Abstract
Summary
This study evaluated the yield of routine laboratory examination in a large population of older women in primary care. The prevalence of laboratory abnormalities was low and the clinical consequences in follow-up were limited. There was a weak association of laboratory abnormalities with osteoporosis but no association with vertebral fractures and recent fractures.
Purpose
Most osteoporosis guidelines advice routine laboratory examination. We have investigated the yield of laboratory examinations in facture risk evaluation of elderly women in primary care.
Methods
We assessed the prevalence of laboratory abnormalities and their association with risk factors for fractures, recent fractures, low bone mineral density (BMD), and prevalent vertebral fracture in 8996 women ≥ 65 years of age participating in a primary care fracture risk screening study. In a sample of 2208 of these participants, we also evaluated the medical consequences in the medical records during a follow-up period of ≥ 1 year.
Results
Vitamin D deficiency (< 30 nmol/L) was present in 13% and insufficiency (< 50 nmol/L) in 43% of the study sample. The prevalence of other laboratory abnormalities (ESR, calcium, creatinine, FT4) was 4.6% in women with risk factors for fractures, 6.1% in women with low BMD (T-score ≤ − 2.5), 6.0% after a prevalent vertebral fracture, 5.2% after a recent fracture and 2.6% in the absence of important risk factors for fractures. Laboratory abnormalities other than vitamin D were associated with low BMD (OR 1.4, 95%CI 1.1–1.8) but not with prevalent vertebral fractures nor recent fractures. Low BMD was associated with renal failure (OR 2.0, 95%CI 1.3–3.4), vitamin D insufficiency (OR 1.2, 95%CI 1.0–1.3) and deficiency (OR 1.3, 95%CI 1.1–.5). In the follow-up period, 82% of the laboratory abnormalities did not result in a new diagnosis or treatment reported in the medical records.
Conclusions
We identified a low prevalence of laboratory abnormalities in a primary care population of older women and the majority of these findings had no medical consequences.
Similar content being viewed by others
Introduction
Osteoporosis is defined as a combination of low bone mineral density (BMD) and loss of microarchitecture. It is an important risk factor for fractures and leads to substantial morbidity and mortality [1, 2]. Most people with osteoporosis have so-called primary osteoporosis, deterioration of the bone caused by multifactorial causes: sex, familial predisposition, life style and aging. Secondary osteoporosis is defined as osteoporosis that is accompanied by a disease or medication that is known to cause decline of BMD and microarchitecture. Some of the causes of secondary osteoporosis can be detected with medical history taking (e.g. corticosteroid use, excessive alcohol use, earlier menopause, rheumatoid arthritis), others can be detected by laboratory examination (e.g. hyperthyroidism, hyperparathyroidism, vitamin D deficiency, renal disease).
Most guidelines advise routine laboratory examination in patients with osteoporosis or in older people with recent fractures [3,4,5,6]. This advice is based on high prevalence of laboratory abnormalities in mainly secondary care studies. A review of these studies shows a prevalence of secondary osteoporosis in 5–48% of the evaluated patients with osteoporosis and in 26–51% after a new fracture [7]. This large distribution in prevalence is caused by several factors: the selection of the population, the inclusion of anamnestic data, the number of performed laboratory examinations and the chosen laboratory thresholds. Secondly, the inclusion of vitamin D deficiency makes a large difference. When vitamin D deficiency was included as abnormality, this percentage is even higher up to 68%. There is a lack of data about the situation in primary care; until now, most data are collected in fracture liaisons and secondary care settings. In addition, in the studies that did evaluate subjects that were referred from primary care, the preselection is unclear leaving room for selection bias [8,9,10]. Beside causes of secondary osteoporosis, there are laboratory results that can influence treatment. Severe kidney failure is a contraindication for bisphosphonate treatment, and vitamin D deficiency can cause osteomalacia and needs primarily supplementation of vitamin D. Also, hypocalcaemia should be corrected before potent anti-osteoporotic treatment is started. Data about the yield of routine laboratory examination in a primary care population is desirable since there is evidence that screening programs in elderly women reduce hip fracture risk which leaded to the recommendation of the implementation of fracture risk screening in primary care [11,12,13]. Beside assessing the prevalence of abnormal laboratory results, it is important to know if these findings are associated with low BMD and fractures. One should question the indication for routine laboratory examination if this association is weak or absent.
The first aim of our study was to assess the prevalence of relevant laboratory abnormalities that are detectable with simple laboratory examination in a population of older women in primary care.
Secondly, we wanted to study the association of the detected laboratory abnormalities with risk factors for fractures, low BMD, prevalent vertebral fractures and recent fractures.
And thirdly, we wanted to quantify the effect of routine laboratory examination on diagnosis and treatment during a 1-year follow-up.
Methods
Participants
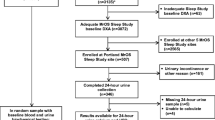
We analysed the data of women that participated in the Salt Osteoporosis Study (SOS). SOS is a pragmatic randomized clinical trial to study the effect of screening women ≥ 65 years in primary care for high fracture risk [14, 15]. In this study, all women in the intervention group with at least one risk factor for fractures were invited for bone densitometry. The exclusion criteria were a short-life expectancy according to the GP, current use of anti-osteoporotic medication or in the preceding 5 years, recent bone densitometry (< 2 years), terminal illness, body weight > 135 kg or current corticosteroid use ≥ 7.5 mg prednisone equivalent per day. In this study, 47.1% of all older women responded and of these women 42.3% with at least one risk factor were invited for bone densitometry. All women that had bone densitometry and laboratory examination (68.0%) were included in the study. Besides the women participating in de SOS trial, a random sample (15%) of the source population without important risk factors were asked to undergo the same examinations.
Measurements
Participants fulfilled a questionnaire that included questions about risk factors for fractures. Measurement of weight, length, bone densitometry of the femoral neck and the lumbar spine, vertebral fracture assessment (Hologic Discovery SL) and laboratory examinations were performed. Low BMD was defined as a T-score ≤ − 2.5 in the femoral neck or lumbar spine. Prevalent vertebral fractures were defined as a reduction in vertebral height of ≥ 20% in the lumbar spine or ≥ 25% in the thoracic spine on the vertebral fracture assessment [16]. A recent fracture was defined as any fracture < 1 year before examination (fractures of fingers, toes and skull excluded).
The serum laboratory examinations were as follows: calcium, albumin, thyroid-stimulating hormon (TSH), free tetraiodiothyronine (FT4), creatinine (Cobas, Roche), erythrocyte sedimentation rate (ESR) (Vesmatic Cube 200, Menarini), 25(OH) vitamine D3 (Diasorin, Stillwater, USA). All test were performed in the same laboratory (SALT, Koog aan de Zaan, ISO certified), except for the 25(OH) vitamine D3 (AML, Antwerpen). The thresholds for the laboratory abnormalities were as follows: ESR > 50 mm/h, calcium corrected for albumin > 2.60 mmol/L, TSH < 0.30 mU/L with FT4 > 21 pmol/L, cockcroft clearance < 30 ml/min, vitamin D deficiency 25(OH)D3 < 30 nmol/L, vitamin D insufficiency 25(OH) D3 < 50 nmol/L.
We have chosen the cut off for severe renal failure (< 30 ml/min), because this has consequences for the prescription of medication. The other thresholds were the regular thresholds of our laboratory.
The questionnaires, biometrics, bone densitometry measurement, vertebral fracture assessment and laboratory measurements have been extensively described previously [14].
Medical files
We analysed the GP medical files of all participants with at least one laboratory abnormality during screening in the period between February 2010 and May 2012. The medical file of the GP was analysed by either a GP or a GP supervised medical doctor in training to become a GP. This was done after at least 1 year had elapsed between the moment the abnormal laboratory examination was reported to the GP. We studied whether the abnormal laboratory examination was previously registered in the medical file. Secondly, we looked for notes in the medical file whether the observed abnormality had led to a new diagnosis or had influenced treatment. The evaluation of the medical files and definitions are shown in Table 1.
Statistical analysis
We assessed the prevalence of laboratory abnormalities and performed univariate logistic regression, to calculate the odds ratios (OR) for all individual laboratory abnormalities and at least one laboratory abnormality in the presence a recent fracture, low BMD or prevalent vertebral fracture. Additionally, we performed a multivariate logistic regression analysis to evaluate possible confounding effect of age and body mass index. We defined a confounding effect as a more than 10% change in regression coefficient of the determinant. In this regression analysis, we made a distinction between either vitamin D deficiency and insufficiency or any other laboratory abnormality combined, because there is a considerably lower prevalence of laboratory abnormalities other than 25(OH) vitamin D, and we wanted to avoid low vitamin D to dominate the other results.
In the patients of whom the medical files were examined, we evaluated the number of new diagnoses/treatments for all participants with one or more per laboratory abnormalities.
Results
Of 8996 women with risk factors for fractures, 2536 had a low BMD, 1044 had a prevalent vertebral fracture and 334 had a new fracture. The prevalence of vitamin D insufficiency in participants with risk factors was 43.0% and vitamin D deficiency was 13.2%. The prevalence of any other abnormality was 4.6% in participant with risk factors for fracture, in participants with low BMD 6.1%, in participants with prevalent vertebral fracture 6.0%, in participants with a recent fracture 5.2% and in the group without risk factors 2.6% (Table 2).
Because of a confounding effect for age but not for BMI, we present the multivariate odds ratios corrected for age.
The presence of risk factors for fractures was associated with any laboratory abnormality other than low 25(OH)D (OR 1.8, 95% CI 1.3–2.5). Also, low BMD was associated with any laboratory abnormality other than low 25(OH)D (OR 1.4, 95% CI 1.1–1.8). There was no significant association of prevalent vertebral fractures (OR 1.1, 95% CI 0.8–1.6) nor with recent fractures (OR 1.1, 95% CI 0.7–1.9) and any laboratory abnormality other than low 25(OH)D. In the analysis of the individual laboratory abnormalities, an association was found of low BMD with creatinine clearance < 30 ml/min (OR 2.0, 95% CI 1.3–3.4), vitamin D insufficiency (OR 1.2, 95% CI 1.0–1.3) and vitamin D deficiency (OR 1.3, 95% CI 1.1–1.5). Low BMD had an inversed correlation with FT4 (OR 0.5, 95% CI 0.3–0.8) (Table 3).
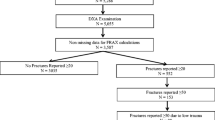
The follow-up group compromised 24.5% of all participants of which 109 (4.5%) had a laboratory abnormality. In 55/109 (50.5%), the same abnormality had also previously been noted in the medical file indicating that the abnormality was no new observation. In one third (n = 16) of the other 54 participants, no note of follow-up was found regarding the abnormality. In a third (n = 18), a follow-up measurement was normal indicating that the initial measurement had normalised or had been false positive. In about one third (n = 20) of the participants, a new diagnosis was made or the abnormal laboratory finding had led to a medical consequence (Table 4). The diagnoses are displayed in Fig. 1.
Discussion
Principal findings
This is the first study in a large primary population of older women with increased fracture risk that has quantified the prevalence of abnormalities during routine laboratory examination and the associations of these abnormalities with risk factors for fractures, recent fractures, low BMD and prevalent vertebral fractures. While the prevalence of low vitamin D is high, the prevalence of other laboratory abnormalities is low. Although we have observed an association between all laboratory abnormalities and the presence of both clinical risk factors and low BMD, we have to conclude that the associations are weak. The only exception is the association with severe renal failure. In this population, recent fractures and prevalent vertebral fractures do not increase the probability of finding laboratory abnormalities. About half of the laboratory abnormalities were previously reported in the medical file. Furthermore, 82% the new laboratory abnormalities did not lead to a new diagnosis or treatment. New diagnoses were as often found in participants with or without low BMD, recent fractures and prevalent vertebral fractures.
If we weigh this limited yield against the costs, the discomfort of examination and unnecessary further examination in false positives, the conclusion might be that there is not enough ground for a routine laboratory panel in fracture risk assessment or screening in primary care. The only exception might be the assessment of vitamin D and renal function. An alternative solution to the assessment of vitamin D would be to advise vitamin D supplementation in all women at risk for fractures since deficiency is very frequent. This is currently the policy in the Netherlands [18]. Another alternative could be to select those with risk factors for vitamin D deficiency for supplementation or measurement [19]. Since severe chronic kidney failure is a contra indication for bisphosphonates, we suggest that the kidney function is routinely verified or assessed before start of treatment.
Leaving out most of the routine laboratory examinations will sound contra-intuitive to many physicians. When you have a patient with osteoporosis you do not want to miss that one patient with hyperparathyroidism or a malignancy. On the other hand, we have to conclude that low BMD, recent fractures and prevalent vertebral fractures turn out to be no strong signals for these conditions in a primary care population. Primary care seems to have particularly primary osteoporosis. Performing additional laboratory examinations should therefore be performed on clinical indication only.
Comparison with other studies
Compared to other studies the number of laboratory abnormalities is low in our study. There are several explanations. In the first place, some studies use a more extensive laboratory panel. Secondly, the threshold in our study for abnormal renal function is lower than in some other studies (some studies include renal failure stage 3, 30–45 ml/min). And in the third place, our study population differs from other studies. The population in studies in a fracture liaison service or secondary care might be a selection of less healthy persons.
Another difference with the previous studies is that of all laboratory abnormalities the proportion of new abnormalities is lower in our population. We think that this is caused by not only collecting information from the participants but also including information from the GP medical file in our study.
Five studies looked at the association between osteoporosis and laboratory abnormalities or newly found secondary osteoporosis, three in a fracture liaison, one in a population of healthy older women and one in an unselected cohort of older men [20,21,22,23,24]. Only one study in the fracture liaison found an association between osteoporosis and newly found secondary causes of osteoporosis. The weak associations we have found in our study population is in concordance with the inconsistence between the studies.
Strengths and limitations
The primary strength of this study is the strictly primary care setting, since information on the prevalence of laboratory abnormalities in women at risk for fractures in primary care is not available. A second strength of the study is the sample size. This opened the opportunity to identify not only associations between risk factors and the complete laboratory panel, but also with the single measurements. The third strength of this study is the follow-up by checking the medical files at least 1 year later. The benefit of the approach is that we could estimate the yield of the routine laboratory examination and get insight in what are true findings and false positives. We think that this approach is important to support solid recommendations in guidelines. The fourth strength in this study is that not only the association with low BMD was taken into account but also with prevalent vertebral fractures and recent fractures. These are important pillars of fracture risk assessment besides the diagnosis low BMD based on bone densitometry alone.
A limitation in this study is that only about 50% of the people invited to the SOS participated in the trial. Furthermore, all women with recent bone densitometry or earlier use of osteoporosis medication were excluded; this comprised 6.6% of the population. This could have led to selection bias. Indeed, we saw a lower participation in higher age in the SOS, indicating a possible selection of the healthier individuals. In a previous pilot study of SOS women of 50 years or older, which was performed during a clinical improvement program without requesting extensive informed consent of the participants, the risk of selection was smaller with a response rate of 83.4% [14]. In the women of 65 years and older (n = 324) with risk factors for fractures, the prevalence of vitamin D deficiency was 44.9% and other laboratory abnormalities was 4.1% (unpublished). Since these percentages are similar to our study, this indicates that the effect of selection bias is probably limited.
In our study, we have focused on laboratory examinations that are easily accessible in primary care and which cover most prevalent and relevant secondary causes. Another limitation of this study is, therefore, that we did not measure PTH, resulting in the inability to identify secondary hyperparathyroidism. However, since we have measured vitamin 25(OH)D and creatinine clearance, the main causes of secondary hyperparathyroidism are covered. With our panel, we have probably also missed some rare causes of secondary osteoporosis, but that is unlikely to have much effect on the results, since the prevalence of the more common causes of secondary osteoporosis are already very low. Furthermore, there are two restrictions regarding generalizability. As this study included only women in primary care, the results cannot be extrapolated to men nor to a fracture liaison or secondary care setting.
Conclusion
In elderly women at risk for fractures in primary care, the prevalence of relevant laboratory abnormalities found in a routine screening is low. Examination of ESR, calcium, creatinine and TSH/FT4 leads to few new diagnoses and clinical consequences. Nevertheless, we found a high prevalence of vitamin D insufficiency and deficiency. There was a weak association between all laboratory abnormalities and low BMD, but in particular severe renal failure was associated with low BMD. We suggest that the routine laboratory examination in primary care in women with low BMD should only imply creatinine measurement and 25(OH) vitamin D, if vitamin D supplementation is not already started. The recommendation in guidelines to add other routine laboratory examinations is not supported by this study.
References
Svedbom A, Borgstrom F, Hernlund E, Strom O, Alekna V et al (2018) Quality of life up to 18 months after low-energy hip, vertebral, and distal forearm fractures- results from the ICUROS. Osteopor Int 29(3):557–566. https://doi.org/10.1007/s00198-017-4317-4
Abrahamsen B, van Staa T, Ariely R, Olsen M, Cooper C (2009) Excess mortality following hip fracture: a systematic epidemiological review. Osteopor Int 20(10):1633–1650. https://doi.org/10.1007/s00198-009-0920-3
LeBoff MA, Greenspan SL, Insogna KL, Lewieki EM, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Ost Int 33(10):2049–2102. https://doi.org/10.1007/s00198-021-05900-y
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS. Farooko A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB (2020) American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 26(Supple 1):1–46. https://doi.org/10.4158/GL-2020-0524SUPPL
Kanis JA, Cooper C, Rizzoli R, Reginser JY (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30(1):3–44. https://doi.org/10.1007/s00198-018-4704-5
Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, McCloskey E, Moss K, Parker J, Paskins Z, Poole K, Reid DM, Stone M, Thomson J, Vine N, Comston J (2022) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporosis 17(1):58. https://doi.org/10.1007/s11657-022-01061-5
Bours SPG, Van de Bergh JPW, Geel TACM, Geusens PPMM (2014) Secondary osteoporosis and metabolic bone disease in patients 50 years and older with osteoporosis or with a recent clinical fracture: a clinical perspective. Curr Opin Rheumatol 26(4):430–439. https://doi.org/10.1097/BOR.0000000000000074
Tannenbaum C, Clark J, Schwartzman K et al (2002) Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 87:4431–4437. https://doi.org/10.1210/jc.2002-020275
Deutschmann H, Weger M, Weger W et al (2002) Search for occult secondary osteoporosis: impact of identified possible risk factors on bone mineral density. J Intern med 252:389–397. https://doi.org/10.1046/j.1365-2796.2002.01040.x
Freitag A, Barzel US (2002) Differential diagnosis of osteoporosis. Gerontology 48:98–102. https://doi.org/10.1159/000048934
Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM (2020) Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Ost Int 31(2):251–257. https://doi.org/10.1007/s00198-019-05226-w
McCloskey EV, Chotiyarnwong P, Harvey NC, Lorentzon M, Kanis JA (2022) Population screening for risk in postmenopausal women- a logical step in reducing the osteoporotic fracture burden. Ost Int 33(8):1631–1637. https://doi.org/10.1007/s00198-022-06419-6
Chotiayarnwong P, McCloskey EV, Harvey NC, Lorentzon M, Prieto-Alhambra D et al (2022) Is it time to consider population screening for fracture risk in postmenopausal women? A position paper from the international Osteoporosis Foundation Epidemiologie/Quality of Life Working Group. Arch Osteoporos 17(1):87. https://doi.org/10.1007/s11657-022-01117-6
Elders PJM, Merlijn T, Swart KMA et al (2017) Design of the SALT Osteoporosis Study: a randomised pragmatic trial, to study a primary care screening and treatment program for the prevention of fractures in women aged 65 years or older. BMC Musculoskelet Disord 18(1):424. https://doi.org/10.1186/s12891-017-1783-y
Merlijn T, Swart KMA, van Schoor NM, Heymans MW, van der Zwaard BC, van der Heijden AA, Rutters F, Lips P, van der Horst HE, Niemeijer C, Netelenbos JC, Elders PJM (2019) The effect of a screening and treatment program for the prevention of fractures in older women: a randomized trial. JBMR 34(11):1993–2000. https://doi.org/10.1002/jbmr.3815
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiqantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
van Boven K, ten Napel H (2021) ICPC-3 International Classification of Primary Care: user manual and classification. CRC Press, Boca Raton. ISBN 9781003197157
Health Council of the Netherlands (2012) Evaluation of the dietary reference values for vitamin D. Health Council of the Netherlands, The Hague
Merlijn T, Swart KMA, Lips P, Heymans MW, Sohl E, Van Schoor NM, Netelenbos CJ, Elders PJM (2018) Prediction of insufficient serum vitamin D status in older women: a validated model. Ost Int 29(7):1539–1547. https://doi.org/10.1007/s00198-018-4410-3
Bours SPG, Geel TACM, Geusens PPMM, Janssen MJW, Janzing HMJ, Hoffland GA, Willems PC, Van de Bergh JPW (2011) Contributors to secondary osteoporosis and metabolic bone disease in patients presenting with a clinical fracture. J Clin Endocrinol Metab 96:1360–1367. https://doi.org/10.1210/jc.2010-2135
Malgo F, Appelman-Dijkstra NM, Termaat MF, Van der Heide HJL, Schipper IB, Rabelink TJ, Hamdy NAT (2016) High prevalence of secondary factors for bone fragility in patients with recent fracture independently of BMD. Arch Osteoporos 11:12. https://doi.org/10.1007/s11657-016-0258-3
de Klerk G, Hegeman JH, van der Velde D, van der Palen J, van Bergeijk L, ten Duis HJ. The value of laboratory tests in diagnosing secondary osteoporosis at a fracture and osteoporosis outpatient clinic. Geriatr Orthop Surg Rehabil 4(2):53–57. https://doi.org/10.1177/2151458513501176
Jamal SA, Leiter RE, Bayoumi AM, Bauer DC, Cummings SR (2005) Clinical utility of laboratory testing in women with osteoporosis. Osteoporos Int 16:534–540. https://doi.org/10.1007/s00198-004-1718-y
Fink HA, Litwack-Harrison S, Taylor BC, Bauer DC, Orwoll ES, Lee CG, Barrett-Connor E, Schousboe JT, Kado DM, Garimella PS, Ensrud (2016) Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOs) Study Group. Osteoporos Int 27(1):331–338. https://doi.org/10.1007/s00198-015-3356-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent informed
Consent was obtained from all individual participants and the study was approved by the Dutch Health Council (OGZ, 2.978.265).
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Merlijn, T., Swart, K.M.A., Niemeijer, C. et al. The yield of routine laboratory examination in osteoporosis evaluation in primary care. Osteoporos Int 35, 911–918 (2024). https://doi.org/10.1007/s00198-024-07042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-024-07042-3