Abstract
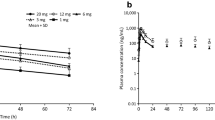
The tolerability and pharmacokinetics of risedronate after a single oral administration and during multiple oral administrations were examined in healthy adult male volunteers. In the single dose study, the dose was increased gradually from 1 mg to 2.5, 5, 10, or 20 mg. Subsequently, risedronate was given by multiple administration, 5 mg per dosing, once daily, for 7 days. The observed adverse events, whose causality was possibly related or unknown, included headache, diarrhea, increased body temperature, increased CK-BB, and increased urinary Β2-microglobulin excretion rate. However, none of these adverse events was clinically significant. The results thus showed that risedronate was well tolerated when delivered as a single administration of up to 20 mg or as a multiple administration of up to 5 mg/day. In the multiple dose study, changes in urinary deoxypyridinoline suggested the bone antiresorptive activity of risedronate. In the single dose study, AUC and Cmax, after the administration of risedronate at 1, 2.5, 5, 10, and 20 mg, increased dose dependently, and the Tmax, t 1/2, and urinary excretion rates were nearly constant. Therefore, the pharmacokinetic profile of risedronate was considered to show linearity in a dosage range of up to 20 mg. Furthermore, the results obtained in the multiple administration study indicated that the plasma concentrations of risedronate reached a steady state on day 4 of administration. The plasma concentrations of risedronate after the administration of 2.5 mg risedronate to the Japanese population were nearly comparable to the serum concentrations after the administration of 5 mg risedronate to the United Kingdom study population.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ogura, Y., Gonsho, A., Cyong, JC. et al. Clinical trial of risedronate in Japanese volunteers: single and multiple oral dose studies. J Bone Miner Metab 22, 111–119 (2004). https://doi.org/10.1007/s00774-003-0458-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00774-003-0458-y