Abstract
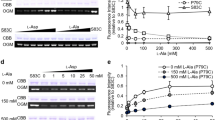
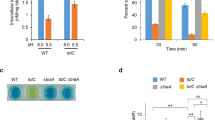
The Escherichia coli alaE gene encodes the l-alanine exporter, AlaE, that catalyzes active export of l-alanine using proton electrochemical potential. The transporter comprises only 149 amino acid residues and four predicted transmembrane domains (TMs), which contain three charged amino acid residues. The AlaE-deficient l-alanine non-metabolizing cells (ΔalaE cells) appeared hypersusceptible to l-alanyl-l-alanine showing a minimum inhibitory concentration (MIC) of 2.5 µg/ml for the dipeptide due to a toxic accumulation of l-alanine. To elucidate the mechanism by which AlaE exports l-alanine, we replaced charged amino acid residues in the TMs, glutamic acid-30 (TM-I), arginine-45 (TM-II), and aspartic acid-84 (TM-III) with their respective charge-conserved amino acid or a net neutral cysteine. The ΔalaE cells producing R45K or R45C appeared hypersusceptible to the dipeptide, indicating that arginine-45 is essential for AlaE activity. MIC of the dipeptide in the ΔalaE cells expressing E30D and E30C was 156 µg/ml and >10,000 µg/ml, respectively, thereby suggesting that a negative charge at this position is not essential. The ΔalaE cells expressing D84E or D84C showed an MIC >10,000 and 78 µg/ml, respectively, implying that a negative charge is required at this position. These results were generally consistent with that of the l-alanine accumulation experiments in intact cells. We therefore concluded that charged amino acid residues (R45 and D84) in the AlaE transmembrane domain play a pivotal role in l-alanine export. Replacement of three cysteine residues at C22, C28 (both in TM-I), and C135 (C-terminal region) with alanine showed only a marginal effect on l-alanine export.



Similar content being viewed by others
References
Abe K, Ohnishi R, Yagi K, Nakajima T, Higuchi T, Sano M, Machida M, Sarker RI, Maloney PC (2002) Plasmid-encoded asp operon confers a proton motive metabolic cycle catalyzed by an aspartate-alanine exchange reaction. J Bacteriol 184:2906–2913
Bay DC, Rommens KL, Turner RJ (2008) Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta 1778:1814–1838
Butler PJ, Ubarretxena-Belandia I, Warne T, Tate CG (2004) The Escherichia coli multidrug transporter EmrE is a dimer in the detergent-solubilised state. J Mol Biol 340:797–808
Dassler T, Maier T, Winterhalter C, Bock A (2000) Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol Microbiol 36:1101–1112
Doroshenko V, Airich L, Vitushkina M, Kolokolova A, Livshits V, Mashko S (2007) YddG from Escherichia coli promotes export of aromatic amino acids. FEMS Microbiol Lett 275:312–318
Fisher R, Tuli R, Haselkorn R (1981) A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci USA 78:3393–3397
Fleming KG, Engelman DM (2001) Specificity in transmembrane helix-helix interactions can define a hierarchy of stability for sequence variants. Proc Natl Acad Sci USA 98:14340–14344
Guan L, Kaback HR (2006) Lessons from lactose permease. Annu Rev Biophys Biomol Struct 35:67–91
Hori H (2011a) Identification and functional analysis of l-alanine exporter in Escherichia coli. Ph.D. Thesis, Tohoku University
Hori H, Ando T, Isogai E, Yoneyama H, Katsumata R (2011b) Identification of an l-alanine export system in Escherichia coli and isolation and characterization of export-deficient mutants. FEMS Microbiol Lett 316:83–89
Hori H, Yoneyama H, Tobe R, Ando T, Isogai E, Katsumata R (2011c) Inducible l-alanine exporter encoded by the novel gene ygaW (alaE) in Escherichia coli. Appl Environ Microbiol 77:4027–4034
Kadner RJ (1996) Cytoplasmic Membrane. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, pp 58–87
Kennerknecht N, Sahm H, Yen MR, Patek M, Saier MH Jr, Eggeling L (2002) Export of l-isoleucine from Corynebacterium glutamicum: a two-gene-encoded member of a new translocator family. J Bacteriol 184:3947–3956
Kim S, Ihara K, Katsube S, Hori H, Ando T, Isogai E, Yoneyama H (2015) Characterization of the l-alanine exporter AlaE of Escherichia coli and its potential role in protecting cells from a toxic-level accumulation of l-alanine and its derivatives. MicrobiologyOpen 4:632–643
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation part I. Production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol 3:193–205
Kutukova EA, Livshits VA, Altman IP, Ptitsyn LR, Zyiatdinov MH, Tokmakova IL, Zakataeva NP (2005) The yeaS (leuE) gene of Escherichia coli encodes an exporter of leucine, and the Lrp protein regulates its expression. FEBS Lett 579:4629–4634
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Livshits VA, Zakataeva NP, Aleshin VV, Vitushkina MV (2003) Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res Microbiol 154:123–135
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A (2006) Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173–179
Nakamura J, Hirano S, Ito H, Wachi M (2007) Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl Environ Microbiol 73:4491–4498
Nandineni MR, Gowrishankar J (2004) Evidence for an arginine exporter encoded by yggA (argO) that is regulated by the LysR-type transcriptional regulator ArgP in Escherichia coli. J Bacteriol 186:3539–3546
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Nikaido H, Pages JM (2012) Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363
Oxender DL (1972) Membrane transport. Annu Rev Biochem 41:777–814
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E (2009) Structure and mechanism of a Na+-independent amino acid transporter. Science 325:1010–1014
Simic P, Sahm H, Eggeling L (2001) l-threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J Bacteriol 183:5317–5324
Vrljic M, Sahm H, Eggeling L (1996) A new type of transporter with a new type of cellular function: l-lysine export from Corynebacterium glutamicum. Mol Microbiol 22:815–826
Yan N (2013) Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem Sci 38:151–159
Yerushalmi H, Schuldiner S (2000) A common binding site for substrates and protons in EmrE, an ion-coupled multidrug transporter. FEBS Lett 476:93–97
Zakataeva NP, Aleshin VV, Tokmakova IL, Troshin PV, Livshits VA (1999) The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett 452:228–232
Acknowledgments
We thank Taiji Nakae for his valuable discussion and critical reading of the manuscript. We also thank Hatsuhiro Hori for his helpful discussion. This study was supported in part by the Institute for Fermentation, Osaka, a grant from the Japan Science and Technology Agency, and the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Djamel Drider.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, S., Ihara, K., Katsube, S. et al. Impact of charged amino acid substitution in the transmembrane domain of l-alanine exporter, AlaE, of Escherichia coli on the l-alanine export. Arch Microbiol 199, 105–114 (2017). https://doi.org/10.1007/s00203-016-1279-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1279-4