Abstract
Summary
Impaired physical performance is associated with increased fracture risk. Performance on four physical functioning tests and prevalence of sarcopenia were assessed for 1789 fracture patients and compared to reference data. Performance was low on all tests, especially for patients with a hip, major or ≥ 1 prevalent vertebral fracture.
Purpose Introduction
Impaired physical performance and sarcopenia are associated with increased fracture risk. This study aims to assess physical performance and the prevalence of sarcopenia in patients with a recent clinical fracture attending the Fracture Liaison Service (FLS) compared to population means.
Methods
In this cross-sectional study, chair stand test (CST), handgrip strength (HGS), timed-up-and-go (TUG), 6-min walking-test (6MWT), and sarcopenia (following EWGSOP2) were assessed. The proportion of patients with impaired/poor performance compared to reference data was calculated (Z-score: ≥ − 2SD to < − 1 (impaired) and < − 2 SD (poor)). Associations of fracture type, sex, age, and time since fracture with Z-scores were assessed using linear regression analyses.
Results
A total of 1789 consecutive FLS patients were included (median age (IQR): 66 (59–74), 70.7% females, 3.9 (± 1.6) months after fracture). The prevalence of impaired/poor performance for CST, HGS, TUG, and 6MWT was 39.2%, 30.4%, 21.9%, and 71.5%, respectively (expected proportion of 16%) and 2.8% had sarcopenia. Lower Z-scores (P < 0.001) were found for hip, major, and ≥ 1 prevalent vertebral fracture (VF) in CST (major: regression coefficient (B) (95%CI) = − 0.25 [− 0.34, − 0.16]; hip: B = − 0.32 [− 0.47, − 0.17], VF: B = − 0.22 [− 0.34, − 0.11]), TUG; (major: B = − 0.54 [− 0.75, − 0.33]; hip: B = − 1.72 [− 2.08, -1.35], VF: B = − 0.61 [− 0.88, − 0.57]), 6MWT (major: B = − 0.34 [− 0.47, − 0.21]; hip: B = − 0.99 [− 1,22, − 0.77], VF: B = − 0.36 [− 0.53, − 0.19]).
Conclusions
Physical performance is significantly lower in FLS patients compared to healthy peers, especially in patients with hip, major or prevalent VF. These findings underline the need to assess and improve the physical performance of FLS patients, despite a low prevalence of sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures are most common in older persons and comprise an increasing health concern with substantial costs, a lower quality of life, and an increased risk of subsequent fractures and mortality in our aging population [1, 2]. The risk of subsequent fractures is the highest immediately after the index fracture [3]. However, a large treatment gap still remains in secondary fracture prevention including fracture risk evaluation and treatment. Consequently, recommendations to optimize post-fracture care were made by several international societies including the International Osteoporosis Foundation (IOF) [4, 5]. These recommendations include the implementation and optimisation of specialised Fracture Liaison Services (FLSs). At the FLS, a standardized evaluation of secondary fracture risk and personalized treatment based on these risks is offered [6, 7]. FLS care has shown to effectively reduce the risk of subsequent fractures and mortality [7]. Fall risk evaluation is advised, but often not yet routinely implemented in FLS care, and has only recently been named as a key performance indicator by the Capture the Fracture network [6]. However, the importance of fall risk evaluation at the FLS is evident; up to 90% of fractures are caused by a fall and patients attending the FLS have a high prevalence of fall risk factors ranging from 60 to 84% [8, 9]. Recently, a prospective study of FLS patients reported a subsequent 3-year fracture incidence of 11%, even though patients were treated according to current fracture prevention guidelines, with extensive fracture risk evaluation and according treatment, e.g., by anti-osteoporotic medication [10]. While evaluating these subsequent fractures, 78% were found to be fall related, half of which was sustained at the first fall and suffering a fall after the initial fracture resulted in a ninefold higher risk of a subsequent fracture [10]. A recent large population-based study found similar patterns of imminent fall and fracture risk after incident fracture, suggesting that the imminent fracture risk might very well be attributable to an imminent fall risk [11]. Fall risk is caused by a variety of risk factors, of which a decline in muscle strength and physical performance are amongst the most important [12, 13].
Muscle strength and physical performance can be quantified by several performance tests, that are also used in the revised algorithm of the European Working Group on Sarcopenia in Older People (EWGSOP2) to assess sarcopenia in clinical practice [14]. Recent efforts in older populations have shown sarcopenia and decreased performance on several tests be associated with a higher future fracture risk [15, 16]. Also, in fracture populations, many studies report on lower functional performance after incident fracture, as summarized in a review by Szulc [17]. However, in this review, large heterogeneity was present among the included studies, e.g., in terms of fracture type, ages (mostly older patients, not including ages 50–65 year olds), and sex of the populations that were studied and interventions such as exercise training that were performed after the fracture. Thus, clinicians lack a comprehensive overview of the physical performance of patients at the time of FLS evaluation, including the ‘younger’ fracture patients, and patients with a broad range of fracture types.
In this study, we present measurements of four physical performance tests the chair stand test (CST), hand grip strength (HGS), the timed up and go (TUG), and the 6-min walking test (6MWT) in patients attending the FLS after a recent clinical fracture. While poor performance on these tests is often defined using cut-off scores, we aim to give a comprehensive overview of physical performance of FLS patients by comparing these measures to age- and sex-related reference data (mean and SD) using Z-scores.
Methods
Study population
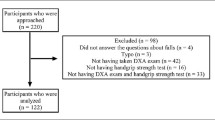
A cross-sectional study was conducted in patients with a clinical vertebral or non-vertebral fracture attending the Fracture liaison Service (FLS) in VieCuri Medical Center located in the south of the Netherlands from November 2013 to June 2016. Subsequent fracture risk evaluation includes clinical evaluation, laboratory tests, medication review, vertebral fracture assessment (VFA), bone mineral density (BMD) measurement using DXA, and ideally also a fall risk assessment [6, 18]. All adults aged 50–90 that visited the emergency department with a radiologically confirmed fracture were screened for fracture risk evaluation by trained nurses. Those eligible for fracture risk evaluation were invited to attend the FLS as part of usual care. Ineligible patients were persons living outside of the region, with a low life expectancy (less than a year), or already under care for osteoporotic or oncological care. Further, patients with facial and skull fractures, prosthetic failure, pathological fracture, or osteomyelitis were not invited. For this study, patients with missing values on weight and height, or missing values on all functional performance tests (CST, HGS, TUG, and 6MWT) due to logistical issues (e.g., issues around availability of equipment, appointment scheduling, or lack of time or personnel) or unknown causes were excluded. Patients who were unable to perform these tests due to physical inability were included. Patients were evaluated and treated according to the Dutch guidelines for osteoporosis and fracture prevention [19].
Measurements
Age at the time of fracture, weight (kg), height (cm), and BMI (kg/m2) were measured during the FLS visit. During the FLS visit, the patients were asked to complete a questionnaire containing information about medical history and medication use, residential status, cause of the fracture, and information on previous fractures. Furthermore, medical history was extracted from medical records of the emergency department visit. Comorbidities were classified according to the tenth revision of International Classification of Disease (ICD-10). Dual-energy X-ray absorptiometry (DXA, Hologic Inc, Bedford, MA, USA), was used to measure bone mineral density (BMD) and measurements of body composition. Osteoporosis was defined as bone mineral density T-score ≤ − 2.5, osteopenia as T-score between − 1.0 and − 2.5, and a normal BMD as a T-score ≥ 1.0 at the hip, femoral neck, or lumbar spine [20]. Prevalent vertebral fractures (VFs) were assessed on lateral spine images acquired with DXA. The grading of VFs was done morphometrically using the classification of Genant [21], based on percentage height loss and were categorized according to the most severe VF, as follows; grade 1 (20 to 25%), grade 2 (> 25 to 40%), or grade 3 (height loss > 40%). Appendicular lean mass (ALM) was calculated as the sum of lean tissue in the arms and legs (kg) [22] and was corrected for squared height [14].
Physical performance tests
Physical functioning tests at baseline included CST, HGS, TUG, and 6MWT. All tests have a good to excellent inter-rater reliability in older populations with and without comorbidities (supplemental Table 1). All tests were conducted by trained nurses. Lower body strength was assessed by 30-s CST [23]. Participants were instructed to start in seated position, to not use the armrests, cross the arms over the chest, stand up, and fully sit back down in-between stands. The measured outcome was the number of times a person can fully stand up from a chair in 30 s time. HGS was measured by handheld dynamometer (JAMAR, Sammons Preston, Bolingbrook, Illinois). HGS testing was performed in seated position, with the elbows flexed at 90°. The maximum handgrip strength from three attempts for the left hand and three attempts for the right hand was used for analysis and was defined as the best score out the six attempts. TUG was used to measure balance, walking ability and overall mobility [24]. Participants were asked to rise from a chair with armrests, walk for three meters, turn, walk back to the chair, and sit down. The use of walking aids was permitted. The measured outcome was time to perform TUG in seconds. The TUG was performed three times and the mean score was calculated. If patients could only perform the test one or two times, the mean of those scores was used. Lastly, 6MWT was used to measure walking ability and functional exercise capacity [25]. Patients were asked to walk up and down between the safety cones on either end of a 10 m level linoleum hallway, at a comfortable speed, while covering as much distance as possible. Use of walking aid was permitted and patients were allowed to rest or stop when needed. No encouragements were given during the test. Patients performed the test one time, and walking distance in meters (6MWD) was scored. Confirmed sarcopenia was defined according to the EWGSOP2 criteria, as having either low HGS or low CST scores, and low ALM, e.g., “confirmed sarcopenia” [14]. “Probable sarcopenia” was defined as having either low CST or low HGS scores. The guideline’s cut-off scores for HGS are < 16 kg for females and < 27 kg for males and of CST > 15 s for five rises. As the 30-s CST was performed in this study, the cut-off score for low performance on CST was adapted to < 10 stands in 30 s. The guideline defines low ALM as < 7.0 kg/m2 for males and < 5.5 kg/m2 for females. Osteosarcopenia was defined as having “confirmed sarcopenia,” combined with having osteoporosis or osteopenia [26].
Fracture classification
Fractures were grouped in three ways; first, according to the center classification into four different fracture categories: 1. hip fractures, 2. major fractures (vertebra, multiple rib, humerus, pelvis, distal femur, and proximal tibia), 3. minor fractures; all remaining except fingers and toes which were grouped separately in group 4 [2]. Multiple fractures were allocated according to the most severe fracture. The center classification groups’ fractures based on mortality risk after the fracture. Second, fractures were grouped according to the most important osteoporotic fractures of the IOF classification: major osteoporotic fracture (MOF) including lower arm, hip, humerus, clinical vertebrae fractures, and non-MOF fractures (all other fractures). The third grouping covered all lower extremity fractures (any fracture from the pelvis down) versus all upper body fractures (all other fractures) and was chosen to differentiate if proximity of the fracture is of influence on the physical performance.
Reference data selection
To adequately assess physical performance of the FLS patients, a comparison to a healthy age- and sex-related reference population using Z-scores was chosen, comparable with the use of T-scores in osteoporosis measurements. While many test cut-off points for poor physical performance are proposed in the literature, some methodological issues could lead, when used, to a biased overview of poor physical performance in a population. First, a wide variety of different cut-off scores exist for all tests without established consensus on the optimal cut-off point. Cut-off scores are developed based on different outcomes for poor performance (e.g., the TUG cut-off score for is falls 15 s, for fractures 13.5 s, and sarcopenia 20 s [14, 15, 27]. Last, most are a “one-size fits all” cut-off score and not stratified to age and sex [14, 15, 27], which could lead to over- or underestimation of test results in specific groups. To secure optimal reference data of the general population, we carried out a semi-structured literature search in March 2022. The search strategy incorporated a combination of “Medical Subject Headings” or “Title/Abstract” terms describing “reference values” and physical performance test, e.g., “TUG, 6MWT, CST or HGS.” The search strategy, outcome and rationale of the literature search is presented in Supplementary Table 1 and 2. For the TUG we applied the reference data from Svinøy et al. [28], a large (N = 5400) Norwegian cross-sectional study including community-dwelling older persons aged 60–84 and published mean and SD’s that were comparable to other study in a Western population of Kenny et al. [29]. Results of Kenny et al. for the age categories 50–60 were studied and it was decided that the reference values of Svinøy et al. for 60 year olds could be extrapolated to 50–60 year olds. For 30CST and 6MWT, reference data was provided by Rikli and Jones et al. including 7183 community dwelling adults from the United States aged 60–94 [30]. The reference data from Rikli and Jones dates from 1999. However, reference data for the 30 s CST were particularly scarce and the testing method of Rikli and Jones was completely identical to ours as they were the first to develop this test. CST outcome of Rikli and Jones were compared to a more recent German cohort study of Albrecht et al. that did not meet inclusion criteria of the age range and results between the two studies were largely comparable [31]. Based on a comparison with other literature [32], it was decided that the reference values of Rikli and Jones et al. for 60 year olds could be extrapolated to 50–60 year olds. For the 6WMT, not all inclusion criteria were met; no study had identical testing methods except for Beekman et al. [33]; however, they did not present results stratified to age and sex groups. Means and SD of Rikli and Jones were used and their sex-stratified outcomes were compared to Beekman et al. and Casanova et al. [34], both of the latter reported higher means in both sexes. Thus, possible low performance of the FLS population might be even higher if other reference populations were used [30]. Finally, for the HGS, the reference data were derived from the meta-analysis of Dodds et al. which included data on HGS of 49,964 persons from 12 general population studies from across the United Kingdom [35], with similar testing methods and high comparability with reference data from other developed countries.
Statistical analysis
In the descriptive analysis mean and SD were calculated for normal distributed data. For non-normally distributed data medians and interquartile ranges were presented. Normality was visually assessed, and due to the large sample size approximately normal distributions were accepted. Patient specific Z-scores were calculated for each test, using the age- and sex-specific means and SD derived from the literature. The mean Z-scores were tested against the theoretical expected value of 0 of the reference population (the healthy age- and sex-related peers) using one-sample T-tests. Fracture group-specific boxplots of the Z-scores were created, according to center classification, stratified to sex and age. All categories shown have > 5 persons included in the analysis. Impaired and poor performance were defined as scoring 1SD and 2SD below age and gender norms, respectively, and proportions of impaired and poor performance were calculated. Patients who were physically unable to perform these tests were included in the poor performance group (> 2SD deviance from normative expectations). Uni- and multivariate linear regression models were used to investigate the association between the different fracture groupings and the Z-scores of the different performance tests. Following groups were tested: center major and hip fractures (with Center Minor as reference group), IOF MOF (non-MOF as reference group), lower extremity (upper extremity fractures as reference group), prevalent VF, excluding all patients with a clinical vertebral fracture (no prevalent VF as reference group). Multivariable analyses were adjusted for age, sex, and time since fracture. As a sensitivity analysis the descriptive analysis of the performance tests, comparison of Z-scores using T-tests and the proportions of impaired and poor performance were also calculated for the population excluding finger and toe fractures, as these fractures are often not included in FLS populations. A P value of < 0.05 was considered statistically significant. Statistical analyses were run in SPSS (IBM SPSS Statistics 28).
Results
Between November 2013 and June 2016, a total of 1947 patients attended the FLS, of whom 92% n = 1789 (70.7% females) were included in this study (flowchart presented in Supplementary Fig. 1). Population characteristics are presented in Table 1. Median age (IQR) was 64 (57–74) and 66 (59–74) for males and females, respectively, with 10% of the patients aged 80 years or older. The FLS visit took place at a mean of 3.9±1.6 months after fracture and 92.6% of patients were evaluated at the FLS within 6 months after fracture. Osteoporosis was diagnosed in 20.4% of males and 30.7% of females and 16.9% and 14.2% had at least one prevalent grade 2–3 vertebral fracture, respectively.
Results of physical performance tests were available for 1759 (90.9%), 1781 (92.0%), 1733 (89.5%), and 1725 (89.1%) of the participants for CST, HGS, TUG, and 6MWT, respectively (supplemental Fig. 1). The characteristics of patients with missing functional performance measures were similar to the total population. Results stratified by sex are shown in Table 2. Based on the EWGSOP2 criteria, 3.3% of males and 2.5% of the females had sarcopenia. The proportion of patients with probable sarcopenia was 30.9% for males and 42.8% for females. Percentages of osteosarcopenia were 3.1% for males and 2.3% for females.
As shown in Table 2, the mean Z-score in our FLS population was significantly lower compared to the reference population (P < 0.0001) for CST − 0.72 (95% CI − 0.76, − 0.69), HGS − 0.54 (95% CI − 0.58, − 0.48), TUG − 0.34 (95% CI − 0.43, − 0.25) and 6MWT − 1.71 (95%CI − 1.76 to − 1.65). Of the FLS patients, 32.6%, 18.9%, 8.5% and 36.4% had an impaired performance (Z-score between − 1 to − 2 SD) on CST, HGS, TUG, and 6MWT, compared to an expected proportion of 13.6% in the reference population, respectively (Table 3). Further, a poor performance (< − 2SD) was shown in 6.6%, 11.4%, and 13.1% and 35.2% on CST, HGS, TUG, and 6MWT respectively, compared to 2.3% in the reference population. Proportions of impaired and poor performance were similar between males and females. Proportions of poor and impaired performance were higher compared to expected proportions throughout all age groups, except for TUG scores of 50–59 year olds in both males and females(data not shown).
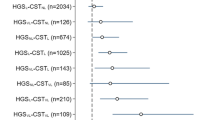
As presented in Fig. 1, for CST, 6MWT, and HGS, a lower performance was shown for all age groups and fracture types (hip, major, minor, and finger and toe) in both males and females. For CST and 6MWT, the interquartile range of all ages and fracture types was below the theoretical population mean of 0. For TUG, low performance was most pronounced in hip fracture patients and patients aged 80 + with a major fracture.
A–D Z-scores for males and females stratified to fracture type and age. Boxplots of Z-scores of FLS patients stratified to age, sex, and fracture type. A CST, B HGS, C TUG, and D 6MWT. Fracture types are grouped according to the Center classification; hip fractures, major fractures (vertebra, multiple rib, humerus, pelvis, distal femur, and proximal tibia), minor fractures; all remaining except fingers and toes which were grouped separately. Multiple fractures were allocated according to the most severe fracture. CST = chair stand test; HGS = handgrip strength; TUG = timed up and go; 6MWT = 6-min walking test. All groups include > 5 patients. Reference line represents theoretical population mean. Ages:
 ,
,
 ,
,
 ,
,

When compared to minor fractures, hip and major fractures were significantly associated (P < 0.001) with lower Z-scores for CST; (major: regression coefficient (B) [95%CI] = − 0.25 [− 0.34 to − 0.16]; hip: B = − 0.32 [− 0.47 to − 0.17]), TUG (major: B = − 0.54 [− 0.75 to − 0.33]; hip: B = − 1.72 [− 2.08 to − 1.35]) and 6MWT (major: B = − 0.34 [− 0.47 to − 0.21]; hip: B = − 0.99 [− 1.22 to − 0.77]), but not for HGS (model 1, outcome adjusted for age, sex and time since fracture) (Table 4). According to the IOF classification, MOF showed significant lower Z-scores compared to non-MOF, but with smaller effect sizes ranging between − 0.09 and − 0.20). Lower extremity fractures have significantly lower Z-scores on TUG, CST and 6MWT, but are especially lower in TUG (B = − 0.42 [− 0.53 to − 0.31]) and 6MWT (B = − 0.36 [− 0.53 to − 0.19]). Participants with at least one prevalent VF grade 2 or 3, in addition to the index, fracture had significantly lower Z-scores compared to the participants without prevalent VF on CST (B = − 0.22 [− 0.34 to − 0.11]), TUG (B = − 0.61 [− 0.88 to − 0.34]), and 6MWT (B = − 0.36 [− 0.53 to − 0.19]).
Sensitivity analysis
Supplementary Table 3 and 4 show the results of the study population excluding finger and toe fractures of overall physical performance, comparisons to the reference population, and proportions of impaired and poor performance. Results were largely similar compared to the main analysis, with a small increase in the mean differences compared to the reference population (Supplementary Table 3), and a small increase of the proportion of patients with impaired and poor performance (Supplementary Table 4).
Discussion
Reporting on strength (HGS, CST, and TUG), balance (TUG and 6MWT), and cardiorespiratory fitness (CST and 6MWT) measures, this study provides a comprehensive evaluation on physical performance of patients with a recent fracture attending the FLS for fracture risk evaluation. This study shows that physical performance of FLS patients four months after fracture was significantly lower compared to their healthy peers, while the prevalence of confirmed sarcopenia was very low. Importantly, stratified analysis showed a high proportion of patients with impaired or poor performance in all age groups and in both sexes, especially on the 6MWT, HGS, and CST measures. Low performance on the TUG was mainly present in patients with hip fractures. Major and hip fractures and the presence of a prevalent VF were associated with lower performance scores for CST, TUG, and 6MWT, but not for HGS.
Physical performance of the FLS patients in our study was low in several domains. These findings are in line with previous research reporting on physical performance tests after fracture, summarized by a recent review of Szulc et al. [17]. However, comparability between the studies included in this review is low as the study populations included in this review are heterogeneous with respect to age, gender, and time since fracture. Our study is unique in its approach to assess physical performance for a wide range of fractures, ages and both sexes, and in its comparison of FLS patients to a predefined reference population of age- and sex-related peers. The results of the stratified Z-scores (Fig. 1) clearly indicate that the physical performance of FLS patients is affected in both males and females and in all age groups for CST, HGS, and 6MWT. As stated, decreased physical performance is often defined using cut-off scores. A wide variety of cut-off scores on muscle strength and physical performance tests for increased fall-and fracture risk are reported in the literature [15, 27]. Cut-off scores for low performance are often based on statistical parameters within the studied population (e.g., by using quartiles or SD deviations from the mean) and are often not stratified to age and sex [15, 27]. However, it is widely established that physical performance declines with age and varies between sexes [28, 30, 35]. Using a one-size fits all cut-off score might lead to an underestimation of physical performance in older ages or females and an overestimation of younger persons and males, and does not give an unbiased overview of physical performance in a population. Our study provides clinicians with insights how to assess physical performance in fracture patients that could be of value to comply with the recommendations of the recent Capture the Fracture network key performance indicators, which recommends fall risk assessment, e.g., based on performance on physical function tests, and appropriate recommendations or referral to falls clinics for all fracture patients [6]. These recommendations are in line with the world guidelines for falls prevention that recommend a multifactorial falls risk assessment, including mobility testing, for all patients with an injurious fall, such as fracture patients [12]. However, consensus on which test is preferred to assess falls and fracture risk remains difficult. The umbrella review on physical performance tests on behalf of the world falls guidelines reports gait speed testing as a possible useful measure to predict falls, but shows inconsistencies in the predictive ability of individual physical performance tests for falls, including the TUG and CST [27]. However, they underline the clinical value of mobility assessment and identification of appropriate targeted interventions, as well as the need to assess their fall predictive ability in subpopulations in the future.
Several approaches to cluster fractures are reported in literature, of which the center classification, the IOF classification and grouping based on fracture location are the most commonly used. When comparing the results of these different fracture classifications, the center classification seems to be the most distinctive in terms of physical performance; it showed the largest differences in effect sizes in the association between fracture site and physical performance. This might be explained by the fact that the center classification groups patients based on mortality risk after a fracture, with a higher risk of death after a major or hip fracture types [2]. Correspondingly, lower physical performance measures have previously been associated with a higher mortality after fracture in osteoporotic males and females [36, 37]. In the center classification, distal radius fractures are classified as minor fractures, while these are classified as MOFs according the to the IOF classification. This might result in a lower performance on the HGS in patients with a MOF, as well as in a relatively weaker association of MOF with all other tests Z-scores. Moreover, our results show that the presence of prevalent vertebral fractures was associated with low physical performance, which is in line with previous findings [17]. These findings imply that assessment of physical performance should have extra emphasis in FLS patients with a major or hip fractures and in patients with prevalent vertebral fractures. Although evidence regarding the optimal intervention for regaining functional fitness and the prevention of future falls after a fracture is still scarce, personalized and multifactorial interventions show some promising results, especially in hip fracture patients [38].
It is known that sarcopenia is associated with falls and Vranken et al. have recently reported that patients with a fracture attending the FLS have a high risk of sustaining new falls and subsequent fractures [10]. Despite the high proportion of poor performance on individual tests, the proportion of FLS patients with confirmed sarcopenia as well as osteosarcopenia is low compared to the literature. A recent global review an meta-analysis on the prevalence of sarcopenia reported a wide ranging prevalence of sarcopenia, varying from 0.2 to 86.6% depending on used classification and population. However, only three studies included in the meta-analysis used the EWGSOP2 (n = 5720, all older persons without a fracture), and an estimated prevalence of 10% (95% CI: 2.0–17.0%) was reported [39]. In a recent study in older fracture patients visiting a fall and fracture evaluation, a prevalence of osteosarcopenia of 25% was reported, while in our study the prevalence was only 3.1% for males and 2.3% for females [40]. This difference could partly be explained by the higher mean age of that study (77.9 ± 0.42). The ALM of our study population is comparable to healthy populations [41], with only 4.2% of males and 4.0% of females having an ALM below the sex-specific cut-off values. Possibly, the high BMI of our study population could impose bias as patients with higher fat tend to have higher ALM, resulting in a lower proportion of sarcopenia in our cohort [14, 42]. Interestingly, when using the definition of “probable sarcopenia,” proportions in our study are much higher compared to large older community dwelling population studies reporting ranges between 23.5% (mean age 75.9 ± 4.0) and 33.1% (mean age 70.1 ± 7.7) [43, 44]. Several studies describes muscle strength as a stronger predictor for mobility impairment and fractures compared to muscle mass [15, 45]; for persons with low strength, McLean et al. reported similar odds of mobility impairment for those with high or low muscle mass [45]. This is in line with EWGSOP2 guidelines that propose the diagnosis of “probable sarcopenia” is enough to trigger further assessment and start interventions [14]. The evaluation of individual physical performance measures using single domains testing has shown to be promising in predicting incident fractures and mortality in several populations [15, 36, 46, 47]. However, more prospective studies are needed, in fracture populations specifically, to test associations between impaired physical performance with clinical outcome measures, such as subsequent fractures and mortality.
Strengths and limitations
We have included a large study population of males and females with a wide range of fracture types and ages, assessing physical performance at the FLS. Our study population seems to be representative for the general FLS population and 92% of patients were able to perform the physical evaluation. However, it is important to note that our study might not be representative for all fracture patients as the FLS population is inherently a selection of all fracture patients; FLS non-attenders are known to be older with a higher proportion of hip fractures [10]. Second, the physical performance reported in our study is subject to the mean time since fracture of four months, in which the rehabilitation after fracture is not always fully completed. We did not have information on the type, length, or intensity of the rehabilitation. It is likely that a large difference in rehabilitation programs was present among FLS patients due to differences in fracture type and fracture treatment that might have influenced the results on physical performance tests. Inevitably, while using reference data from population studies, the outcome depends on the reference values that are used. Determining optimal reference data for the 6MWT was especially challenging, due to high variability in execution of the test as well as in the reported outcomes (i.e., reference equations). However, recent reference studies have reported similar or higher means per age and sex stratification compared to the reference data of Rikli et al. which we have used in our study [30, 33, 34]. Finally, the large proportion of poor performers on 6MWT would be similar, or even higher, when using these more recently published reference values.
Conclusion
Patients with a recent fracture attending the FLS four months after fracture had a significantly lower performance on CST, HGS, TUG, and 6MWT as compared to peers. There is a low prevalence of sarcopenia as defined by the EWGSOP2. These findings underline the need for the assessment of physical performance during the FLS visit, and personalized physical therapy to regain their physical performance should be considered for all FLS patients.
Data availability
Research data are not shared due to privacy or ethical restrictions.
References
Willers C, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Borgström F, Kanis JA (2022) Osteoporosis in Europe: a compendium of country-specific reports. Arch Osteoporos 17:23. https://doi.org/10.1007/s11657-021-00969-8
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882. https://doi.org/10.1016/s0140-6736(98)09075-8
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68:99–102. https://doi.org/10.1136/ard.2008.092775
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24:2135–2152. https://doi.org/10.1007/s00198-013-2348-z
Geusens P, Bours SPG, Wyers CE, van den Bergh JP (2019) Fracture liaison programs. Best Pract Res Clin Rheumatol 33:278–289. https://doi.org/10.1016/j.berh.2019.03.016
Javaid MK, Sami A, Lems W et al (2020) A patient-level key performance indicator set to measure the effectiveness of fracture liaison services and guide quality improvement: a position paper of the IOF Capture the Fracture Working Group, National Osteoporosis Foundation and Fragility Fracture Network. Osteoporos Int 31:1193–1204. https://doi.org/10.1007/s00198-020-05377-1
Li N, Hiligsmann M, Boonen A, van Oostwaard MM, de Bot R, Wyers CE, Bours SPG, van den Bergh JP (2021) The impact of fracture liaison services on subsequent fractures and mortality: a systematic literature review and meta-analysis. Osteoporos Int 32:1517–1530. https://doi.org/10.1007/s00198-021-05911-9
Vranken L, Wyers CE, van den Bergh JPW, Geusens P (2017) The phenotype of patients with a recent fracture: a literature survey of the Fracture Liaison Service. Calcif Tissue Int 101:248–258. https://doi.org/10.1007/s00223-017-0284-1
Van Helden S, van Geel AC, Geusens PP, Kessels A, Kruseman ACN, Brink PR (2008) Bone and fall-related fracture risks in women and men with a recent clinical fracture. JBJS 90:241–248
Vranken L, Wyers CE, Van der Velde RY et al (2022) Association between incident falls and subsequent fractures in patients attending the fracture liaison service after an index fracture: a 3-year prospective observational cohort study. BMJ Open 12:e058983. https://doi.org/10.1136/bmjopen-2021-058983
Schene MR, Wyers CE, Driessen AMH, Souverein PC, Gemmeke M, van den Bergh JP, Willems HC (2023) Imminent fall risk after fracture. Age Ageing 52:afad201. https://doi.org/10.1093/ageing/afad201
Montero-Odasso M, van der Velde N, Martin FC et al (2022) World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 51:afac205. https://doi.org/10.1093/ageing/afac205
Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, Criss M, Verma S, Osborne J, Chui KK (2017) Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther 40:1–36. https://doi.org/10.1519/jpt.0000000000000099
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Alajlouni DA, Bliuc D, Tran TS, Blank RD, Center JR (2023) Muscle strength and physical performance contribute to and improve fracture risk prediction in older people: a narrative review. Bone 172:116755. https://doi.org/10.1016/j.bone.2023.116755
Harvey NC, Orwoll E, Kwok T et al (2021) Sarcopenia definitions as predictors of fracture risk independent of FRAX(®), Falls, and BMD in the osteoporotic fractures in men (MrOS) study: a meta-analysis. J Bone Miner Res. https://doi.org/10.1002/jbmr.4293
Szulc P (2020) Impact of bone fracture on muscle strength and physical performance-narrative review. Curr Osteoporos Rep 18:633–645. https://doi.org/10.1007/s11914-020-00623-1
van den Bergh JP, van Geel TA, Geusens PP (2012) Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol 8:163–172. https://doi.org/10.1038/nrrheum.2011.217
Dutch Institute for Healthcare Improvement CBO (2011) Richtlijn Osteoporose en Fractuurpreventie, Derde Herziening [Dutch] Dutch Institute for Healthcare Improvement CBO. The Netherlands, Utrecht
World Health Organisation (2003) Prevention and management of osteoporosis. World Health Organization, World Health Organization Technical Report Series
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Cawthon PM (2015) Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom 18:467–471. https://doi.org/10.1016/j.jocd.2015.05.063
Jones CJ, Rikli RE, Beam WC (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 70:113–119. https://doi.org/10.1080/02701367.1999.10608028
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
Rikli RE, Jones CJ (1998) The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act 6:363–375
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790. https://doi.org/10.1007/s00198-017-4151-8
Beck Jepsen D, Robinson K, Ogliari G, Montero-Odasso M, Kamkar N, Ryg J, Freiberger E, Masud T (2022) Predicting falls in older adults: an umbrella review of instruments assessing gait, balance, and functional mobility. BMC Geriatr 22:615. https://doi.org/10.1186/s12877-022-03271-5
Svinøy OE, Hilde G, Bergland A, Strand BH (2021) Timed up and go: reference values for community-dwelling older adults with and without arthritis and non-communicable diseases: the Tromsø study. Clin Interv Aging 16:335–343. https://doi.org/10.2147/cia.S294512
Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM (2013) Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. J Am Geriatr Soc 61(2):S279-290. https://doi.org/10.1111/jgs.12195
Rikli RE, Jones CJ (1999) Functional fitness normative scores for community-residing older adults, ages 60–94. J Aging Phys Act 7:162. https://doi.org/10.1123/japa.7.2.162
Albrecht BM, Stalling I, Bammann K (2021) Sex- and age-specific normative values for handgrip strength and components of the Senior Fitness Test in community-dwelling older adults aged 65–75 years in Germany: results from the OUTDOOR ACTIVE study. BMC Geriatr 21:273. https://doi.org/10.1186/s12877-021-02188-9
Warden SJ, Liu Z, Moe SM (2021) Sex- and age-specific centile curves and downloadable calculator for clinical muscle strength tests to identify probable sarcopenia. Phys Ther. https://doi.org/10.1093/ptj/pzab299
Beekman E, Mesters I, Gosselink R, Klaassen MP, Hendriks EJ, Van Schayck OC, de Bie RA (2014) The first reference equations for the 6-minute walk distance over a 10 m course. Thorax 69:867–868. https://doi.org/10.1136/thoraxjnl-2014-205228
Casanova C, Celli BR, Barria P et al (2011) The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J 37:150–156. https://doi.org/10.1183/09031936.00194909
Dodds RM, Syddall HE, Cooper R et al (2014) Grip strength across the life course: normative data from twelve British studies. PLoS ONE 9:e113637. https://doi.org/10.1371/journal.pone.0113637
Alajlouni DA, Bliuc D, Tran TS, Blank RD, Cawthon PM, Ensrud KE, Lane NE, Orwoll ES, Cauley JA, Center JR (2022) Muscle strength and physical performance are associated with risk of postfracture mortality but not subsequent fracture in men. J Bone Miner Res 37:1571–1579. https://doi.org/10.1002/jbmr.4619
Pham HM, Nguyen SC, Ho-Le TP, Center JR, Eisman JA, Nguyen TV (2017) Association of muscle weakness with post-fracture mortality in older men and women: a 25-year prospective study. J Bone Miner Res 32:698–707. https://doi.org/10.1002/jbmr.3037
Wilson N, Hurkmans E, Adams J et al (2020) Prevention and management of osteoporotic fractures by non-physician health professionals: a systematic literature review to inform EULAR points to consider. RMD Open 6:e001143. https://doi.org/10.1136/rmdopen-2019-001143
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C (2022) Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 13:86–99. https://doi.org/10.1002/jcsm.12783
Sepúlveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, Conzade R, Kirk B, Al Saedi A, Probst V, Duque G (2020) The Joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc 21:220–225. https://doi.org/10.1016/j.jamda.2019.09.005
Kirk B, Bani Hassan E, Brennan-Olsen S, Vogrin S, Bird S, Zanker J, Phu S, Meerkin JD, Heymsfield SB, Duque G (2021) Body composition reference ranges in community-dwelling adults using dual-energy X-ray absorptiometry: the Australian Body Composition (ABC) Study. J Cachexia Sarcopenia Muscle 12:880–890. https://doi.org/10.1002/jcsm.12712
Schautz B, Later W, Heller M, Müller MJ, Bosy-Westphal A (2012) Total and regional relationship between lean and fat mass with increasing adiposity–impact for the diagnosis of sarcopenic obesity. Eur J Clin Nutr 66:1356–1361. https://doi.org/10.1038/ejcn.2012.138
Swan L, Warters A, O’Sullivan M (2022) Socioeconomic disadvantage is associated with probable sarcopenia in community-dwelling older adults: findings from the english longitudinal study of ageing. J Frailty Aging 11:398–406. https://doi.org/10.14283/jfa.2022.32
Kim M, Won CW (2019) Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing 48:910–916. https://doi.org/10.1093/ageing/afz091
McLean RR, Shardell MD, Alley DE et al (2014) Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 69:576–583. https://doi.org/10.1093/gerona/glu012
Alajlouni D, Tran T, Bliuc D, Blank RD, Cawthon PM, Orwoll ES, Center JR (2021) Muscle strength and physical performance improve fracture risk prediction beyond Garvan and FRAX: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. https://doi.org/10.1002/jbmr.4483
Larsson BAM, Johansson L, Johansson H et al (2021) The timed up and go test predicts fracture risk in older women independently of clinical risk factors and bone mineral density. Osteoporos Int 32:75–84. https://doi.org/10.1007/s00198-020-05681-w
Acknowledgements
This work was supported by the Scientific Fund of Viecuri Medical Centre and the Weijerhorst Foundation, grant number not applicable.
Funding
Stichting De Weijerhorst
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The Weijerhorst Foundation was not involved in conducting the study, analyzing the data, or writing the manuscript.
Ethical approval
This research was approved by the medical ethics board of the Maastricht University Medical Centre (ref. nr. 2022–3319).
Conflicts of Interest
MS, LV, CW, RV, HJ, and JD declare that they have no conflict of interest. JB reports grands for lectures and the position in the advisory board from UCB and Amgen, HW reports a speaker’s fee for lectures from UCB, and FLH reports a speaker’s fee from Amgen, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schene, M.R., Willems, H.C., Driessen, J.H.M. et al. Physical performance and sarcopenia assessment in patients with a recent fracture visiting the Fracture Liaison Service. Osteoporos Int 35, 851–862 (2024). https://doi.org/10.1007/s00198-023-07009-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-07009-w