of culture media. Approximately 50 L of culture media is required to produce 1 kg of cultured meat (approximately 5 × 1010 cells) (Post 2014). The increased demand for cultured meat leads to increased media production. However, the mass production of culture media is limited by the treatment of the resulting waste and the availability of materials, such as nutrients.
The culture media consist of nutrients such as glucose, amino acids, and animal sera such as fetal bovine serum (FBS). Currently, these nutrients are directly or indirectly derived from grains. The use of grain-derived nutrients for cultured meat would compete with the needs for food production and potentially increase grain prices. In addition, large amounts of energy and natural resources are expended on producing grains, which require chemical fertilizers and agrochemicals. The most commonly used chemical fertilizer is ammonia, which is industrially produced through the Haber–Bosch process using fossil-fuel-derived hydrogen. Ammonia production consumes 1–2% of the annual global energy supply and 3–5% of the global natural gas and is related to 3% of global carbon dioxide emission (Ye et al. 2018; Song et al. 2018). Additionally, most agrochemicals are derived from petroleum, and the increase in their production can cause significant environmental stress. Furthermore, animal sera are essential components of the culture medium. However, their use is directly linked to the high cost of cultured meat and may cause zoonoses (Gottipamula et al. 2013). Moreover, the use of animal sera contradicts the idea of cultured meat, which aims to generate a new source of proteins independently from the livestock. Thus, cultured meat production using culture media containing grain-derived nutrients and animal sera can eventually lead to a high environmental burden.
The second issue associated with culture media is the mode of waste treatment. While mammalian myoblasts vigorously deplete the glucose and glutamine in the culture media, only 26% of other amino acids are consumed (Haraguchi and Shimizu 2021). In addition, the amounts of pyruvate, vitamins, and inorganic salts in the waste media after the culturing step remain unchanged (Haraguchi and Shimizu 2021). In contrast, the amount of ammonia, which adversely affects mammalian cells, increases after culturing (Schneider et al. 1996; Price 2017; Haraguchi and Shimizu 2021). Generally, the waste medium is discarded and replaced with a new culture medium because of the reduction in nutrients and accumulation of waste products. The discarded medium can lead to environmental problems, including the eutrophication of water bodies (Mattick et al. 2015). The large volumes of waste media generated during cultured meat production will represent an inevitable issue in the future.
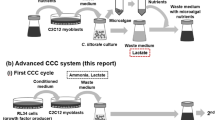
Thus, cultured meat production faces diverse issues associated with the culture media. To address this issue, we propose a novel mammalian cell culture system using microalgae. We have previously reported that mammalian cells can be cultivated using nutrients extracted from microalgae (Okamoto et al. 2020 and 2022). In addition, we have also demonstrated successful microalgal culture in mammalian cell waste medium (Haraguchi and Shimizu 2021). In this study, we aimed to demonstrate an unprecedented circular cell culture (CCC) system that combines mammalian muscle cell culture with microalgal nutrients and microalgal culture with mammalian muscle cell waste medium as a more advanced and sustainable technology for cultured meat production. Microalgae are used for supplying nutrients to the mammalian cell culture and as a recycler of the waste medium in the CCC system. The whole process of the CCC system is illustrated in comparison with the conventional culture meat production system in Fig. 1.
Materials and methods
The study design and detailed experimental process are summarized in Fig. 2.
Mammalian cell culture
C2C12 mouse myoblasts were used as muscle cells, and RL34 rat hepatocytes were used as producers of growth factors for muscle cell proliferation. These cells were cultured according to previous methods (Nishihashi et al. 2006; Haraguchi et al. 2012a) RL34 cells (5.0 × 106 cells/dish) suspended in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FBS (Thermo Fisher Scientific, MA, USA) and 1% penicillin/streptomycin (PS; Invitrogen, Carlsbad, CA, USA) were seeded on a 100 mm-diameter polystyrene dish (Greiner bio one, Gasthaus Huthmayr, Austria) and cultured for 16 h for cells to adhere to the dish. After disposing of the medium, the cells were washed twice with phosphate-buffered saline (PBS; Sigma-Aldrich) cultured in serum-free DMEM for 24 h. The three processes of (i) medium change, (ii) 24-h cultivation, and (iii) medium harvesting were repeated daily until seven days after cell seeding. Subsequently, the conditioned medium was centrifuged (2300 × g for 5 min), and the supernatant was filtered (0.2 µm; Thermo Fisher Scientific). For the C2C12 cell culture, the cells suspended in DMEM (Sigma-Aldrich) supplemented with 10% FBS and 1% PS were seeded on a 100 mm-diameter polystyrene dish (1.0 × 106 cells/dish) (Greiner bio one). After 4 h of culture, the medium was discarded, and the cells were washed twice with PBS. For myoblast culture in the first cycle, the C2C12 cells were cultured for 48 h with a mixture of RL34 conditioned medium and DMEM (vol:vol = 1:1). In contrast, for myoblast culture in the second cycle, the cells were cultured for 48 h with a mixture of the waste medium from the first cycle and the newly extracted nutrients from microalgae. Then, for both first and second cycles, cultured cells were harvested using a trypsin–EDTA solution. The cells were centrifuged at 4 °C (300 × g and 5 min) and suspended in DMEM. Trypan blue staining solution (Nacalai Tesque, Kyoto, Japan) was added to an aliquot of cell suspension (vol:vol = 1:1). Unstained cells were considered viable cells. The viable cell count was determined using a hemacytometer (Waken B Tech, Kyoto, Japan), and the medium volumes were measured using micropipettes (M&S Instruments, Osaka, Japan). Viable cell counts were calculated based on the cell concentrations and the medium volumes. The C2C12 culture supernatant was centrifuged (2300 × g and 5 min), and the centrifugal supernatant was filtered (0.2 µm, Thermo Fisher Scientific). This C2C12 waste medium was used for microalgal culture and biochemical analyses. Mammalian cell images were captured using a microscope (ECLIPSE TS2; Nikon, Tokyo, Japan) and the NIS-Elements BR software (Nikon).
Microalgal culture
The seawater Chlorophyta, C. littorale (NBRC 102,761, National Institute of Technology and Evaluation, Tokyo, Japan) (Satoh et al. 2004) was maintained using an Erlenmeyer flask (AsOne, Osaka, Japan) capped with a silicone plug (AsOne) in a mixture of Daigo IMK medium (Nihon Pharmaceutical, Tokyo, Japan) and Daigo artificial seawater SP (Nihon Pharmaceutical) in a plant growth chamber (Biotorn; Nippon Medical & Chemical Instruments, Osaka, Japan) at 25℃ and under 1% CO2 concentration and continuous light (photosynthetic photon flux density, PPFD: approximately 30 µmol/m2/s) and was agitated using a stirrer (60 rpm, As One). PPFD was measured using a quantum light meter (Ogawa Seiki, Tokyo, Japan). For microalgal culture in the first and second cycles of the CCC, C. littorale was cultured with a waste medium after the cultivation of C2C12 cells in the same condition as described above. Microalgae were observed by a microscope (ECLIPSE TS2; Nikon) using the NIS-Elements BR software (Nikon) (Supplementary Fig. 1). Microalgal growth was analyzed by measuring turbidity and wet weight as described below:
(i) Turbidity: The microalgal suspension (100 µL) at 0 or 48 h after the cultivation was seeded in a 96-well plate (AGC Techno Glass, Shizuoka, Japan) and was measured at an optical density of 750 nm (OD750) using a spectrophotometer (Nivo F; PerkinElmer, Waltham, MA, USA). The OD750, which was subtracted by the OD of blank without microalgae, was measured at 0 or 48 h. The average values of duplicate experiments were used.
(ii) Wet weight: Microalgae were harvested in a centrifuge tube (Greiner Bio-One) by centrifugation (2300 × g, 5 min), and the supernatant was harvested using a micropipette (M&S Instruments). The weights of the centrifuge tube without/with microalgae were measured using an electronic analytical scale (Mettler Toledo, Columbus, OH, USA), and the wet weight of microalgae was calculated by subtracting the weight of the centrifuge tube without microalgae from that of the tube with microalgae.
Nutrient extraction/analysis from microalgae cultured by waste medium
After C. littorale was cultured in the mammalian cell waste medium for 48 h in the first and second cycles, the microalgae were harvested by centrifugation (2300 × g and 5 min). Nutrients were extracted from the microalgae using hydrochloric acid, as previously described (Okamoto et al. 2020 and 2022). Briefly, the microalgae were treated with 0.5 N hydrochloric acid (Fujifilm Wako Pure Chemical, Osaka, Japan) at a concentration of 330 g (wet weight)/L at 100℃ for 24 h. After neutralization using sodium hydroxide (Fujifilm Wako Pure Chemical) and centrifugation (12,500 × g and 5 min), the supernatant was harvested and used for mammalian cell culture. Microalgal extraction was added at 5% concentration (V/V) in C2C12 cell culture.
Glucose, amino acids, and ammonia levels of the microalgal extract and waste medium were analyzed by the hexokinase method, liquid chromatography-mass spectrometry, and a colorimetric method, respectively, as described in previous reports (Haraguchi et al. 2017; Okamoto et al. 2020 and 2022).
Statistical analysis
Two-group or multi-group comparisons were conducted by unpaired Student’s t test and one-way ANOVA with post-hoc Tukey’s HSD test, respectively.
Results
Serum-free myoblast culture (first myoblast culture process)
In this study, we used RL34 cell-conditioned medium as an alternative for FBS. Almost all C2C12 myoblasts adhered to the culture dish surface after 4 h from cell seeding; the cells efficiently proliferated when cultured in the RL34 cell-conditioned medium even in serum-free conditions (3.7-fold in 44 h; Fig. 3a, 3b). After 48-h cultivation, C2C12 cells amplified 4.0 ± 1.2-fold (Fig. 3b). The results showed that the conditioned medium of RL34 cells was a valuable alternative to FBS.
Amplification of C2C12 cells with serum-free medium (first myoblast culture process). The photomicrographs of C2C12 cells at 4 (a-i) and 48 h (a-ii) after cell seeding. The cell numbers of C2C12 cells at 4 and 48 h after cell seeding (b). Initiation cell numbers: 1 × 106 cells/100 mm-diameter dish. Data are presented as the mean ± standard deviation (n = 3)
Microalgal culture using myoblast waste medium (first microalgae culture process)
C. littorale cells were cultured with the waste medium of C2C12 myoblasts generated from the first myoblast culture process described above. After 48 h, the turbidity and wet weight of the microalgae increased 2.8-fold (Fig. 4a-i and 4b-i) and 2.2-fold (Fig. 4a-ii and b-ii), respectively, indicating that C. littorale efficiently proliferated in the culture waste medium without the use of an algal-specific medium. Moreover, while C. littorale effectively consumed ammonia (91.8 ± 8.6%; Fig. 4c-i), the microalgae hardly consumed glucose and amino acids (Fig. 4c-ii and c-iii). Therefore, the microalgae culture promoted the clearance of ammonia from the medium and resulted in effective microalgal proliferation without reducing the amount of remaining glucose and amino acids, which are essential nutrients for mammalian cells.
Cultivation of C. littorale using waste medium of myoblasts (first microalgal culture process). The photographs of Erlenmeyer flasks in which C. littorale was cultured in the waste medium of C2C12 cells at 0 (Before cultivation) and 48 h (After cultivation) after microalgal seeding (a-i). b Harvested microalgae at 0 (Before cultivation) and 48 h (After cultivation) after the seeding. After culturing for 48 h, the turbidity of the waste medium increased 2.8-fold (b-i), and the wet weight of microalgae increased 2.2-fold (b-ii). c Fluctuation of waste product and nutrient levels in the microalgal culture. The fluctuation of ammonia (c-i), glucose (c-ii), and proteinogenic amino acids (c-iii) levels in the waste medium without or with C. littorale for two days. Data are presented as the mean ± standard deviation (n = 5)
Nutrients extracted from microalgae cultured with myoblast waste medium (first nutrient extraction process)
While ammonia was cleared in the microalgal culture, whether the nutrients in the myoblast waste medium from the first culture step would be sufficient was determined by extracting nutrients from microalgae that proliferated in the waste medium. The glucose yield from C. littorale was 42.7 ± 8.4 mM; this concentration was higher than that in DMEM (24.6 ± 0.4 mM) (Fig. 5a). Moreover, 14 of 15 proteinogenic amino acids were present in DMEM, and five more proteinogenic amino acids—aspartic acid, asparagine, glutamic acid, proline, and alanine—although absent from DMEM, were detected in the microalgal extract (Fig. 5b). While glutamine was absent in the microalgal extract, highly concentrated glutamic acid was detected (Fig. 5b), indicative of glutamine oxidation to glutamic acid during acid hydrolysis. The total amount of proteinogenic amino acids in the microalgal extract was two-fold more than that in DMEM (Fig. 5b). These results indicate that the nutrients required for myoblast culture were efficiently extracted from the microalgae cultured with the waste medium.
Nutrients extracted from C. littorale cultured in the waste medium after myoblast cultivation (first nutrient extraction process). The concentrations of glucose (a) and proteinogenic amino acids (b-i) in extracts obtained by acid hydrolysis of C. littorale cultured in the C2C12 cell waste medium were higher than those in Dulbecco’s modified Eagle’s medium (DMEM). Fourteen of 15 amino acids present in the DMEM and five more proteinogenic amino acids, which were absent in the DMEM, were extracted from the microalgae (b-ii). Data are presented as the mean ± standard deviation (n = 3)
Myoblast cultivation by recycling of waste medium combined with microalgal nutrients (second myoblast culture process)
Next, C2C12 myoblasts were cultured by combining the waste medium generated in the first microalgal culture process with microalgal nutrients extracted in the first nutrient extraction process. C2C12 cell proliferation increased 5.0 ± 1.2-fold when cultured in DMEM with FBS for 48 h, whereas the cells hardly proliferated in DMEM without FBS (Fig. 6). C2C12 cells amplified in the waste medium from the first microalgal culture (2.2 ± 0.4-fold, Fig. 6). However, when the microalgal nutrient extracts were added to the waste medium, the proliferation rate of C2C12 cells was markedly enhanced (4.5 ± 1.5-fold, Fig. 6). The growth rate was comparable to that of DMEM, containing 10% FBS (Fig. 6). The growth rate of the second myoblast culture process was also comparable to that of the first myoblast culture process (Figs. 3 and 6). These results showed that RL34 cell-derived growth factors were maintained without degradation even after the first CCC cycle, and myoblasts proliferated without the use of animal sera. Moreover, the nutrients that were depleted in the second myoblast culture were successfully supplied by adding the microalgal-derived nutrients.
Cultivation of myoblasts by recycling of waste medium combined with microalgal extracts (second myoblast culture process). After 48 h of cultivation, the proliferation rates of C2C12 cells by recycling the C2C12 cell waste medium combined with the microalgal extracts were comparable to those using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. In contrast, the proliferation rates of C2C12 cells cultured in only the waste medium were similar to those cultured in DMEM supplemented without FBS. Data are presented as the mean ± standard deviation (n = 3)
Microalgal culture with the second myoblast culture waste medium (second microalgae culture process)
Next, C. littorale was cultured with the waste medium generated in the second myoblast culture process. After 48 h, the turbidity and wet weight of the microalgae increased 2.4- and 2.7-fold, respectively (Fig. 7a and b). The result shows that C. littorale also efficiently proliferated in the waste medium after the second myoblast culture. In the cultivation, C. littorale also consumed ammonia effectively (93.2 ± 4.0%, n = 6) and hardly consumed glucose and amino acids (Fig. 7c). Therefore, the second microalgal culture also led to the clearance of a waste product, ammonia, from the waste medium and led to effective microalgal proliferation without causing a reduction in glucose and amino acid levels.
Microalgal proliferation in myoblast re-cultured waste medium (second microalgal culture process). The photographs of Erlenmeyer flasks in which C. littorale were cultured in the waste medium of C2C12 cells at 0 (Before cultivation) and 48 h (After cultivation) after microalgal seeding (a-i). b Harvested microalgae at 0 (Before cultivation) and 48 h (After cultivation) after seeding. After culturing for 48 h, the turbidity of the waste medium (b-i) and the wet weight of microalgae (b-ii) were measured. c Fluctuation of re-cultured waste products and nutrients in the microalgal culture. The fluctuation in ammonia (c-i), glucose (c-ii), and proteinogenic amino acid (c-iii) levels in the waste medium without or with C. littorale for two days. Data are presented as the mean ± standard deviation (n = 3–6)
Nutrients extracted from microalgae proliferated in the second culture (second nutrient extraction process)
Finally, nutrients were extracted from the microalgae that proliferated in the second microalgal culture process. The glucose concentration of the C. littorale extract was 66.3 ± 17.6 mM, which was higher than that of DMEM (Fig. 8a). Nineteen proteinogenic amino acids, excluding glutamine, were present in the extract (Fig. 8b). The total amount of proteinogenic amino acids in the microalgal extract was over two-fold that in DMEM (Fig. 8b). The efficiency of the second nutrient extraction process was similar to that of the first extraction. These results highlight that the nutrients necessary for myoblast culture were efficiently extracted from the microalgae cultured in the waste medium from both the first and second nutrient extraction processes.
Nutrients extracted from C. littorale cultured in re-cultured myoblast waste medium (second nutrient extraction process). The concentrations of glucose (a) and proteinogenic amino acids (b-i) in acid-hydrolyzed extracts of C. littorale cultured in the C2C12 cell waste medium were higher than those in Dulbecco’s modified Eagle’s medium (DMEM). Fourteen of 15 amino acids present in the DMEM and five more proteinogenic amino acids, which were absent from the DMEM, were extracted from the microalgae (b-ii). Data are presented as the mean ± standard deviation (n = 3)
Discussion
To establish a sustainable cultured meat production system, we developed the CCC system, which combines mammalian cell culture and microalgal culture. The use of microalgae is a distinctive feature of the CCC system. Microalgae are used as both a nutrient supplier for mammalian cell culture and a recycler of the waste medium in the system. Another feature of the system is that the culture medium is circulated throughout the mammalian cell and microalgal cultures. The critical factors affecting mammalian cell proliferation include inorganic salts, nutrients such as glucose and amino acids, growth factors, and waste products (Schneider et al. 1996; Price 2017; Hegde and Behr 2012).
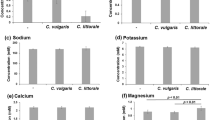
The optimum concentration of inorganic salts in culture media varies with mammalian cells and microalgae. Since mammalian cells lack a cell wall, they are susceptible to these differences. Therefore, we used a mammalian cell culture medium as the base medium in the CCC system. The medium contained approximately 160 mM sodium chloride, and the osmotic pressure of the waste medium was approximately 330 mOsm/KgH2O11. Therefore, seawater or euryhaline microalgae18 rather than freshwater microalgae are suitable for this system. In this study, seawater microalgae, C. littorale, were selected based on previous data (Haraguchi and Shimizu 2021). Because mammalian cells and microalgae hardly consume inorganic salts, such as sodium, calcium, and potassium, during their cultivation (Haraguchi and Shimizu 2021), these inorganic salts need not be added until several cycles of CCC are completed.
Next, mammalian cells consumed glucose and amino acids for their proliferation. Because these nutrients decrease with each CCC cycle, they had to be added to the culture medium before the mammalian cell culture (Fig. 2). Both glucose and amino acids were efficiently extracted from C. littorale that proliferated in the myoblast waste medium (Figs. 5 and 8) and were added to the microalgal waste medium for the subsequent mammalian cell culture (Fig. 6). Regarding the composition of amino acids, DMEM contains glutamine and lacks glutamic acid; in contrast, C. littorale-derived extracts obtained by acid hydrolysis had a high concentration of glutamic acid and lacked glutamine (Figs. 5b and 8b) since glutamine is converted into glutamic acid during the acid hydrolysis process. However, as we have previously reported, C2C12 cells can proliferate using glutamic acid as an alternative to glutamine (Okamoto et al. 2020). Additionally, the total proteinogenic amino acid concentrations of the first and second mammalian cell culture wastes were almost equivalent (first cycle: 8.1 ± 0.6 mM; second cycle: 8.5 ± 0.2 mM), indicating an efficient supplementation of microalgal extracts (Figs. 4c-iii and 7c-iii). In contrast, the glucose concentration (9.3 ± 1.2 mM) of the waste medium after the second myoblast culture was lower than that (14.8 ± 4.8 mM) after the first myoblast culture (Figs. 4c-ii and 7c-ii). Therefore, considering the amount of microalgal nutrient added, glucose can become insufficient after further cycling in the CCC process. In each process of the CCC system, the concentrations of critical nutrients such as glucose and amino acids will need to be monitored, and the amount of added microalgal nutrients will need to be modified accordingly.
Alternative growth factors to FBS are required for cultured meat production. Stem cells such as mesenchymal stem cells, which produce multiple growth factors and cytokines, have the ability to regenerate tissue via a “paracrine effect” (Miyahara et al. 2006; Haraguchi et al. 2012b). Mammalian cells produce secretomes that contain growth factors such as fibroblast growth factor-2, which promotes the proliferation of mammalian myoblasts (Shima et al. 2020). We examined the growth effect of the conditioned medium and determined that C2C12 myoblasts efficiently proliferated in the conditioned medium from RL34 cells (Fig. 3). The proliferation rate of C2C12 myoblasts using the first myoblast culture process (4.0 ± 1.2-fold) was comparable to that using DMEM with FBS (5.0 ± 1.2-fold, Fig. 4c). The proliferation rate using the second myoblast culture process (4.5 ± 1.5-fold) was also maintained without a reduction as compared with that of the first myoblast culture process, indicating that the activities of the RL34 cells-derived growth factors were maintained during at least two cycles. However, their growth factors could become inactivated during multiple CCC cycles. Therefore, adding RL34 cell-derived growth factors will be necessary every few cycles. We also succeeded in the efficient proliferation of primary bovine myoblasts, which are the main cell source of cultured meat, in the conditioned medium of RL34 cells (Unpublished observation). The serum-free culture system using the RL34 cell supernatant will contribute to the establishment of a sustainable cultured meat production system.
Mammalian cells excrete ammonia generated from the amino acid metabolism as waste products, adversely affecting cell viability at concentrations higher than 0.6 mM (Schneider et al. 1996; Price 2017). In contrast, ammonia is an essential source for microalgal proliferation and is used by microalgae to synthesize all proteinogenic amino acids (Lupatini et al. 2017). Therefore, culture media waste, which is harmful to mammalian cells due to the accumulation of waste products, is a nutrient-rich culture medium for microalgae. C. littorale effectively consumed ammonia in the C2C12 myoblast waste medium (more than 90%, Fig. 4c-i and 7c-i). In contrast, the microalgae hardly consumed the nutrients such as glucose and amino acids still present in the waste medium (Figs. 4c and 7c). Therefore, microalgal culture using the mammalian cell waste medium allows the clearance of harmful wastes, and the remaining nutrients can be recycled in the next mammalian cell culture.
In the CCC system, carbon dioxide obtained through microalgal photosynthesis (Vecchi et al. 2020) is the source of carbon; therefore, the CCC system requires no additional carbon sources such as glucose. However, the system bears a limitation by requiring the replenishment of nitrogen and phosphorus sources as they decrease. Using microalgae with nitrogen-fixing capacity (Kumar et al. 2010) could eliminate the requirement for additional chemically synthesized nitrogen compounds. We are currently working on further improvements to optimize the system thoroughly.
In the present study, the growth rate of microalgae cultured with mammalian cell culture waste medium was significantly higher than that in our previous report (this report: 2.2–2.8-fold after two days, previous report: 2.2-fold after seven days) (Haraguchi and Shimizu 2021). In this study, we examined and optimized the culture conditions of microalgae, which differed in some aspects from those in the previous report: (i) While in the previous study, microalgae were cultured statically on a mammalian cell culture dish, in this study, a spinner culture system was used on an Erlenmeyer flask. (ii) In the previous study, microalgae were cultured in a CO2 incubator for mammalian cell culture, whereas in this study, microalgae were cultured in a plant growth chamber. (iii) In the previous study, the light irradiation source was only placed above, whereas, in this study, the light was irradiated from all directions. Thus, the improvement in culture conditions increased microalgal proliferation. This implies increased carbon dioxide fixation in the CCC system, which may lead to more efficient mammalian cell proliferation.
By changing meat production from using livestock to mammalian cells cultured with microalgal nutrients, the environmental load and resource/energy consumption will drastically decrease, that is, (i) 99% of the land, (ii) 78–96% of GHG emissions, (iii) 82–96% of water, and (iv) 7–45% of energy will be saved (Tuomisto and Teixeira de Mattos 2011). The CCC system might contribute to a more efficient cultured meat production system because microalgae are cultured using the muscle cell waste medium without an algal-specific medium. Additionally, FBS is replaced by a culture supernatant of growth factor-producing cells. These features minimize the cost of the culture medium required to produce cultured meat and drastically reduce the disposal volume of the culture media. Therefore, the CCC system will drastically reduce the environmental load, resource/energy consumption, and cost associated with cultured meat production.
This is the first report on the potential application of a CCC system using microalgae as a nutrient supplier for mammalian cell culture and simultaneously as a recycler of the mammalian cell culture waste medium. Further research on the factors that are depleted and accumulated during further CCC cycles and whether those factors affect cell proliferation is required. Identifying and controlling these factors will allow the development of an ideal CCC system for producing cultured meat sustainably. In the future, the CCC system may contribute not only to cultured meat production but also to other fields such as biopharmaceuticals, antibody therapeutics, and vaccine development.
References
Ahmad I, Hellebust JA (1984) Osmoregulation in the extremely euryhaline marine micro-alga Chlorella autotrophica. Plant Physiol 74:1010–1015. https://doi.org/10.1104/pp.74.4.1010
Cassia R, Nocioni M, Correa-Aragunde N, Lamattina L (2018) Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front Plant Sci 9:273. https://doi.org/10.3389/fpls.2018.00273
Environmental Protection Agency of United States (EPA) (2021) Overview of greenhouse gases. https://www.epa.gov/ghgemissions/overview-greenhouse-gases.
Food and Agriculture Organization of the United States (FAO) (2021). Key facts and fndings. http://www.fao.org/news/story/pt/item/197623/icode/#:~:text=Total%20emissions%20from%20global%20livestock,of%20all%20anthropogenic%20GHG%20emissions.
Gottipamula S, Muttigi MS, Kolkundkar U, Seetharam RN (2013) Serum-free media for the production of human mesenchymal stromal cells: a review. Cell Prolif 46:608–627. https://doi.org/10.1111/cpr.12063
Haraguchi Y, Kagawa Y, Sakaguchi K, Matsuura K, Shimizu T, Okano T (2017) Thicker three-dimensional tissue from a “symbiotic recycling system” combining mammalian cells and algae. Sci Rep 7:41594. https://doi.org/10.1038/srep41594
Haraguchi Y, Shimizu T (2021) Microalgal culture in animal cell waste medium for sustainable “cultured food” production. Arch Microbiol 203:5525–5532. https://doi.org/10.1007/s00203-021-02509-x
Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T (2012a) Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc 7:850–858. https://doi.org/10.1038/nprot.2012.027
Haraguchi Y, Shimizu T, Yamato M, Okano T (2012b) Concise review: cell therapy and tissue engineering for cardiovascular disease. Stem Cells Transl Med 1:136–141. https://doi.org/10.5966/sctm.2012-0030
Hegde A, Behr B (2012) Media composition: growth factors. Methods Mol Biol 912:177–198. https://doi.org/10.1007/978-1-61779-971-6_11
Kumar K, Mella-Herrera RA, Golden JW (2010) Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol 2:a000315. https://doi.org/10.1101/cshperspect.a000315
Lupatini AL, Colla LM, Canan C, Colla E (2017) Potential application of microalga Spirulina platensis as a protein source. J Sci Food Agric 97:724–732. https://doi.org/10.1002/jsfa.7987
Mattick CS, Landis AE, Allenby BR, Genovese NJ (2015) Anticipatory life cycle analysis of in vitro biomass culture for cultured meat production in the United States. Environ Sci Technol 49:11941–11949. https://doi.org/10.1021/acs.est.5b01614
Miranda ND, Tuomisto HL, McCulloch MD (2015) Meta-analysis of greenhouse gas emissions from anaerobic digestion processes in dairy farms. Environ Sci Technol 49:5211–5219. https://doi.org/10.1021/acs.est.5b00018
Miyahara Y et al (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12:459–465. https://doi.org/10.1038/nm1391
Nishihashi H, Kanno Y, Tomuro K, Nakahama T, Inouye Y (2006) Primary structure and organ-specific expression of the rat aryl hydrocarbon receptor repressor gene. Biol Pharm Bull 29:640–647. https://doi.org/10.1248/bpb.29.640
Okamoto Y, Haraguchi Y, Sawamura N, Asahi T, Shimizu T (2020) Mammalian cell cultivation using nutrients extracted from microalgae. Biotechnol Prog 36:e2941. https://doi.org/10.1002/btpr.2941
Okamoto Y, Haraguchi Y, Yoshida A, Takahashi H, Yamanaka K, Sawamura N, Asahi T, Shimizu T (2022) Proliferation and differentiation of primary bovine myoblasts using Chlorella vulgaris extract for sustainable production of cultured meat. Biotechnol Prog 38:e3239. https://doi.org/10.1002/btpr.3239
Pandurangan M, Kim DH (2015) A novel approach for in vitro meat production. Appl Microbiol Biotechnol 99:5391–5395. https://doi.org/10.3389/fpls.2018.00273
Post MJ (2014) An alternative animal protein source: cultured beef. Ann NY Acad Sci 1328:29–33. https://doi.org/10.1111/nyas.12569
Price PJ (2017) Best practices for media selection for mammalian cells. In Vitro Cell Dev Biol Anim 53:673–681. https://doi.org/10.1007/s11626-017-0186-6
Satoh A, Kurano N, Harayama S, Miyachi S (2004) Effects of chloramphenicol on photosynthesis, protein profiles and transketolase activity under extremely high CO2 concentration in an extremely-high-CO2-tolerant green microalga, Chlorococcum littorale. Plant Cell Physiol 45:1857–1862. https://doi.org/10.1093/pcp/pch196
Schneider M, Marison IW, von Stockar U (1996) The importance of ammonia in mammalian cell culture. J Biotechnol 46:161–185. https://doi.org/10.1016/0168-1656(95)00196-4
Shima A, Itou A, Takeuchi S (2020) Cell fibers promote proliferation of co-cultured cells on a dish. Sci Rep 10:288. https://doi.org/10.1038/s41598-019-57213-0
Simdi H, Seker A (2022) A change is gonna come: will traditional meat production end? Environ Sci Pollut Res 29:30470–30485. https://doi.org/10.1007/s11356-021-17829-0
Song Y, Johnson D, Peng R, Hensley DK, Bonnesen PV, Liang L, Huang J, Yang F, Zhang F, Qiao R, Baddorf AP, Tschaplinski TJ, Engle NL, Hatzell MC, Wu Z, Cullen DA, Meyer HM 3rd, Sumpter BG, Rondinone AJ (2018) A physical catalyst for the electrolysis of nitrogen to ammonia. Sci Adv 4:e1700336. https://doi.org/10.1126/sciadv.1700336
Tuomisto HL (2019) The eco-friendly burger: Could cultured meat improve the environmental sustainability of meat products? EMBO Rep 20:e47395
Tuomisto HL, Teixeira de Mattos MJ (2011) Environmental impacts of cultured meat production. Environ Sci Technol 45:6117–6123. https://doi.org/10.1021/es200130u
Vecchi V, Barera S, Bassi R, Dall’Osto L (2020) Potential and challenges of improving photosynthesis in algae. Plants (basel) 9:67. https://doi.org/10.3390/plants9010067
Ye Y, Ngo HH, Guo W, Liu Y, Chang SW, Nguyen DD, Liang H, Wang J (2018) A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour Technol 268:749–758. https://doi.org/10.1016/j.biortech.2018.07.111
Acknowledgements
This work was supported by Cabinet Office, Government of Japan, Cross-ministerial Moonshot Agriculture, Forestry and Fisheries Research and Development Program, “Technologies for Smart Bio-industry and Agriculture” (funding agency: Bio-oriented Technology Research Advancement Institution).
Funding
Cross-ministerial Moonshot Agriculture,Forestry and Fisheries Research and Development Program,“Technologies for Smart Bio-industry and Agriculture” (funding agency: Bio-oriented Technology Research Advancement Institution)
Author information
Authors and Affiliations
Contributions
Y.H., Y.O., and T.S. discussed and defined the project. Y.H. initiated and led the project in experimental design, sample fabrication, and procurement and performed most of the measurements. Y.H., Y.O., and T.S. discussed results and interpretation and prepared and finalized the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Tokyo Women’s Medical University was receiving research funds from IntegriCulture Inc.
Ethical approval
This study does not include any experimental procedure performed on humans or animals.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haraguchi, Y., Okamoto, Y. & Shimizu, T. A circular cell culture system using microalgae and mammalian myoblasts for the production of sustainable cultured meat. Arch Microbiol 204, 615 (2022). https://doi.org/10.1007/s00203-022-03234-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03234-9