Abstract
Introduction and hypothesis
The aim of the study is to demonstrate the impact of the size of implanted mesh in relation to its immunohistochemical reaction implanted into animal models.
Methods
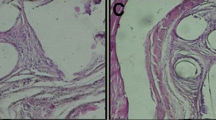
An experimental study utilizing 54 female Sprague Dawley (SD) rats was divided into five groups: control, sham, and study groups (mesh-small [M-S], mesh-medium [M-M], mesh-large [M-L]). The M-S group used a mesh size of 0.2 × 0.2 cm, the M-M group a mesh size of 0.5 × 0.5 cm, and the M-L a mesh size of 0.7 × 1.0 cm. The sham group underwent vaginal dissection with no mesh implantation. The rats were sacrificed using isoflurane overdose on days 7 and 30. The mesh with the surrounding vaginal and bladder wall tissues were removed and processed for histochemical and western blot analysis.
Results
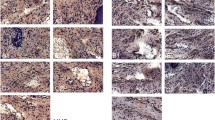
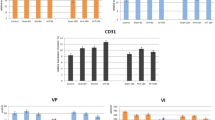
There is a significant increase in IL-1 and TNF-α immunoreactivity in the M-M and M-L groups on day 7 when compared with the sham group with p values of 0.001 and < 0.001 respectively. M-L showed significantly higher immunoreactivity to TNF-α persisting until day 30. All study groups presented a significantly higher immunoreactivity to MMP-2 and NGF on day 7. However, reactivity to NGF does not persist to day 30 in all groups. Immunoreactivity to CD 31 on days 7 and 30 appears significantly greater in the M-M and M-L groups, with the reaction in the M-L group continuing until day 30.
Conclusion
Mesh size is directly proportional to the inflammatory reaction in the host tissue. The prolonged inflammatory process leads to delayed tissue remodeling and angiogenesis, which could delay mesh–tissue integration.





Similar content being viewed by others
References
US Food and Drug Administration (FDA). Urogynecologic surgical mesh: update on the safety and effectiveness of vaginal placement for pelvic organ prolapse. Silver Spring, MD:FDA; 2011.
Ugianskiene A, Davila GW, Su TH, FIGO Urogynecology and Pelvic Floor Committee. FIGO review of statements on use of synthetic mesh for pelvic organ prolapse and stress urinary incontinence. Int J Gynaecol Obstet. 2019;147(2):147–55.
Mangir N, Roman S, MacNeil S. The changing regulatory landscape for biomedical implants and its relationship to withdrawal of some vaginal mesh products. Curr Opin Urol. 2019;29(4):414–8.
Abbott S, Unger CA, Evans JM, Jallad K, Mishra K, Karram MM, et al. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: a multicenter study. Am J Obstet Gynecol. 2014;210(2):163.e1–8.
Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG, et al. Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J. 2011 Jul;22(7):789–98.
Glazener CM, Breeman S, Elders A, Hemming C, Cooper KG, Freeman RM, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381–92.
Patel H, Ostergard DR, Sternschuss G. Polypropylene mesh and the host response. Int Urogynecol J. 2012;23(6):669–79.
Kelly M, Macdougall K, Olabisi O, McGuire N. In vivo response to polypropylene following implantation in animal models: a review of biocompatibility. Int Urogynecol J. 2017;28(2):171–80.
Ito Y, Kaneko N, Iwasaki T, Morikawa S, Kaneko K, Masumoto J. IL-1 as a target in inflammation. Endocr Metab Immune Disord Drug Targets. 2015;15(3):206–11.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100.
Kawamoto K, Matsuda H. Nerve growth factor and wound healing. Prog Brain Res. 2004;146:369–84.
Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor levels in overactive bladder syndrome and lower urinary tract disorders. J Formos Med Assoc. 2010;109(12):862–78.
Sheng W, Zhang H, Ruth KH. Could urinary nerve growth factor be a biomarker for overactive bladder? A meta-analysis. Neurourol Urodyn. 2017;36(7):1703–10.
Lo TS, Lin YH, Chu HC, Cortes EFM, Pue LB, Tan YL, et al. Association of urodynamics and lower urogenital tract nerve growth factor after synthetic vaginal mesh implantation on a rat model. J Obstet Gynaecol Res. 2017;43(1):173–8.
Lo TS, Lin YH, Uy-Patrimonio MC, Chu HC, Hsieh WC, Chua S. Dissecting of the paravesical space associated with lower urinary tract dysfunction—a rat model. Sci Rep. 2020;10(1):1718.
La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184(6):2311–26.
Nguyen T, Mobashery S, Mayland C. Roles of matrix metalloproteinases in cutaneous wound healing. In: Alexandrescu (ed) Wound healing: new insights into ancient challenges. 2016. https://doi.org/10.5772/64611.
Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA. Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque. Am J Obstet Gynecol. 2015;212(2):174.e1–7.
Ayuk SM, Abrahamse H, Houreld NN. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J Diabetes Res. 2016;2016:2897656.
Hilger WS, Walter A, Zobitz ME, Leslie KO, Magtibay P, Cornella J. Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol. 2006;195(6):1826–31.
Pierce LM, Rao A, Baumann SS, Glassberg JE, Kuehl TJ, Muir TW. Long-term histologic response to synthetic and biologic graft materials implanted in the vagina and abdomen of a rabbit model. Am J Obstet Gynecol. 2009;200(5):546.e1–8.
Endo M, Urbankova I, Vlacil J, Sengupta S, Deprest T, Klosterhalfen B, et al. Cross-linked xenogenic collagen implantation in the sheep model for vaginal surgery. Gynecol Surg. 2015;12(2):113–22.
Feola A, Endo M, Urbankova I, Vlacil J, Deprest T, Bettin S, et al. Host reaction to vaginally inserted collagen containing polypropylene implants in sheep. Am J Obstet Gynecol. 2015;212(4):474.e1–8.
Lo TS, Lin YH, Yusoff FM, Chu HC, Hsieh WC, Uy-Patrimonio MC. The immunohistochemical and urodynamic evaluation towards the collagen-coated and non-coated polypropylene meshes implanted in the pelvic wall of the rats. Sci Rep. 2016;6:38960.
Manodoro S, Endo M, Uvin P, Albersen M, Vláčil J, Engels A, et al. Graft-related complications and biaxial tensiometry following experimental vaginal implantation of flat mesh of variable dimensions. BJOG. 2013;120(2):244–50.
Prudente A, Favaro WJ, Latuf Filho P, Zanettini Riccetto CL. Host inflammatory response to polypropylene implants: insights from a quantitative immunohistochemical and birefringence analysis in a rat subcutaneous model. Int Braz J Urol. 2016;42(3):585–93.
Bronzatto E, Riccetto CLZ. Pro-inflammatory cytokines and metalloproteinase activation in polypropylene mesh implant in rat subcutaneous tissue. Int Braz J Urol. 2018;44(4):819–25.
Acknowledgements
This study was supported by Chang Gung University Hospital Research Grants CMRPG2G0381, and approved by Chang Gung Hospital’s Institutional Animal Care and Use Committee IACUC No. 2016 093003.
Financial disclaimer
This study was supported by Chang Gung University Hospital Research Grants CMRPG2G0381.
Author information
Authors and Affiliations
Contributions
T.S. Lo: protocol/project development, data collection, data analysis, manuscript editing; Y.H. Lin: data collection/data analysis; S. Chua: data collection, data analysis and manuscript editing; H.C. Chu: laboratory handling, data collection; M.C. Uy-Patrimonio: manuscript writing; K.L. Ng: data analysis and manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
No further conflicts of interest are claimed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lo, TS., Lin, YH., Chua, S. et al. Immunochemical analysis on polypropylene mesh: does mesh size make a difference?. Int Urogynecol J 32, 47–55 (2021). https://doi.org/10.1007/s00192-020-04399-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04399-x