Abstract
Purpose
Among acute respiratory distress syndrome (ARDS) patients in intensive care units, the efficacy of lung recruitment maneuver (LRM) use is uncertain taking into account the most recent randomized controlled trials (RCTs). We aimed to estimate the effect of LRMs on mortality from ARDS.
Methods
In this systematic review and meta-analysis, we searched for RCTs comparing mechanical ventilation with and without LRMs in adults with ARDS. We generated pooled relative risks (RR), mean difference, performed trial-sequential-analysis and cumulative meta-analysis. The primary outcome was 28-day mortality. The secondary outcomes were oxygenation evaluated by PaO2/FiO2 ratio, rate of rescue therapy and rate of hemodynamic compromise.
Results
In 14 RCTs including 3185 patients, LRMs were not associated with reduced 28-day mortality (RR = 0.92, 95% confidence interval (95% CI) 0.82–1.04, P = 0.21), compared to no-LRM. Trial-sequential-analysis showed that the required information size has been accrued. PaO2/FiO2 ratio was significantly higher in the LRMs group in comparison to the no-LRM group (mean difference = 47.6 mmHg, 95% CI 33.4–61.8, P < 0.001). LRMs were associated with a decreased rate of rescue therapy (RR = 0.69 95% CI 0.56–0.84, P < 0.001), and an increased rate of hemodynamic compromise (RR = 1.19, 95% CI 1.06–1.33, P = 0.002), compared to no-LRM group. Using cumulative meta-analysis, a significant change for effect on mortality was observed after 2017.
Conclusions
The results suggest that in ARDS patients, systematic use of LRMs does not significantly improve 28-day mortality. However, LRM use was associated with positive effects such as an oxygenation improvement and a less frequent use of rescue therapy. Nevertheless, LRM use was associated with negative effects such as hemodynamic impairment.
Similar content being viewed by others
The present meta-analysis involving a total of 3185 patients suggest that in ARDS patients, systematic use of lung recruitment maneuver (LRMs) does not significantly improve 28-day mortality. However, LRM use was associated with positive effects such as an oxygenation improvement, decreased driving pressure and less use of rescue therapy; and LRM use was associated with negative effects such as hemodynamic impairment. |
Introduction
Acute respiratory distress syndrome (ARDS) is a common life-threatening condition in critically ill patients, associated with a high mortality [1, 2]. Atelectasis formation in patients with ARDS can reduce the proportion of aerated lung available for ventilation and further exacerbate ventilation-induced lung injury (VILI) by amplifying stretching forces at margins between aerated and atelectatic regions [3]. The nonaerated lung volume can be separated in recruitable lung volume, which can be aerated, applying an appropriate level of pressure to the lung, and consolidated lung volume, which remains unrecruitable no matter the applied pressure [4]. Lung recruitment maneuver (LRM), which involves transient increase in transpulmonary pressure, aims to reopen recruitable lung units [5]. Along with the positive end-expiratory pressure (PEEP), which helps to keep the lung units recruited and to reduce the outset of atelectasis, LRM has been used to manage ARDS by opening alveoli and keeping them open [6]. Different LRMs have been described, from high continuous positive airway pressure (CPAP) to increases in PEEP at constant driving pressure (Pdrive), or high Pdrive at constant PEEP [7]. LRMs are low-cost, simple and feasible bedside interventions. However, if LRM might be associated with positive effect on oxygenation and lung compliance [5], as well as reduced use of rescue therapies [8], it may result in hemodynamic risks [5]. Higher intrathoracic pressure implies lower cardiac output, increase of pulmonary vascular resistance, which might alter right ventricular ejection, and increased barotrauma [4, 5]. Studies have shown that the increase mechanical power corresponding to an increased pressure (as applied during a LRM) is associated with higher risks of VILI [9]. Moreover, discrepancies in the response to LRM from one patient to another are described [10, 11], with responders and nonresponders to LRMs and positive or negative effects on oxygenation and lung compliance (Fig. 1a, b). Clinical practice guidelines have provided conditional recommendation suggesting the use of LRM in adult patients with ARDS [12, 13] while others did not recommend LRM [14]. Two recent meta-analyses [15, 16] have shown discrepancies, one [15] suggesting a positive effect of LRM on mortality in ARDS patients, while the other [16] did not find a significant difference on mortality between a LRM strategy and a no-LRM strategy. However, since the publication of the two last meta-analyses, additional recent randomized controlled trials (RCTs) (including ART, PHARLAP and LIVE Studies) [17,18,19] have been published in 2017 and 2019. Moreover, Trial Sequential Analysis (TSA) [20] and cumulative meta-analyses have never been performed in the previous systematic reviews and meta-analyses.
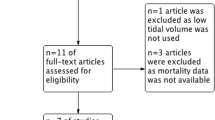
Physiological effects of LRM and study flow diagram. a Effects of lung recruitment on oxygenation and lung compliance among ARDS patients. Panel A illustrates nonresponder patient to lung recruitment, as defined by no effect or worsening on oxygenation and lung compliance after lung recruitment. Panel B illustrates responder patient to lung recruitment, as defined by oxygenation and lung compliance improvement after lung recruitment. b Balance between positive and negative effects of LRM in responders and nonresponders. Positive effects of LRM illustrates the recruitment of new lung units and its consequences in terms of improvement of oxygenation and compliance. Negative effects of LRM illustrates the harmful consequences of the increased intrathoracic pressure, simultaneously on the lung, the cardiovascular system and the brain. c Study flow diagram
We designed this systematic review and meta-analysis of RCTs to assess the effect of LRMs on the mortality in ARDS patients. We hypothesized that, in ARDS patients, systematic use of LRMs was not associated with a reduction of mortality. We also aimed to assess the effect of LRMs on oxygenation, use of rescue therapy, rates of hemodynamic impairment and barotrauma.
Materials and methods
We conducted a systematic review and meta-analysis of RCTs comparing mortality rates between a LRMs group and a no-LRM group, in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [21]. The meta-analysis was registered on the PROSPERO register of systematic review (CRD42018108694).
Search strategy, selection criteria and outcome measures
The search strategy is detailed in the electronic supplementary material. We screened for relevant RCTs that enrolled adult patients with ARDS, defined as in the Berlin Definition [22], as an acute (less than 7 days) hypoxemia based on a PaO2/FiO2 lower than 300 mmHg, with lung edema assessed by chest X-ray, not due or only partially due to a left heart failure. Those studies had to compare a LRMs group with a no-LRM group and to report mortality among the patients. Then we made a quantitative synthesis performing a meta-analysis and systematic review. The main outcome was 28-day mortality. Whenever 28-day mortality was not available, we selected 30-day mortality, and then ICU mortality, as endpoint. The secondary outcomes were respiratory values (PaO2/FiO2 ratio (Pa/Fi) at day 1 and day 7, plateau pressure (Pplat) at day 1 and day 7, PEEP at day 1 and day 7, driving pressure (Pdrive) at day 1 and day 7, PaCO2 at day 1 and day 7), use of rescue therapies (defined as use of nitric oxide inhalation, extracorporeal membrane oxygenation, high frequency oscillatory ventilation, jet ventilation, intravenous almitrin or prone positioning if not considered as first-line treatment), incidence of barotrauma, rate of hemodynamic compromise, other endpoints of mortality (ICU mortality, hospital mortality, 60-day mortality), duration of ICU stay and duration of hospital stay.
Data collection and analysis
First, two authors (JP and ADJ) independently screened the retrieved studies by title and then by abstract for exclusion. They assessed the full text of the possible relevant studies for inclusion and exclusion criteria. Disagreement was resolved by discussion and arbitrated, if necessary, by a third author (SJ). Data were then added to an excel database, specifically designed for this review and analyzed in RevMan 5.3 software and Trial Sequential Analysis viewer version 0.9.5.10 Beta.
Statistical analysis
Data were extracted as they were reported in the original paper or based on the answers of the authors to our queries. Included studies were appraised for their risk of bias by two independent authors (JP, ADJ) using the Cochrane Collaboration’s tool for assessing risk of bias in RCTs. Statistical heterogeneity was quantified by the Q-Cochrane heterogeneity test [Q statistic with degree of freedom (df)] and the I2 statistic [21]. A random-effect model was performed. In case of heterogeneity, the cause was explored in sensitivity analyses. A priori, we decided to perform subgroups analysis (PaO2/FiO2 ratio ≤ 100 mmHg and PaO2/FiO2 > 100 mmHg). A priori, we decided to perform sensitivity analyses on mortality and Pplat outcomes, excluding studies which did not use a protective lung protocol in control group, defined as a setting of tidal volume lower than 8 ml/kg of ideal theoretic weight (ITW). A priori, we decided to perform more focused sensitivity analyses on 28-day mortality, excluding studies which did not use a protective lung protocol in control group, according to the type of the LRM used in the intervention groups (CPAP or other type of LRM). To further explore heterogeneity, post hoc sensitivity analyses were performed according to the first results reported. A funnel plot (plot of treatment effect against trial precision) was also created to determine the presence of publication bias and other possible biases (English language, citation and multiple publication), true heterogeneity, data irregularities and choice of effect measure in the meta-analysis. In the presence of bias that usually leads to an overestimate of the treatment effect, the funnel plot is skewed and asymmetrical.
We conducted a cumulative meta-analysis according to publication year, by updating the pooled risk ratio each time a result of a new trial was published for the primary outcome [23]. This statistical method is used to detect the dynamic trend of the association result or further stabilize the meta-analysis conclusion. To explore more thoroughly the primary end point, we used TSA to assess the risk of random errors due to sparse data and multiple testing of accumulating data [20] and to calculate the required information size. The calculated required information size takes into account the control event proportion, the anticipated heterogeneity variance (D2) of the meta-analysis, and the assumption of a plausible relative risk reduction (RRR) or relative risk increase (RRI). We used an alpha risk of 5%, a beta risk of 10%, and a D2 as suggested by the trials in the meta-analysis. As anticipated intervention effects for the primary outcome in the TSA, we used a realistic a priori RRR or RRI of 20%. All tests were two-sided and p values less than 0.05 were considered statistically significant.
Results
Study selection
We identified 747 articles using the search strategy. We excluded 180 citations because of duplications and 542 citations on the initial abstract screen because inclusion criteria were not met. After examination of the full text of the 25 selected papers, we included 14 RCTs [6, 8, 17,18,19, 24,25,26,27,28,29,30,31,32] for the meta-analysis. Figure 1c shows the study selection flowchart.
Study description
The fourteen studies [6, 8, 17,18,19, 24,25,26,27,28,29,30,31,32] involved a total of 3185 patients from seven countries (China = 4, Taiwan = 3, Australia n = 2, Brazil n = 2, Canada n = 1, France n = 1, Spain n = 1), 1517 patients were analyzed in the LRMs group and 1668 in the no-LRM group (Table 1).
Risk of bias and quality assessment
All RCTs were identified with low to moderate risk of bias according to the Cochrane collaboration’s tool. Figures S1 and S2 present the risk of bias assessment of included studies. The 28-day mortality outcome was reported in all 14 studies. Pa/Fi at day 1, Pa/Fi at day 7, PEEP at day 1 and PEEP at day 7 were reported in nine studies. Pplat at day 1, Pplat at day 7, Pdrive at day 1, Pdrive at day 7 were reported in, respectively, eight, seven, three and three studies. PaCO2 at day 1 and PaCO2 at day 7 were, respectively, reported in eight and seven studies. Use of rescue therapy was reported in five studies. Incidences of barotrauma and hemodynamic compromise were reported, respectively, in twelve and four studies. ICU mortality, hospital mortality and 60-day mortality were reported, respectively, in ten, eleven and three studies. Duration of ICU stay and duration of hospital stay were reported, respectively, in seven and five studies. Details of the described studies for each item are reported in the Electronic Supplemental Material.
Primary outcome: 28-day mortality
Fourteen studies presented results for the 28-day mortality. The pooled RR across all studies was 0.92 (95% Confidence Interval (CI) 0.82–1.04, P = 0.21), indicating no significant difference in 28-day mortality in the LRMs group when compared to the no-LRM group (Fig. 2a). There was no significant heterogeneity for this outcome (df = 13, P = 0.25) with a corresponding I2 statistic of 18%.
The effect of LRMs on 28-day mortality in ARDS patients, compared to a no-LRM group. a Forest plot. b Cumulative meta-analysis. Pooled risk ratios are updated each time a new study was published. c Trial Sequential Analysis of all trials of the effect of LRMs on 28-day mortality. Control event proportion of 38.6%, diversity (D2) of 39%, alpha of 5%, power of 90% and relative risk reduction of 20%. As the cumulative Z-curve reached the trial sequential monitoring boundary for futility, we may reject a 20% RRR with high level of certainty. LRM lung recruitment maneuver, CI confidence interval, df degrees of freedom, I2 heterogeneity statistic, M-H Mantel–Haenszel
A cumulative meta-analysis was conducted to assess changes over time (Fig. 2b). A statistically significant reduction in 28-day mortality was first observed in the studies performed from 1998 to 2017 (RR = 0.83 95% CI 0.72–0.95). After adding the additional recent RCTs [17,18,19], no more significant reduction in 28-day mortality was observed (RR = 0.92 95% CI 0.82–1.04).
TSA showed that the required information size to reject a RRR/RRI of at least 20% had been accrued. The certainty of evidence was high (Fig. 2c).
A first sensitivity analysis was performed, excluding one study [24] that did not use a protective ventilation protocol in control group (Figure S3). It showed no significant difference in 28-day mortality (13 studies, 3132 patients, RR = 1.00 95% CI 0.92–1.09, P = 0.99). There was no significant heterogeneity. The second sensitivity analysis (Figure S4) including only studies using CPAP LRMs, excluding studies with no protective ventilation in control group, showed no significant difference in 28-day mortality (four studies, 1414 patients, RR = 0.87 95% CI 0.74–1.01, P = 0.07), without heterogeneity. The sensitivity analysis (Figure S4) including only studies using other LRMs showed no significant difference in 28-day mortality (nine studies, 1605 patients, RR = 1.07 95% CI 0.96–1.18, P = 0.23) without heterogeneity. A subgroup analysis was performed, according to the severity of ARDS at baseline. It showed no significant difference in 28-day mortality neither in patients with a PaFi lower or equal than 100 mmHg nor in patients with a PaFi higher than 100 mmHg (Figure S5).
Secondary outcomes
Oxygenation and ventilation parameters
Nine studies presented results on PaFi at day 1 (Fig. 3a). PaFi at day 1 was found significantly higher in the LRMs group when compared to the no-LRM group (MD = 47.6 mmHg (95% CI 33.4–61.8, P < 0.001), indicating better oxygenation. Nine studies presented results on PaFi at day 7 (Figure S6). The MD was 34.2 mmHg (95% CI 8.0–60.4, P = 0.01), indicating better oxygenation at day 7 in the LRMs group when compared to the no-LRM group.
The effect of LRMs on oxygenation, compared to a no-LRM group. a Forest plot of the effect of LRMs on Pa/Fi at day 1 in ARDS patients, compared to a no-LRM group. b Forest plot of the effect of LRMs on use of rescue therapy for hypoxemia in ARDS patients, compared to a no-LRM group. PaFi PaO2/FiO2 ratio, LRM lung recruitment maneuver, CI confidence interval, df degrees of freedom, I2 heterogeneity statistic, IV inverse variance, M-H Mantel–Haenszel
The results on PEEP, Pplat and Pdrive, at day 1 and day 7 after randomization, are presented on electronic supplemental material (Table S1, Figures S7-S15). Sensitivity analyses were performed on Pplat outcomes, excluding one RCT [24] which did not use lung-protective ventilation in the control group. A post hoc sensitivity analysis was performed on the Pdrive at day 1 outcome, excluding one study [19] which contained significant difference in Pdrive at baseline characteristics between groups.
Use of rescue therapy
Five studies presented results for the use of rescue therapy (Fig. 3b). There was a significantly lower rate of rescue therapy in the LRMs group, when compared to the no-LRM group (2013 patients, RR = 0.69 95% CI 0.56–0.84, P < 0.001).
Adverse events: incidence of barotrauma
Twelve studies presented results for the incidence of barotrauma (Fig. 4a). The pooled RR across all studies was 0.99 (3133 patients, 95% CI 0.60–1.63, P = 0.96), indicating no significant difference concerning the incidence of barotrauma whether LRMs were applied or not. A sensitivity analysis was performed excluding one study [24] which did not use protective ventilation in the control group (Figure S16). It showed no significant difference in barotrauma (11 studies, 3080 patients, RR = 1.21 95% CI 0.80–1.83, P = 0.36).
The effect of LRMs on adverse events, compared to a no-LRM group. a Forest plot of the effect of LRMs on incidence of barotrauma in ARDS patients, compared to a no-LRM group. b Forest plot of the effect of LRMs on the rate of hemodynamic compromise in ARDS patients, compared to a no-LRM group. LRM lung recruitment maneuver, CI confidence interval, df degrees of freedom; I2 heterogeneity statistic, M-H Mantel–Haenszel
Adverse events: rate of hemodynamic compromise
Four studies reported rate of hemodynamic compromise (Fig. 4b). The pooled RR was 1.19 (95% CI 1.06–1.33, P = 0.002), indicating higher rate of hemodynamic compromise in the LRMs group when compared to the no-LRM group.
Other secondary results are available in the electronic supplementary material (Table S1–S3, Figures S1–S27).
Discussion
The present systematic review and meta-analysis of fourteen RCTs showed that systematic LRMs do not reduce mortality in ARDS patients compared to no-LRM, with a high certainty of evidence using TSA. The analysis of secondary outcomes brought out opposite effects, depending on the studied outcome. Higher Pa/Fi ratios and a less frequent use of rescue therapy for hypoxemia were reported in the LRMs group, compared to the no-LRM group. A significantly higher risk of hemodynamic instability in the LRMs group compared to the no-LRM group was found. Using cumulative meta-analysis, a significant change for effect on mortality was observed after adding the additional recent RCTs (ART, PHARLAP and LIVE Studies) [17,18,19].
In comparison to the results reported in the meta-analysis of Goligher et al. [15] which showed an improvement on 28-day mortality with the use of LRMs, the present meta-analysis including the most recent RCT showed that systematic LRMs do not reduce mortality in ARDS patients compared to no-LRM, with a high certainty of evidence using TSA. Only six trials were included in the meta-analysis of Goligher et al. [15], which could explain that it had not reached the Required Information Size to conclude with a high level of certainty, unlike our analysis (Fig. 2c) and the meta-analysis of Bhattacharjee et al. [16]. Contrary to the meta-analysis of Bhattacharjee et al. [16] in which the studies performed without protective ventilation were excluded, we aimed to perform a global overview of LRM in ARDS management (Table 1), even if standards of care for ARDS have changed since the earliest trials. To overcome this bias, we conducted a cumulative meta-analysis for the primary outcome, 28-day mortality (Fig. 2b). Even if the oldest RCT [24] showed a significant effect of the intervention group, it is worth noting that a lung-protective ventilation strategy was not used in the control group. Since lung protective strategy has become the standard of care in the last decade [33] the ventilatory settings might be an important confusion bias. When considering the studies performed between 2008 and 2017, a statistically significant reduction in 28-day mortality was first found (RR = 0.83 95% CI 0.72–0.95). After adding the more recent RCTs published after 2017, no more significant reduction in 28-day mortality was observed (RR = 0.96; 95% CI 0.88–1.05).
One explanation of the discrepancies over time could be the rate of adverse events, such as hemodynamic instability. In the ART study [17], LRMs were associated with an increased rate of hemodynamic compromise. The differences in levels of pressure applied to the lungs might explain this higher rate of hemodynamic compromise in the four studies reporting this outcome [6, 17,18,19]. In addition, pooling the studies describing the adverse events during the LRM (Table S2), we found that only 69% of the patients randomized in the intervention groups received LRM to its completion according to the protocol. In 17% of the patients, the LRM was stopped before its completion due to an adverse event (hypotension, desaturation, cardiac arrythmia or cardiac arrest). The remaining 14% of the patients did not receive LRM because of a contraindication (at clinician’s decision). Moreover, even if barotrauma was not significantly different between the LRMs group and the no-LRM group, significantly higher Pplat was found at day 1 in the LRMs group, when excluding in a sensitivity analysis the study that did not use lung-protective ventilation in the control group [24], thus increasing the mechanical power applied to the lung [9]. However, recent data suggest that more importantly than the Pplat, the Pdrive is strongly and independently associated with mortality in ARDS patients [1, 34, 35]. In the present meta-analysis, Pdrive was found significantly lower in the LRMs group at day 1 (sensitivity analysis) and day 7 (primary analysis) in comparison to the no-LRM group. These results are consistent with the positive effects of LRMs observed on oxygenation (Fig. 3a), accompanied by reduced use of rescue therapies (Fig. 3b).
One of the strengths of the present systematic review and meta-analysis is the high sample size, which allowed to reach a high level of certainty, as demonstrated by TSA analysis when assessing that LRMs-based strategies do not improve 28-day mortality (Fig. 2c). Moreover, sensitivity analysis did not show any significant differences between groups for mortality endpoints, suggesting that the absence of benefit of mortality using LRMs is not biased depending upon the type of LRM used.
Our systematic review and meta-analysis has some limitations. First of all, eleven trials used high PEEP as a systematic co-intervention with LRM. Nevertheless, one might argue that LRMs and higher PEEP act synergistically to prevent ventilator-induced lung injury, which is consistent with the ventilatory strategy used by the authors. Second, a high statistical heterogeneity was found throughout different secondary outcomes, reflecting the clinical heterogeneity of the LRMs protocols. Indeed, Table 1 shows a high clinical heterogeneity in the type of LRM (CPAP or incremental PEEP) used, the maximum airway pressure applied (from 35 to 65 cmH2O) and the indications of LRM (at randomization only, systematically during several days or at desaturation or ventilator disconnections). Those differences between protocols of LRM might imply different consequences on oxygenation, ventilation efficiency or adverse events. Moreover, the existence of responders and nonresponders to LRM [10, 11] might also explain discrepancies between studies (Fig. 1a, b). Lung recruitability is known to vary widely from one patient to another [4]. For the same amount of nonaerated lung tissue prior to LRM, the proportion of recruitable and consolidated lung volume might explain the discrepancies in the response to LRM. To overcome this bias, we performed several sensitivity analyses to decrease the observed heterogeneity. Third, various types of LRM were used. However, after separating CPAP LRMs from other LRMs, no significant difference was found regarding the mortality outcome. Last, even if prone positioning has been strongly recommended in the recent guidelines [13, 14] and can be considered as a standard of care, only six studies stated its place in the protocol (Table S3). Most of them placed prone positioning as a rescue therapy, and its overall use in those studies concerned less than 20% of the patients. When looking at the lower use of rescue therapy in the LRM group, that we identified in this meta-analysis, the place of prone positioning must be considered.
Those considerations invite physicians to be extremely careful regarding the use of LRMs in all ARDS patients, in particular among patients with hemodynamic impairment. Applying too high airway pressures to a patient with low vascular filling might result in a post-maneuver cardiorespiratory arrest, as reported in the ART study applying airway pressures up to 60 cmH2O [17]. Among all the ARDS patients, some patients could present a favorable balance between positive effects (improvement in oxygenation and lung compliance) and negative consequences (lung overdistension and hemodynamic risks) of LRMs. One strategy may not fit all, especially a strategy with such important downsides as LRMs (Fig. 1a, b). A personalized rather than systematic use of LRM must be further investigated and could explain discrepancies between studies. Alveolar recruitability is known to vary widely between patients with ARDS. The LIVE study [19] aimed to explore this theory, differentiating ARDS according to lung morphology [36]: focal ARDS, exposed to overdistension of the already opened lung areas, and non-focal ARDS, with more collapsed tissue and higher potential oxygenation benefit, in which LRMs could occupy a useful place. Although the study [19] did not find a significant difference between personalized group and control group, secondary outcomes suggest a high rate of misclassification and a both statistically and clinically significant difference was found when analyzing only rightly classified patients.
Conclusions
The results of the present meta-analysis suggest that in ARDS patients, systematic use of LRMs does not significantly improve 28-day mortality, or hospital mortality, or duration of ICU stay or duration of hospital stay. However, LRM use was associated with positive effects such as an oxygenation improvement, decreased Pdrive and a less frequent use of rescue therapy. Nevertheless, LRM use was associated with negative effects such as hemodynamic impairment. One strategy may not fit all. Considering the numerous physiological and clinical downsides of systematic LRM, and the lack of evidence on clinical outcomes in spite of 20 years of studies, these results support an individualized rather than a systematic use of LRMs. Further studies are needed to evaluate whether selected groups of ARDS patients might benefit from LRMs.
References
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800
Jaber S, Bellani G, Blanch L, Demoule A, Esteban A, Gattinoni L, Guérin C, Hill N, Laffey JG, Maggiore SM, Mancebo J, Mayo PH, Mosier JM, Navalesi P, Quintel M, Vincent JL, Marini JJ (2017) The intensive care medicine research agenda for airways, invasive and noninvasive mechanical ventilation. Intensive Care Med 43:1352–1365
Godet T, Constantin JM, Jaber S, Futier E (2015) How to monitor a recruitment maneuver at the bedside. Curr Opin Crit Care 21:253–258
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, Meade MO, Ferguson ND (2008) Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med 178:1156–1163
Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martinez D, Hernandez M, Tucci M, Borges JB, Lubillo S, Santos A, Araujo JB, Amato MB, Suarez-Sipmann F, Open Lung Approach N (2016) Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med 44:32–42
Constantin JM, Jaber S, Futier E, Cayot-Constantin S, Verny-Pic M, Jung B, Bailly A, Guerin R, Bazin JE (2008) Respiratory effects of different recruitment maneuvers in acute respiratory distress syndrome. Crit Care 12:R50
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE, Lung Open Ventilation Study I (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637–645
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M (2016) Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 42:1567–1575
Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32:24–33
Suzumura EA, Figueiro M, Normilio-Silva K, Laranjeira L, Oliveira C, Buehler AM, Bugano D, Passos Amato MB, Ribeiro Carvalho CR, Berwanger O, Cavalcanti AB (2014) Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 40:1227–1240
Fan E, Brodie D, Slutsky AS (2018) Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 319:698–710
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ, American Thoracic Society ESoICM, Society of Critical Care M (2017) An official american thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195:1253–1263
Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, Forel JM, Guerin C, Jaber S, Mekontso-Dessap A, Mercat A, Richard JC, Roux D, Vieillard-Baron A, Faure H (2019) Formal guidelines: management of acute respiratory distress syndrome. Annals of intensive care 9:69
Goligher EC, Hodgson CL, Adhikari NKJ, Meade MO, Wunsch H, Uleryk E, Gajic O, Amato MPB, Ferguson ND, Rubenfeld GD, Fan E (2017) Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 14:S304–S311
Bhattacharjee S, Soni KD, Maitra S (2018) Recruitment maneuver does not provide any mortality benefit over lung protective strategy ventilation in adult patients with acute respiratory distress syndrome: a meta-analysis and systematic review of the randomized controlled trials. J Intensive Care 6:35
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I, Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, Romano ER, Regenga MM, Taniguchi LNT, Teixeira C, Pinheiro de Oliveira R, Machado FR, Diaz-Quijano FA, Filho MSA, Maia IS, Caser EB, Filho WO, Borges MC, Martins PA, Matsui M, Ospina-Tascon GA, Giancursi TS, Giraldo-Ramirez ND, Vieira SRR, Assef M, Hasan MS, Szczeklik W, Rios F, Amato MBP, Berwanger O, de Carvalho CR (2017) Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 318:1335–1345
Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, Brickell K, Davies A, Fahey C, Fraser J, McGuinness S, Murray L, Parke R, Paul E, Tuxen D, Vallance S, Young M, Nichol A (2019) Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP): A Phase II, multicenter, randomized, controlled trial. Am J Respir Crit Care Med [Article in press]
Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, Ferrandiere M, Grelon F, Seguin P, Ichai C, Veber B, Souweine B, Uberti T, Lasocki S, Legay F, Leone M, Eisenmann N, Dahyot-Fizelier C, Dupont H, Asehnoune K, Sossou A, Chanques G, Muller L, Bazin JE, Monsel A, Borao L, Garcier JM, Rouby JJ, Pereira B, Futier E (2019) Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med 7(10):870–880
Barbateskovic M, Marker S, Granholm A, Anthon CT, Krag M, Jakobsen JC, Perner A, Wetterslev J, Moller MH (2019) Stress ulcer prophylaxis with proton pump inhibitors or histamin-2 receptor antagonists in adult intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 45:143–158
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC (1992) Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 327:248–254
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y (2009) Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care 13:R22
Xi XM, Jiang L, Zhu B, Group RM (2010) Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl) 123:3100–3105
Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, Keating JL, Pilcher DV, Westbrook AJ, Cooper DJ, Nichol AD (2011) A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care 15:R133
Kung SC, Hung YL, Chen WL, Wang CM, Chang HC, Liu WL (2019) effects of stepwise lung recruitment maneuvers in patients with early acute respiratory distress syndrome: a prospective, randomized, controlled trial. J Clin Med 8(2):231
Chung FT, Lee CS, Lin SM, Kuo CH, Wang TY, Fang YF, Hsieh MH, Chen HC, Lin HC (2017) Alveolar recruitment maneuver attenuates extravascular lung water in acute respiratory distress syndrome. Medicine 96:e7627
Yu S, Hu TX, Jin J, Zhang S (2017) Effect of protective lung ventilation strategy combined with lung recruitment maneuver in patients with acute respiratory distress syndrome (ARDS). J Acute Dis 6:163–168
Wang XZ, Lu CJ, Gao FQ, Li XH, Hao D, Ning FY (2007) Comparison of the effects of BiPAP ventilation combined with lung recruitment maneuvers and low tidal volume A/C ventilation in patients with acute respiratory distress syndrome. Zhonghua jie he he hu xi za zhi Zhonghua jiehe he huxi zazhi Chin J Tuberc Respir Dis 30:44–47
Liu W-L, Wang C-M, Chen W-L (2011) Effects of recruitment maneuvers in patients with early acute lung injury and acute respiratory distress syndrome. Respirology 16(Suppl 2):1–326
Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A (2018) Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care 22:122
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
De Jong A, Cossic J, Verzilli D, Monet C, Carr J, Conseil M, Monnin M, Cisse M, Belafia F, Molinari N, Chanques G, Jaber S (2018) Impact of the driving pressure on mortality in obese and non-obese ARDS patients: a retrospective study of 362 cases. Intensive Care Med 44:1106–1114
Constantin JM, Grasso S, Chanques G, Aufort S, Futier E, Sebbane M, Jung B, Gallix B, Bazin JE, Rouby JJ, Jaber S (2010) Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med 38:1108–1117
Funding
The study is an investigator-initiated trial. No funder had a role in the design or conduct of the study, data collection, analysis or interpretation, the writing of the report or in the decision to submit for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Author information
Authors and Affiliations
Contributions
SJ contributed to the conception and design of the study; to the analysis and interpretation of data; to drafting the submitted article, and to provide final approval of the version to be published. JP contributed to conception and design of the study, to the acquisition of the data, to the analysis of the data, to drafting the submitted article, and to provide final approval of the version to be published. ADJ contributed to conception and design of the study, to the acquisition of the data, to the analysis of the data, to drafting the submitted article, and to provide final approval of the version to be published. GC contributed to the conception and the design of the study, to the acquisition of the data, to drafting the submitted article and to provide final approval of the version to be published. NM contributed to the analysis and interpretation of data, to drafting the submitted article, and to provide final approval of the version to be published. All authors provide agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
Pr. Jaber reports receiving consulting fees from Drager, Medtronic, Baxter and Fisher & Paykel. No potential conflict of interest relevant to this article was reported for the other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pensier, J., de Jong, A., Hajjej, Z. et al. Effect of lung recruitment maneuver on oxygenation, physiological parameters and mortality in acute respiratory distress syndrome patients: a systematic review and meta-analysis. Intensive Care Med 45, 1691–1702 (2019). https://doi.org/10.1007/s00134-019-05821-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05821-9