Abstract
Summary
Analyses of healthcare data from 30 million individuals in three countries showed that current use of bisphosphonates may be associated with a small increased risk of cardiac valvulopathy (vs. those not exposed within the previous year), although confounding cannot be entirely ruled out. The observed tendency for decreased valvulopathy risk with cumulative duration of bisphosphonate use >6 months may even indicate a protective effect with prolonged use. Further studies are still needed to evaluate whether bisphosphonates increase or decrease the risk of valvulopathy.
Introduction
A signal of cardiac valve disorders with use of bisphosphonates was identified in the literature and EudraVigilance database, which contains reports of suspected adverse drug reactions from worldwide sources. The aim of this study was to evaluate the association using population-based healthcare data.
Methods
This was a case-control study among users of bisphosphonates and other drugs for osteoporosis in six healthcare databases covering over 30 million individuals in Italy, Netherlands and the UK from 1996 to 2012. Prescriptions/dispensations were used to assess drug exposure. Newly diagnosed cases of cardiac valvulopathy were identified via disease codes/free-text search. Controls were matched to each case by age, sex, database and index date. Adjusted odds ratios (ORs) were estimated using conditional logistic regression for the pooled data and meta-analysis of individual database risk estimates.
Results
A small but statistically significant association was found between exposure to bisphosphonates as a class and risk of valvulopathy. Overall risk was 18 % higher (95 % CI 12–23 %) in those currently exposed to any bisphosphonate (mainly alendronate and risedronate) vs. those not exposed within the previous year. Risk of valve regurgitation was 14 % higher (95 % CI 7–22 %). Decreased valvulopathy risk was observed with longer cumulative duration of bisphosphonate use, compared to use of less than 6 months. Meta-analyses of database-specific estimates confirmed results from pooled analyses.
Conclusions
The observed increased risks of cardiac valvulopathy with bisphosphonate use, although statistically significant, were quite small and unlikely to be clinically significant. Further studies are still needed to evaluate whether bisphosphonates increase or decrease the risk of valvulopathy and to investigate possible mechanisms for the association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates are drugs widely used in the treatment and prevention of bone‐related disorders including osteoporosis, Paget’s disease and complications related to metastatic bone cancer and multiple myeloma [1–3]. A signal of cardiac valve calcification leading to valve incompetence associated with exposure to bisphosphonates was found in the post‐authorisation analysis of EudraVigilance (http://eudravigilance.ema.europa.eu), which contains spontaneous reports of suspected adverse drug reactions from Europe and the rest of the world. This statistical signal of disproportionate reporting was associated with four products of the class (alendronate, ibandronate, pamidronate and zoledronate) [4]. The signal was supported by an earlier study conducted within the Multi-Ethnic Study of Atherosclerosis (MESA) cohort which showed that prevalence of aortic valve (AV) and vascular calcification was higher among female users of bisphosphonates compared to non-users [5].
Valvulopathy is responsible for an increasing proportion of cardiovascular surgical interventions, especially in the setting of an aging population in the USA and Europe, where age-related valve degeneration is the prevailing cause [6]. Drug exposure has been recognised as a cause of valvulopathy since the 1960s when methysergide and ergotamine were found to induce valve regurgitation [7–9]. Other drugs implicated later were fenfluramine and dexfenfluramine [10–12], pergolide and cabergoline [13–15] and most recently benfluorex [16, 17].
Most of the literature on bisphosphonates and cardiovascular outcomes has investigated the possible role of bisphosphonates in reducing cardiac calcification and atherosclerotic burden [18, 19] and in delaying progression of AV stenosis [20]. However, concerns have been recently raised regarding cardiovascular safety of bisphosphonates (several studies reporting inconsistent associations with atrial fibrillation and heart failure) [21–23]. The aim of this study was to determine the risk of newly diagnosed cardiac valvulopathy, including valve regurgitation and calcification, among users of bisphosphonates.
Methodology
Data sources
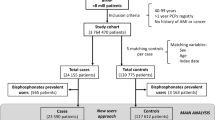
This study used data from six population-based healthcare databases from three countries during the period 1996–2012: (1) Health Search Database (HSD, Italy), (2) Integrated Primary Care Information (IPCI, Netherlands), (3) The Health Improvement Network (THIN, UK), (4) PHARMO (Netherlands), (5) Lombardy (Italy) and (6) Tuscany (Italy). HSD, IPCI and THIN are general practitioner (GP) databases where clinical information including consultations and diagnoses, referrals to secondary care/hospitalisations and drug prescriptions are recorded. PHARMO and the regional databases of Tuscany and Lombardy are record-linkage administrative (claims) systems where drug dispensations are linked to registries containing hospitalisations and other services. A more detailed description of the databases can be found in earlier publications [24–31]. Standardised software (Jerboa©) was used to extract and pool data using a distributed network [24]. The respective Scientific and Ethics committees of each database approved this study. Key characteristics of the databases are summarised in Table S1 (Appendix, available as supplementary material online).
Study design
This was a case-control study nested in a cohort of new users of bisphosphonates (primary cohort). A secondary assessment was conducted by performing similar analyses in a cohort of users of bisphosphonates and other drugs used in treatment of osteoporosis (extended cohort). All individuals registered in the databases who had at least 1 year of valid medical history were eligible for study entry. Eligibility ended with de-registration, death or end of study period. From all eligible subjects, the primary cohort was defined as all patients receiving first prescription/dispensation of any bisphosphonate after start of eligibility period. The extended cohort comprised all patients who received any prescription/dispensation of bisphosphonate as well as any other drug used in osteoporosis treatment (i.e. strontium ranelate, denosumab, teriparatide, raloxifene, calcitonin, parathyroid hormone, vitamin D/calcium preparations).
All subjects who received (at any time prior to or during the study period) prescription of a drug known to potentially cause cardiac valve fibrosis (i.e. pergolide, cabergoline, amiodarone, fenfluramine, dexfenfluramine, phentermine, benfluorex) were excluded, as were patients with history of valvulopathy, endocarditis, valve replacement, carcinoid syndrome or acromegaly.
Case identification
All newly diagnosed cases of valvulopathy were identified using disease codes extracted from hospital discharge diagnoses (claims databases) and primary care physician-recorded diagnoses (GP databases). In addition, free-text search of unstructured clinical narratives was performed in GP databases. Diagnostic codes (Table S2, supplementary material) were harmonised from the following disease terminologies: (1) International Classification of Primary Care (ICPC)—IPCI; (2) International Classification of Diseases, 9th revision-Clinical Modification (ICD-9CM)—Lombardy, Tuscany, HSD, PHARMO; and (3) Read code classification—THIN. Valve regurgitation and valve calcification were investigated separately. Only HSD, IPCI and THIN provided data on valve calcification as no specific code exists in ICD9-CM or ICPC; the outcome was identified with free-text search in HSD and IPCI and with Read code (code specific for AV calcification only) in THIN. Validation of a random sample of cases through manual review of medical records was conducted in IPCI and HSD to verify that diagnoses were confirmed by a cardiologist and/or by evidence from echocardiography or other procedure. Incidence rates of outcomes were calculated overall and per database, age-standardised according to the World Health Organisation Standard Population.
Controls (matched by age, sex and database) were selected from each cohort by incidence density sampling. In each case-control set, index date was defined as date of diagnosis of the case.
Exposure assessment
Prescriptions/dispensations were used to assess drug exposure. Duration covered by each prescription/dispensation was estimated according to legend duration (if dosing regimen is available) or otherwise based on defined daily dose [32]. In line with previous publications that investigated drug-induced valvulopathy using observational databases, a carry-over period of 180 days was included for each prescription to account for induction time and possible delay in recording of the event after initial clinical manifestations [14, 15]. The following exposure categories were defined: (1) current use, referring to prescription duration that lasts up to index date or ends ≤180 days before; (2) past use, i.e. duration of prescription ending between 180 and 365 days before index date; and (3) distant past, i.e. no exposure within 365 days before index date.
Statistical analyses
Different case-control sets were generated for each of the three outcomes. Using conditional logistic regression, odds ratios (ORs) and 95 % confidence intervals (95 % CIs), with ‘distant past use’ as reference, were estimated for current and past use of bisphosphonates as a class and of individual bisphosphonates. The following co-morbidities were considered as potential confounders: heart failure, hypertension, arrhythmias, coronary heart disease, cerebrovascular disorders, peripheral arterial disease, venous thromboembolism, obesity, autoimmune disorders, chronic obstructive pulmonary disease, chronic renal failure and diabetes mellitus. Prior use of lipid-lowering drugs and of gastroprotective agents was also taken into account. Only those confounders associated (p value <0.10) with the outcome in a univariate analysis were subsequently entered in the final model and selected using backward selection. ORs were likewise estimated for use of other anti-osteoporosis drugs, as previously described. Analyses were performed for each database separately and for the (unweighted) pooled data. Meta-analyses (random and fixed effects) of single database results were done. Heterogeneity was evaluated using Cochran’s Q statistic. All statistical analyses were done using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Additional analyses were conducted to determine whether age (≥ or <65 years) and sex are effect modifiers of the association between valvulopathy and bisphosphonates. Effects of duration of treatment and switching from bisphosphonate to other anti-osteoporosis drug (and vice versa) were also evaluated. Effect of dosage could not be assessed, as this information was not consistently available across the databases.
Sensitivity analyses
Post-hoc sensitivity analyses were conducted to investigate the impact of residual confounding on the observed association. Both the array approach and rule-out approach, as recommended by Schneeweiss, were used. Using the array approach, we considered severity of osteoporosis as an unmeasured confounder, assuming a (fixed) 10 % prevalence of severe osteoporosis among distant past users of any bisphosphonate and varying the prevalence among current users between 0 and 50 %. Using the rule-out approach, considering all possible unmeasured confounders (which may include smoking and over-the-counter use of calcium supplements) as a single factor, we looked at how strong unmeasured confounding has to be in order to explain observed association, assuming prevalence of the confounder to be 20 %.
Results
The overall study population comprised 30,332,837 individuals with 225,027,048 person-years of follow-up. The primary study cohort included 872,872 new users of bisphosphonates while the extended cohort included 1,597,135 users of any drug for osteoporosis. Most of the exposure to bisphosphonates was accounted for by alendronate (55 %) and risedronate (24 %), with an average exposure of 12 months per patient. There were 234,285 cases of valvulopathy overall (independent of any drug exposure), with age-standardised incidence rate of 95.4/100,000 person-years. Corresponding background incidence rates of valve regurgitation and valve calcification were 51.9/100,000 person-years and 1.4/100,000 person-years, respectively.
Within the primary cohort, a total of 8757 cases of valvulopathy were identified. There were 4914 cases (56 %) of valve regurgitation and 57 cases (1.2 %) of valve calcification. Table 1 shows the characteristics of cases and their matched controls for each outcome separately. Majority were female and between 65 and 80 years of age. Cases had a higher prevalence of several co-morbidities, including cardiovascular co-morbidities, compared to matched controls.
Within the extended cohort, 17,362 cases of valvulopathy were identified, including 9544 cases (55 %) of valve regurgitation and 144 cases (0.8 %) of valve calcification. In general, the characteristics of cases in extended cohort were similar to those of the primary cohort (Table 1). All ORs reported below are adjusted; crude ORs are specified in the tables.
Risk of valvulopathy among new bisphosphonate users
Patients with valvulopathy were predominantly current users of any bisphosphonate (61 %) (see Table 2). Pooled analyses showed a very small, although statistically significant, increased risk of valvulopathy with both current (OR 1.18, 95 % CI 1.12–1.23) and past use of any bisphosphonate (OR 1.11, 95 % CI 1.03–1.21), when compared to distant past use (i.e. no bisphosphonate use in the previous year). Alendronate (36.3 % of current users) and risedronate (14.4 %) together accounted for the largest exposure to bisphosphonates among cases. After adjusting for relevant covariates, increased risk of valvulopathy was observed with current use of alendronate (OR 1.19, 95 % CI 1.12–1.25) and with current use of risedronate (OR 1.20, 95 % CI 1.12–1.28). No increased risk was observed for any other individual bisphosphonate, except for the fixed combination of alendronate/cholecalciferol: OR 1.18 (95 % CI 1.07–1.31).
The adjusted ORs observed per database and results of meta-analyses are shown in Fig. 1. A slightly increased valvulopathy risk with current use of any bisphosphonate was found in all databases (except IPCI, which reported a wide 95 % CI due to a low number of exposed cases), and meta-analysis confirmed this small increased risk: random-effects OR 1.19 (95 % CI 1.11–1.28). A similarly small increased risk with current use of ≥1 bisphosphonate was observed in the Lombardy database; however, because of Lombardy’s large data contribution to the entire database network (42 %), meta-analysis retained the increased risk: OR 1.31 (95 % CI 1.11–1.54). Meta-analyses likewise confirmed increased risk with current use of alendronate, risedronate and alendronate/cholecalciferol.
Risk of cardiac valvulopathy among new users of bisphosphonates, results of meta-analyses (all ORs are adjusted for relevant confounders). a Current use of any bisphosphonate (test for heterogeneity: Q statistic = 8.489; p value = 0.1313; I2 = 41.1). b Past use of any bisphosphonate (test for heterogeneity: Q statistic = 3.3874; p value = 0.6405; I2 = 0). c Current use of >1 bisphosphonate (test for heterogeneity: Q statistic = 5.0599; p value = 0.4086; I2 = 1.18). d Current use of alendronate (test for heterogeneity: Q statistic = 7.6714; p value = 0.1753; I2 = 34.82). e Current use of risedronate (test for heterogeneity: Q statistic = 4.4672; p value = 0.4843; I2 = 0). f Current use of alendronate/cholecalciferol (test for heterogeneity: Q statistic = 10.603; p value = 0.0314; I2 = 62.27. LCL95 lower limit of 95 % confidence interval, UCL95 upper limit of 95 % confidence interval, ARS Tuscany regional database, HSD Health Search database, IPC IPCI database, LOM Lombardy regional database, PHA PHARMO database, THI THIN database, Fix fixed-effect meta-analysis, Ran random-effects meta-analysis
Risk of valve regurgitation among new bisphosphonate users
As shown in Table 2, exposure pattern of patients with valve regurgitation was similar to that of valvulopathy overall. Estimates from pooled analyses show that both current use (OR 1.14, 95 % CI 1.07–1.22) and past use (OR 1.21, 95 % CI 1.09–1.35) of any bisphosphonate were associated with a slightly increased risk of valve regurgitation. Specifically, current use of alendronate (OR 1.15, 95 % CI 1.07–1.24), risedronate (OR 1.18, 95 % CI 1.07–1.29) and alendronate/cholecalciferol (OR 1.16, 95 % CI 1.01–1.33) was associated with this small increased risk. Figure 2 shows the database-specific and meta-analysis estimates for valve regurgitation.
Risk of cardiac valve regurgitation among new users of bisphosphonates, results of meta-analyses (all ORs are adjusted for relevant confounders). Current use of any bisphosphonate (test for heterogeneity: Q statistic = 11.2028; p value = 0.0475; I2 = 55.37). a Past use of any bisphosphonate (test for heterogeneity: Q statistic = 2.8689; p value = 0.7202; I2 = 0). b Current use of >1 bisphosphonate (test for heterogeneity: Q statistic = 6.7041; p value = 0.2436; I2 = 25.42). c Current use of alendronate (test for heterogeneity: Q statistic = 7.7652; p value = 0.1697; I2 = 35.61). d Current use of risedronate (test for heterogeneity: Q statistic = 11.562; p value = 0.04131; I2 = 56.76). e Current use of etidronate/calcium (test for heterogeneity: Q statistic = 3.3754; p value = 0.1850; I2 = 40.75). LCL95 lower limit of 95 % confidence interval, UCL95 upper limit of 95 % confidence interval, ARS Tuscany regional database, HSD Health Search database, IPC IPCI database, LOM Lombardy regional database, PHA PHARMO database, THI THIN database, Fix fixed-effect meta-analysis, Ran random-effects meta-analysis
Risk of valve calcification among new bisphosphonate users
Neither current nor past use of bisphosphonates—either individually or as a class—was significantly associated with risk of valve calcification (Table 1) in any of the three databases that provided data for this outcome.
Risk of valvulopathy among users of drugs for osteoporosis
Secondary analyses looking at valvulopathy risk among users of all osteoporosis drugs resulted in risk estimates consistent with those derived from the primary analysis. Both pooled and meta-analyses showed increased valvulopathy risk with current use of any bisphosphonate and with past use of any osteoporosis drug, when compared with distant past use of any drug (see summary in Appendix, available as supplementary material online).
Effects of switching and duration of use
As shown in Table 3, valvulopathy risk appears to be lower when bisphosphonates are used for ≥6 months compared to <6 months. Switching from bisphosphonate to non-bisphosphonate, or switching from non-bisphosphonate to bisphosphonate, did not significantly increase risk of valvulopathy when compared to non-bisphosphonate alone. Neither age (<65 vs. ≥65) nor sex modified the risk of valvulopathy among bisphosphonate users (Table S4, supplementary material).
Sensitivity analyses
As shown in Figure S3 (Appendix, available as supplementary material online), as the imbalance (between those currently exposed to bisphosphonates vs. those not exposed within the previous year) of the unmeasured confounder increases, the adjusted relative risk moves closer to 1 and even further below 1 (0.6).
The curve shown in Figure S4 (Appendix, available as supplementary material online) denotes the various combinations of strength of association of the unmeasured confounder with valvulopathy and with bisphosphonate use that are required to bring down the observed OR of 1.18 to null. The unknown confounding would have to increase the relative odds of valvulopathy by a factor of 2.5 and simultaneously increase the odds of bisphosphonate exposure by a factor of 4.2 in order for adjustment to completely remove the observed association.
Discussion
This study combined data from six population-based healthcare databases in three countries (source population >30 million individuals) to investigate the relationship between use of bisphosphonates and cardiac valvulopathy. The overall risk of (non-rheumatic) valvulopathy was found to slightly increase—by 18 %—among current users of bisphosphonates compared to those who discontinued bisphosphonate therapy (or had no bisphosphonate exposure at all) longer than 1 year prior. Risk of valve regurgitation specifically increased by 14 % among current users. These estimates were based on analysis of pooled data and meta-analytic pooling of database-specific estimates. Among individual bisphosphonates, alendronate, alendronate/cholecalciferol and risedronate were associated with increased risk, which was observed not only among new bisphosphonate users but also among users of any drug for osteoporosis. These increased risks were similar between those ≥65 years and those who are younger as well as in both sexes. Duration of bisphosphonate use did not change the association, although there was a tendency of lower risk with longer use (>6 months). Switching from bisphosphonate to a non-bisphosphonate (or vice versa) within 180 days before index date did not change risk of valvulopathy.
The observed increased risks, although statistically significant, were quite small and are unlikely to be clinically significant. No biological mechanism accounting for the association of bisphosphonates with cardiac valvulopathy could be identified. The predominant pathology that has been described in drug-induced valvulopathy involves proliferative and fibrotic tissue changes, the mechanism being largely attributed to action of serotonin which promotes fibroblast and smooth muscle cell proliferation via upregulation of target proteins including G protein and transforming growth factor beta [33, 34]. All drugs known to induce valvulopathy (or their metabolites, in the case of fenfluramine and benfluorex) have been demonstrated to be agonists of the specific serotonin receptor 5HT2B, which abound in valvular tissue [35]. The bisphosphonates are not known to act as substrate for this receptor, however; neither are bisphosphonates structurally similar to ergot-derived dopamine agonists or amphetamine-like anorexiants. The cross-sectional MESA study [5] found higher prevalence of AV and vascular calcification among bisphosphonate users compared to non-users, although no potential mechanisms that could explain this observation were proposed (prevalence of calcification of aortic valve ring and mitral annulus was similar in users and non-users). Moreover, in this study, the prevalence of calcification overall among bisphosphonate users was higher in women <65 years compared to those older [5].
Valve calcification is associated with upregulation of morphogenetic proteins, a process similar to bone formation, and has been shown to be integral to disease onset and progression in histopathologic studies of AV stenosis, which itself becomes more prevalent with the aging population [36]. Earlier studies have hypothesised that bisphosphonates actually have the opposite effect: inhibition of vascular calcification and slowing of progression of aortic stenosis. This effect is attributed to bisphosphonates’ inhibition of farnesyl pyrophosphate synthase, a key enzyme in the mevalonate pathway affecting both protein prenylation and cholesterol biosynthesis; inhibition of the mevalonate pathway leads to tissue effects similar to those seen with statin therapy [37]. Moreover, bisphosphonates prevent bone resorption and slow the release of calcium phosphate particles from the bone, which possibly retard the deposition of calcium in vascular and valvular tissues. The number of cases of valve calcification identified in this current study was too low to enable useful inference, but the observed tendency for decreased valvulopathy risk with longer duration of bisphosphonate use in this study may actually indicate a protective effect with prolonged use, supporting findings in previous studies that bisphosphonates reduce cardiac calcification and delay progression of valve stenosis. Such protection may reflect reduced vascular calcification with more prolonged inhibition of the mevalonate pathway; further studies are necessary to investigate this, including the effect of dose and dosing intervals.
As this study was observational, there are several limitations. Misclassification of the outcome may have occurred, although validation by review of medical records was done in some databases to limit the effects of possible misclassification. Individual echocardiographic reports were not systematically reviewed since such information was not consistently available across all databases. Very early stages of the disease, during which a patient is asymptomatic, may not have received medical attention and thus not documented in the healthcare records. Prescriptions/dispensations were considered as proxy for actual drug consumption, which may not always be a reliable assumption, particularly because adherence to bisphosphonate therapy remains notoriously poor [38, 39]. Information bias from potential misclassification of the outcome and exposure may have resulted in an underestimation of the effect, as it is unlikely to be differential. More importantly, a paradoxical calcification has been described among those with reduced bone density or increased bone turnover, suggesting that osteoporosis (the indication for taking bisphosphonates) itself may account for the observed association [40, 41]. The finding that switching from bisphosphonate to non-bisphosphonate (and vice versa) does not significantly increase the risk of valvulopathy, when compared to non-bisphosphonate alone, also reinforces the possible confounding by osteoporosis. Berksonian bias could be another possible explanation for the observed association: patients who use bisphosphonates (for osteoporosis or other condition, such as malignancy) are often elderly and have other co-morbidities and are thus more likely to be subjected to more extensive clinical monitoring and investigation, increasing the likelihood of detecting cardiac valve disorders (that may be asymptomatic). Reliable data on smoking and severity of osteoporosis (as well as use of over-the-counter calcium supplements) were not available and thus were not adjusted for in the analyses. Although residual confounding cannot be entirely ruled out, the sensitivity analyses conducted indicate that unknown confounding would need to have relatively strong associations with the outcome and exposure to explain the observed association (smoking, for example, has been shown to increase the risk of valvulopathy by no more than 2).
In conclusion, while this study among users of drugs for osteoporosis in three countries showed that current use of bisphosphonates (mainly alendronate and risedronate) may be associated with a small increased risk of cardiac valvulopathy, the risk is unlikely to be clinically significant. The observed tendency for decreased valvulopathy risk with duration of bisphosphonate use greater than 6 months could even indicate a protective effect with prolonged use. Further studies are still needed to evaluate whether bisphosphonates increase or decrease the risk of cardiac valvulopathy and to investigate possible mechanisms for the association.
References
O’Connell MB (2006) Prescription drug therapies for prevention and treatment of postmenopausal osteoporosis. J Manag Care Pharma: JMCP 12(6 Suppl A):S10–S19, quiz S26-18
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119(Suppl 2):S150–S162. doi:10.1542/peds.2006-2023H
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83(9):1032–1045. doi:10.4065/83.9.1032
European Medicines Agency 2011 EudraVigilance-Human Annual Report. Accessible at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/06/WC500144560.pdf (2013)
Elmariah S, Delaney JA, O’Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, Kronmal RA, Halperin JL (2010) Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 56(21):1752–1759. doi:10.1016/j.jacc.2010.05.050
d’Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B (2011) Valvular heart disease: the next cardiac epidemic. Heart 97(2):91–93. doi:10.1136/hrt.2010.205096
Graham JR, Suby HI, LeCompte PR, Sadowsky NL (1966) Fibrotic disorders associated with methysergide therapy for headache. N Engl J Med 274(7):359–368. doi:10.1056/NEJM196602172740701
Graham JR (1967) Cardiac and pulmonary fibrosis during methysergide therapy for headache. Am J Med Sci 254(1):1–12
Misch KA (1974) Development of heart valve lesions during methysergide therapy. Br Med J 2(5915):365–366
Gardin JM, Schumacher D, Constantine G, Davis KD, Leung C, Reid CL (2000) Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine. JAMA: J Am Med Assoc 283(13):1703–1709
Seghatol FF, Rigolin VH (2002) Appetite suppressants and valvular heart disease. Curr Opin Cardiol 17(5):486–492
Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE (1998) A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med 339(11):719–724. doi:10.1056/NEJM199809103391102
Corvol JC, Anzouan-Kacou JB, Fauveau E, Bonnet AM, Lebrun-Vignes B, Girault C, Agid Y, Lechat P, Isnard R, Lacomblez L (2007) Heart valve regurgitation, pergolide use, and Parkinson disease: an observational study and meta-analysis. Arch Neurol 64(12):1721–1726. doi:10.1001/archneur.64.12.1721
Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007) Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 356(1):29–38. doi:10.1056/NEJMoa062222
Trifiro G, Mokhles MM, Dieleman JP, van Soest EM, Verhamme K, Mazzaglia G, Herings R, de Luise C, Ross D, Brusselle G, Colao A, Haverkamp W, Schade R, van Camp G, Zanettini R, Sturkenboom MC (2012) Risk of cardiac valve regurgitation with dopamine agonist use in Parkinson’s disease and hyperprolactinaemia: a multi-country, nested case-control study. Drug Saf 35(2):159–171. doi:10.2165/11594940-000000000-00000
Gueffet JP, Piriou N, Trochu JN (2010) Valvular heart disease associated with benfluorex. Arch Cardiovasc Dis 103(5):342–343. doi:10.1016/j.acvd.2009.09.015
Le Ven F, Tribouilloy C, Habib G, Gueffet JP, Marechaux S, Eicher JC, Blanchard-Lemoine B, Rousseau J, Henon P, Jobic Y, Etienne Y (2011) Valvular heart disease associated with benfluorex therapy: results from the French multicentre registry. Europ J Echocardiograp: J Working Group Echocardiography Europ Soc Cardiol 12(4):265–271. doi:10.1093/ejechocard/jeq172
Bevilacqua M, Dominguez LJ, Rosini S, Barbagallo M (2005) Bisphosphonates and atherosclerosis: why? Lupus 14(9):773–779
Fiore CE, Pennisi P, Pulvirenti I, Francucci CM (2009) Bisphosphonates and atherosclerosis. J Endocrinol Investig 32(4 Suppl):38–43
Aksoy O, Cam A, Goel SS, Houghtaling PL, Williams S, Ruiz-Rodriguez E, Menon V, Kapadia SR, Tuzcu EM, Blackstone EH, Griffin BP (2012) Do bisphosphonates slow the progression of aortic stenosis? J Am Coll Cardiol 59(16):1452–1459. doi:10.1016/j.jacc.2012.01.024
John Camm A (2010) Review of the cardiovascular safety of zoledronic acid and other bisphosphonates for the treatment of osteoporosis. Clin Ther 32(3):426–436. doi:10.1016/j.clinthera.2010.03.014
Grove EL, Abrahamsen B, Vestergaard P (2013) Heart failure in patients treated with bisphosphonates. J Intern Med 274(4):342–350. doi:10.1111/joim.12087
Bunch TJ, Anderson JL, May HT, Muhlestein JB, Horne BD, Crandall BG, Weiss JP, Lappe DL, Osborn JS, Day JD (2009) Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol 103(6):824–828. doi:10.1016/j.amjcard.2008.11.037
Coloma PM, Schuemie MJ, Trifiro G, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Giaquinto C, Corrao G, Pedersen L, van der Lei J, Sturkenboom M (2011) Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf 20(1):1–11. doi:10.1002/pds.2053
Coloma PM, Trifiro G, Schuemie MJ, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Picelli G, Corrao G, Pedersen L, van der Lei J, Sturkenboom M (2012) Electronic healthcare databases for active drug safety surveillance: is there enough leverage? Pharmacoepidemiol Drug Saf. doi:10.1002/pds.3197
Mazzaglia G, Mantovani LG, Sturkenboom MC, Filippi A, Trifiro G, Cricelli C, Brignoli O, Caputi AP (2005) Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens 23(11):2093–2100
Vlug AE, van der Lei J, Mosseveld BM, van Wijk MA, van der Linden PD, Sturkenboom MC, van Bemmel JH (1999) Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 38(4–5):339–344. doi:10.1267/METH99040339
Goettsch WG, de Jong RB, Kramarz P, Herings RM (2007) Developments of the incidence of osteoporosis in The Netherlands: a PHARMO study. Pharmacoepidemiol Drug Saf 16(2):166–172. doi:10.1002/pds.1245
Corrao G, Zambon A, Conti V, Nicotra F, La Vecchia C, Fornari C, Cesana G, Contiero P, Tagliabue G, Nappi RE, Merlino L (2008) Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol 19(1):150–155. doi:10.1093/annonc/mdm404
Barchielli A, Balzi D, Marchionni N, Carrabba N, Margheri M, Santoro GM, Olivotto I, Buiatti E (2007) Early discharge after acute myocardial infarction in the current clinical practice. Community data from the AMI-Florence Registry. Italy Int J Cardiol 114(1):57–63. doi:10.1016/j.ijcard.2006.01.006
Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S (2009) Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 67(1):99–109. doi:10.1111/j.1365-2125.2008.03308.x
WHO WHO DDD Classification. http://www.whocc.no/ddd/definition_and_general_considera/
Bhattacharyya S, Schapira AH, Mikhailidis DP, Davar J (2009) Drug-induced fibrotic valvular heart disease. Lancet 374(9689):577–585. doi:10.1016/S0140-6736(09)60252-X
Andrejak M, Tribouilloy C (2013) Drug-induced valvular heart disease: an update. Arch Cardiovasc Dis 106(5):333–339. doi:10.1016/j.acvd.2013.02.003
Cosyns B, Droogmans S, Rosenhek R, Lancellotti P (2013) Drug-induced valvular heart disease. Heart 99(1):7–12. doi:10.1136/heartjnl-2012-302239
Mohler ER 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH (1999) Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 8(3):254–260
Elmariah S, Rajamannan NM (2010) Medical therapy for calcific aortic stenosis: the use of bisphosphonates. Cardiology 117(3):229–230. doi:10.1159/000322902
Vieira HP, Leite IA, Araujo Sampaio TM, de Dos Anjos PJ, do Nascimento AA, de Abreu LC, Valenti VE, Goulart FC, Adami F (2013) Bisphosphonates adherence for treatment of osteoporosis. Int Arch Med 6(1):24. doi:10.1186/1755-7682-6-24
Reynolds K, Muntner P, Cheetham TC, Harrison TN, Morisky DE, Silverman S, Gold DT, Vansomphone SS, Wei R, O’Malley CD (2013) Primary non-adherence to bisphosphonates in an integrated healthcare setting. Osteoporos Int 24(9):2509–2517. doi:10.1007/s00198-013-2326-5
Persy V, D’Haese P (2009) Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 15(9):405–416. doi:10.1016/j.molmed.2009.07.001
Dweck MR, Newby DE (2012) Osteoporosis is a major confounder in observational studies investigating bisphosphonate therapy in aortic stenosis. J Am Coll Cardiol 60(11):1027. doi:10.1016/j.jacc.2012.04.048, author reply 1027
Acknowledgments
The research leading to these results has been funded by the European Medicines Agency (EMA/2011/39/CN).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The respective Scientific and Ethics committees of each database used approved this study.
Conflicts of interest
Miriam Sturkenboom has received various unconditional research grants unrelated to this work from pharmaceutical companies (Merck, Pfizer, Johnson and Johnson, Amgen, Roche, Altana, GSK) and has served as consultant to Pfizer, Celgene, Servier and Sanofi Aventis. Ron MC Herings and Irene Bezemer belong to an organisation that performs studies for various pharmaceutical companies; there are no other relationships or activities that could appear to have influenced the submitted work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 603 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Coloma, P.M., de Ridder, M., Bezemer, I. et al. Risk of cardiac valvulopathy with use of bisphosphonates: a population-based, multi-country case-control study. Osteoporos Int 27, 1857–1867 (2016). https://doi.org/10.1007/s00198-015-3441-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3441-2