Abstract
Summary
To develop a population pharmacokinetic model that describes the absorption and low plasma levels of risedronate in the body. The impact of patients’ characteristics on risedronate kinetics is investigated. Simulations revealed the high variability in the concentration levels after different dosage schemes. No dosage adjustment is required in renal impairment.
Introduction
Risedronate exhibits very low plasma levels and high residence time in the body. The aim of this study is to describe and explain the risedronate transit through the body. The impact of volunteers’ characteristics on the kinetics of risedronate is also investigated. Simulations are used to compare the risedronate plasma levels after different dosage schemes and assess the need for dose adjustment in patients with impaired kidney functionality.
Methods
Plasma concentration—time data were obtained from a four-period, two sequence, single-dose, crossover bioequivalence study. The effects of several covariates (e.g., weight, albumin, creatinine, alkaline phosphatase, and calcium) on model parameters were tested. Non-linear mixed-effect modeling was applied and a variety of models were evaluated placing emphasis on absorption and disposition properties. The modeling and simulation work was implemented in MonolixTM 2020R1.
Results
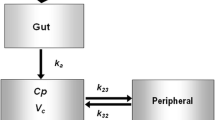
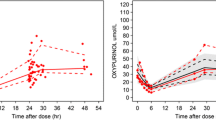
Following oral administration, the kinetics of risedronate was best described by a two-compartment model with lag time, first-order absorption, and elimination. The extent of peripheral distribution (i.e., bones) was found to be remarkably high. No volunteer characteristics were identified to affect significantly the disposition of risedronate. Using simulations, risedronate plasma profiles were obtained for different doses and frequencies of administration.
Conclusion
The absorption and disposition kinetics of risedronate were successfully characterized. Simulations revealed the high discrepancy in the concentration levels observed after different dosage regimens, implying the safety profile of risedronate. In virtual patients with renal impairment, the blood levels of risedronate are increased, but not in an extent requiring dose adaptation.



Similar content being viewed by others
References
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C (2020) The epidemiology of osteoporosis. Br Med Bull 133:105–117. https://doi.org/10.1093/bmb/ldaa005
Ruiz-Adame M, Correa M (2020) A systematic review of the indirect and social costs studies in fragility fractures. Osteoporos Int 31:1205–1216. https://doi.org/10.1007/s00198-020-05319-x
Reid IR (2020) Management of Paget's disease of bone. Osteoporos Int 31:827–837. https://doi.org/10.1007/s00198-019-05259-1
Khosla S, Hofbauer LC (2017) Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5:898–907. https://doi.org/10.1016/S2213-8587(17)30188-2
Anastasilakis AD, Polyzos SA, Makras P (2018) Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol 179:R31–R45. https://doi.org/10.1530/EJE-18-0056
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) Pharmacological management of osteoporosis in postmenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 104:1595–1622. https://doi.org/10.1210/jc.2019-00221
Cremers S, Drake MT, Ebetino FH, Bilezikian JP, Russell RGG (2019) Pharmacology of bisphosphonates. Br J Clin Pharmacol 85:1052–1062. https://doi.org/10.1111/bcp.13867
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008 Jun) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19(6):733–759. https://doi.org/10.1007/s00198-007-0540-8
Nayak S, Greenspan SL (2019) A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos Int 30:705–720. https://doi.org/10.1007/s00198-018-4791-3
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056. https://doi.org/10.1359/jbmr.2003.18.6.1051
Ogura Y, Gonsho A, Cyong JC, Orimo H (2004) Clinical trial of risedronate in Japanese volunteers: single and multiple oral dose studies. J Bone Miner Metab 22:111–119. https://doi.org/10.1007/s00774-003-0458-y
Actonel® Full prescribing information. 2008 Procter & Gamble Pharmaceuticals, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020835s035lbl.pdf. Accessed 31 December 2020
Risedronate sodium 35mg. Summary of product characteristics. Sandoz limited. Date of revision of the text 27/11/2020. https://www.medicines.org.uk/emc/medicine/25017#gref. Accessed 31 December 2020
Risedronate sodium 75mg. Summary of product characteristics. Sandoz limited. https://mri.cts-mrp.eu/human/downloads/ES_H_0358_001_FinalPI_2of2.pdf. Accessed 11 February 2021
Soares AP, do Espírito Santo RF, Line SR, Md P, Santos Pde M, Toralles MB, do Espírito Santo AR (2016) Bisphosphonates: pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ Toxicol Pharmacol 42:212–217. https://doi.org/10.1016/j.etap.2016.01.015
Cremers SC, Pillai G, Papapoulos SE (2005) Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 44:551–570. https://doi.org/10.2165/00003088-200544060-00001
Perazella MA, Markowitz GS (2008) Bisphosphonate nephrotoxicity. Kidney Int 74:1385–1393. https://doi.org/10.1038/ki.2008.356
Mitchell DY, Barr WH, Eusebio RA, Stevens KA, Duke FP, Russell DA, Nesbitt JD, Powell JH, Thompson GA (2001) Risedronate pharmacokinetics and intra- and inter-subject variability upon single-dose intravenous and oral administration. Pharm Res 18:166–170. https://doi.org/10.1023/a:1011024200280
Bonate PL (2011) Pharmacokinetic-Pharmacodynamic Modeling and Simulation, 2nd edn. Springer US, New York
MonolixTM, Simulation Plus. URL: https://www.simulations-plus.com/software/monolix. Accessed 31 December 2020
Dziak JJ, Coffman DL, Lanza ST, Li R, Jermiin LS (2020) Sensitivity and specificity of information criteria. Brief Bioinform 21:553–565. https://doi.org/10.1093/bib/bbz016
Kasting GB, Francis MD (1992) Retention of etidronate in human, dog, and rat. J Bone Miner Res 7:513–522. https://doi.org/10.1002/jbmr.5650070507
Cremers S, Ebetino FH, Phipps R (2020) On the pharmacological evaluation of bisphosphonates in humans. Bone 139:115501. https://doi.org/10.1016/j.bone.2020.115501
Weiss M (1999) The anomalous pharmacokinetics of amiodarone explained by nonexponential tissue trapping. J Pharmacokinet Biopharm 27:383–396. https://doi.org/10.1023/a:1020965005254
Lin JH (1996) Bisphosphonates: a review of their pharmacokinetic properties. Bone 18:75–85. https://doi.org/10.1016/8756-3282(95)00445-9
US Food and Drug Administration. Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review(s). Application number: 022560ORIG1S000", 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022560orig1s000clinpharmr.pdf. Accessed 31 December 2020
Hens B, Corsetti M, Spiller R, Marciani L, Vanuytsel T, Tack J, Talattof A, Amidon GL, Koziolek M, Weitschies W, Wilson CG, Bennink RJ, Brouwers J, Augustijns P (2017) Exploring gastrointestinal variables affecting drug and formulation behavior: Methodologies, challenges and opportunities. Int J Pharm 519:79–97. https://doi.org/10.1016/j.ijpharm.2016.11.063
Traynard P, Ayral G, Twarogowska M, Chauvin J (2020) Efficient pharmacokinetic modeling workflow with the monolixsuite: a case study of remifentanil. CPT Pharmacometrics Syst Pharmacol 9:198–210. https://doi.org/10.1002/psp4.12500
Pillai G, Gieschke R, Goggin T, Jacqmin P, Schimmer RC, Steimer JL (2004) A semimechanistic and mechanistic population PK-PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol 58:618–631. https://doi.org/10.1111/j.1365-2125.2004.02224.x
Allen MR (2018) Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep 16:198–204. https://doi.org/10.1007/s11914-018-0430-3
Mitchell DY, St Peter JV, Eusebio RA, Pallone KA, Kelly SC, Russell DA, Nesbitt JD, Thompson GA, Powell JH (2000) Effect of renal function on risedronate pharmacokinetics after a single oral dose. Br J Clin Pharmacol 49:215–222. https://doi.org/10.1046/j.1365-2125.2000.00135.x
Mitchell DY, Eusebio RA, Sacco-Gibson NA, Pallone KA, Kelly SC, Nesbitt JD, Brezovic CP, Thompson GA, Powell JH (2000) Dose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers. J Clin Pharmacol 40:258–265. https://doi.org/10.1177/00912700022008928
Acknowledgements
The authors wish to thank Verisfield Pharmaceuticals SA for providing the concentration-time data to perform the current modeling analysis
Code availability
A software (MonolixTM) was used for the modeling and simulation task of this study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardozo, B., Karatza, E. & Karalis, V. Osteoporosis treatment with risedronate: a population pharmacokinetic model for the description of its absorption and low plasma levels. Osteoporos Int 32, 2313–2321 (2021). https://doi.org/10.1007/s00198-021-05944-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-05944-0