Abstract
Summary
Strontium ranelate use, compared with oral bisphosphonates, is not associated with increased risk of AMI in patients with no contraindications for SR use. However, current strontium ranelate (compared with current bisphosphonate) appears associated with 25–30% excess risk of VTE and 35% excess risk of CVDeath.
Introduction
Evaluate the risk of cardiac and thromboembolic events among new users of SR and oral BPs without contraindications for SR.
Methods
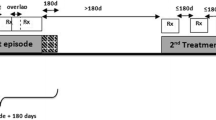
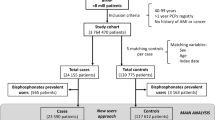
We conducted three multi-national, multi-database (Aarhus-Denmark, HSD-Italy, IPCI-Netherlands, SIDIAP-Spain, THIN-UK) case-control studies nested within a cohort of new users of SR/BP. We matched cases of acute myocardial infarction (AMI), venous thromboembolism (VTE), and cardiovascular death (CVDeath), up to 10 controls on gender, year of birth, index date, and country. Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CIs) according to current SR vs current BP use and current vs past SR use, adjusting for potential confounders. Data were pooled using random effects meta-analysis.
Results
No excess risk of AMI (5477 cases/54,674 controls) was found with current SR vs current BP (OR 0.89 (95%CI 0.70, 1.12)) nor with current vs past SR use (0.71(0.56, 0.91)). For VTE (5614 cases/6036 controls), an excess risk was found with current SR compared with current BP use, 1.24 (0.96, 1.61), and current vs past SR use, 1.30 (1.04, 1.62). For CVDeath (3019 cases/29,871 controls), an increased risk was seen with current SR vs current BP use, 1.35 (1.02, 1.80), but not with current vs past SR use (0.68 (0.48, 0.96)).
Conclusion
In patients without contraindications for SR, we found no evidence of an increased risk of AMI but a 25–30% excess risk of VTE and a 35% excess risk of CVDeath with current SR vs current BP users. This is despite a reduction in risk in CVDeath with current vs past SR users. The latter disparity could still be partially explained by cessation of preventative therapies in end-of-life or residual confounding by indication.
Similar content being viewed by others
Date availability
The different data sources have provided the research team with data subject to different licences, which do not allow sharing.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
References
European Medicines Agency (2013) PSUR assessment report. EMA/PRAC/136656/2013. Strontium ranelate
European Medicines Agency (2013) Recommendation to restrict the use of Protelos / Osseor (strontium ranelate)
European Medicines Agency (2014) Protelos/Osseor to remain available but with further restrictions
European Medicines Agency (2014) Protelos and Osseor. CHMP scientific conclusions and PRAC Assessment report of the Review under Article 20 of Regulation (EC) No 726/2004
Abrahamsen B, Grove E, Vestergaard P (2014) Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporos Int 25(2):757–762
Svanström H, Pasternak B, Hviid A (2014) Use of strontium ranelate and risk of acute coronary syndrome: cohort study. Ann Rheum Dis 73(6):1037–1043
Cooper C, Fox K, Borer J (2014) Ischaemic cardiac events and use of strontium ranelate in postmenopausal osteoporosis: a nested case–control study in the CPRD. Osteoporos Int 25(2):737–745
Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, Molokhia M, Mosseveld M, Morabito P, Schuemie MJ, van der Lei J, Sturkenboom M, Trifirò G, on behalf of the EU-ADR Consortium (2013) Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open 3(6):e002862
Ramos R, Balló E, Marrugat J, Elosua R, Sala J, Grau M, Vila J, Bolíbar B, García-Gil M, Martí R, Fina F, Hermosilla E, Rosell M, Muñoz MA, Prieto-Alhambra D, Quesada M (2012) Validity for use in research on vascular diseases of the SIDIAP (information system for the development of research in primary care): the EMMA study. Rev Esp Cardiol (English Edition) 65(1):29–37
Lawrenson R, Todd JC, Leydon G, Williams T, Farmer R (2000) Validation of the diagnosis of venous thromboembolism in general practice database studies. Br J Clin Pharmacol 49(6):591–596
Severinsen MT, Kristensen SR, Overvad K, Dethlefsen C, Tjønneland A, Johnsen SP (2010) Venous thromboembolism discharge diagnoses in the Danish National Patient Registry should be used with caution. J Clin Epidemiol 63(2):223–228
Reyes C, Aragon M, Rijnbeek P, van der Lei J, Verhamme K, Prieto-Alhambra D (2018) Validation of COPD ICD10 diagnostic codes in the SIDIAP primary health care research database using a combination of spirometry measures, symptoms, drug use, and free text review. Value Health 21:S368
R Core Team (2013) R: A language and environment for statistical computing
Berencsi K, Sami A, Ali MS, Marinier K, Deltour N, Perez-Gutthann S, Pedersen L, Rijnbeek P, van der Lei J, Lapi F, Simonetti M, Reyes C, Sturkenboom MCJM, Prieto-Alhambra D (2020) Impact of risk minimisation measures on the use of strontium ranelate in Europe: a multi-national cohort study in 5 EU countries by the EU-ADR Alliance. Osteoporos Int 31(4):721–755
Martín-Merino E, Petersen I, Hawley S et al (2018) Risk of venous thromboembolism among users of different anti-osteoporosis drugs: a population-based cohort analysis including over 200,000 participants from Spain and the UK. Osteoporos Int 29(2):467–478
Sing C-W, Wong AYS, Kiel DP et al (2018) Association of alendronate and risk of cardiovascular events in patients with hip fracture. J Bone Miner Res 33(8):1422–1434
Acknowledgements
We thank to our colleagues Eva Molero and Natasha Yefimenko (SYNAPSE Research Management Partners S.L.) who provided the project management of this study and helped with formatting of the related full study report.
Role of funding source
The study funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors had full access to all of the data in the study and take responsibility for the integrity of such data and the accuracy of the data analysis.
Code availability (software application or custom code)
Not Applicable.
Funding
This work was funded by Servier through a research grant to the EU-ADR Alliance.
DPA is funded by a National Institute for Health Research Clinician Scientist award (CS-2013-13-012). This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. This work was partially supported by the NIHR Biomedical Research Centre, Oxford.
At the time of study, Miriam Sturkenboom was co-coordinating the work from Erasmus University Medical Center.
Author information
Authors and Affiliations
Contributions
DPA, MS, SPG, KM, ND, and MSA conceived and planned the study. MSA, KB, LP, PR, FL, MS, CR, JVdL, and MS extracted and/or prepared the data for the statistical analyses. MSA conducted the statistical analysis. MSA, DPA, and SPG completed the interpretation of the results with contributions from all authors. MSA drafted of the manuscript, which was reviewed, commented, and approved by all authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Conflicts of interest
The manuscript is accompanied by completed ‘Authorship & Disclosure Form’.
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare SPG as employee of RTI Health Solutions receives grants and consultation from pharmaceutical companies including Servier for other regulatory safety studies and for supporting the role in the scientific advisory board for this study; DPA’s department has received fees for speaker services and advisory board membership from Amgen, consultancy fees from UCB Biopharma, and research grants from UCB Biopharma and Amgen; KM and ND are employees of Servier.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located; and, vi) licence any third party to do any or all of the above.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, M., Berencsi, K., Marinier, K. et al. Comparative cardiovascular safety of strontium ranelate and bisphosphonates: a multi-database study in 5 EU countries by the EU-ADR Alliance. Osteoporos Int 31, 2425–2438 (2020). https://doi.org/10.1007/s00198-020-05580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05580-0