Abstract
Summary
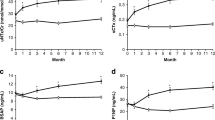
In patients discontinuing ALN after a median of 7.0 years (range 5.0–20.0 years), BMD decreased, and bone turnover markers increased within the premenopausal reference range over 2 years. Increased p-CTX after 3 months was associated with greater bone loss at the hip confirming that maintenance of BMD is dependent on continued suppression of bone turnover.
Introduction
It is unknown how to monitor patients discontinuing alendronate (ALN) after more than 5 years. We investigated if BTM measured before or during treatment discontinuation with ALN predict bone loss after 1 or 2 years.
Methods
PROSA was a cohort study conducted at Aarhus University Hospital including postmenopausal women and men above 50 years treated with ALN ≥ 5 years who had osteopenia at the hip and BMD T-score at the lumbar spine > − 4. ALN was discontinued and BTMs were measured at baseline, months (M) 1, 3, 6, and 12, and DXA was performed at baseline, M6, and M12. We extended the study and measured BTMs and performed DXA at M24.
The primary endpoint was if changes in p-CTX at M3 or M6 predict changes in THBMD after 1 year (Clinicaltrials.gov: NCT03051620).
Results
We enrolled 136 participants discontinuing ALN after a median of 7.0 years (range 5.0–20.0 years) in PROSA and 124 participants in PROSA Extension. There was a significant decrease in LSBMD − 0.74% ± 0.27, THBMD − 2.65% ± 0.39, FNBMD − 2.35% ± 0.33, and trabecular bone score − 0.97% ± 0.35 and an increase in p-CTX by 61.1% ± 4.7 (p < 0.05 for all) after 24 months. Increase in p-CTX at M3 was associated with bone loss at the hip sites at M12 and M24.
Conclusion
In patients discontinuing ALN, BMD decreased significantly and BTMs increased within the reference range over 2 years. An increase in p-CTX after 3 months was associated with greater bone loss at the hip confirming that maintenance of BMD during treatment discontinuation is dependent on continued suppression of bone turnover.



Similar content being viewed by others
Data Availability
The study protocol is available in the appendix.
References
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296(24):2927–2938
Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, Wallace RB, Hochberg MC, Feldstein AC, Lombardi A, Black DM, for the FLEX Research Group (2010) Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX trial. J Bone Miner Res 25(5):976–982
Saag K et al (2012) Resolution of effects on bone turnover markers and bone mineral density after discontinuation of long-term bisphosphonate use. ACR/ARHP. Annual. Meeting. Abstract. 1971.
Hansen C, Pedersen BD, Konradsen H, Abrahamsen B (2013) Anti-osteoporotic therapy in Denmark - predictors and demographics of poor refill compliance and poor persistence. Osteoporos Int 24(7):2079–2097
Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA (2018) Diagnosis of endocrine disease: Bone turnover markers: are they clinically useful? Eur J Endocrinol 178(1):R19–R31
Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NFA, McCloskey EV, Walsh JS, Eastell R (2016) Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 27(1):21–31
Diez-Perez A et al (2017) International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 28(3):767–774
Diez-Perez A et al (2012) Treatment failure in osteoporosis. Osteoporos Int 23(12):2769–2774
McNabb BL, Vittinghoff E, Schwartz AV, Eastell R, Bauer DC, Ensrud K, Rosenberg E, Santora A, Barrett-Connor E, Black DM (2013) BMD changes and predictors of increased bone loss in postmenopausal women after a 5-year course of alendronate. J Bone Miner Res 28(6):1319–1327
Paggiosi MA, Peel N, McCloskey E, Walsh JS, Eastell R (2014) Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 25(12):2729–2741
Naylor KE, Bradburn M, Paggiosi MA, Gossiel F, Peel NFA, McCloskey EV, Walsh JS, Eastell R (2018) Effects of discontinuing oral bisphosphonate treatments for postmenopausal osteoporosis on bone turnover markers and bone density. Osteoporos Int 29(6):1407–1417
Naylor KE, McCloskey EV, Jacques RM, Peel NFA, Paggiosi MA, Gossiel F, Walsh JS, Eastell R (2019) Clinical utility of bone turnover markers in monitoring the withdrawal of treatment with oral bisphosphonates in postmenopausal osteoporosis. Osteoporos Int 30(4):917–922
Vasikaran S et al (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22(2):391–420
Acknowledgments
We would like to thank all the participants for their extraordinary enthusiasm and cooperativeness throughout the study. Additionally, we would like to thank the foundations that supported the study financially.
Funding
The study was initiated by the investigators. The Foundation of Carl and Ellen Hertz, The Foundation of Aase and Ejnar Danielsen, The Svend Fælding Foundation, and Aarhus University have granted financial support. The funders of the study had no role in the study design, data collection, data interpretation, or writing of the report. The authors had full access to all the data and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare financial support from The Foundation of Carl and Ellen Hertz, The Foundation of Aase and Ejnar Danielsen, The Svend Fælding Foundation, and Aarhus University during the conduct of the study.
Additionally, Torben Harsløf received lecture fees from Amgen, Astra Zeneca, and Eli Lilly.
Bente Langdahl has received research funding to her institution from Amgen and Novo Nordisk. Bente Langdahl serves on advisory boards and speaker’s bureau for Eli Lilly, Amgen, UCB, Gedeon-Richter, and Gilead.
Niels Henrik Bruun and Anne Sophie Sølling have no additional competing interest.
Ethics approval
The Regional Ethics Committee (1-10-72-202-16), the Danish Data Protection Agency (1-16-02-485-16), the Danish Health and Medicines Authority (2016-0728-85), and The Danish Health Data Authority (FSEID-00003034) approved the study. The trial is registered at clinicaltrials.gov on 14 February 2017 (identifier NCT03346395). We conducted the study according to the Helsinki Declaration and the Danish Health Law.
Consent to participate
All participants gave a written informed consent at baseline and month 12.
Consent for publication
All participants gave a written informed consent at baseline and month 12.
Code availability
Not applicable.
Dissemination to participants
After completion of the study, the participants will receive written information about the results. Also, the participants can seek further information about the project by contacting the investigator.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 175 kb)
Rights and permissions
About this article
Cite this article
Sølling, A., Harsløf, T., Bruun, N. et al. The predictive value of bone turnover markers during discontinuation of alendronate: the PROSA study. Osteoporos Int 32, 1557–1566 (2021). https://doi.org/10.1007/s00198-021-05835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-05835-4