Abstract
Aims/hypothesis
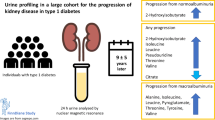
The aim of this study was to describe the metabolome in diabetic kidney disease (DKD) and its association with incident CVD in type 2 diabetes, and identify prognostic biomarkers.
Methods
From a prospective cohort of individuals with type 2 diabetes, baseline sera (N=1991) were quantified for 170 metabolites using NMR spectroscopy with median 5.2 years of follow-up. Associations of chronic kidney disease (CKD, eGFR<60 ml/min per 1.73 m2) or severely increased albuminuria with each metabolite were examined using linear regression, adjusted for confounders and multiplicity. Associations between DKD (CKD or severely increased albuminuria)-related metabolites and incident CVD were examined using Cox regressions. Metabolomic biomarkers were identified and assessed for CVD prediction and replicated in two independent cohorts.
Results
At false discovery rate (FDR)<0.05, 156 metabolites were associated with DKD (151 for CKD and 128 for severely increased albuminuria), including apolipoprotein B-containing lipoproteins, HDL, fatty acids, phenylalanine, tyrosine, albumin and glycoprotein acetyls. Over 5.2 years of follow-up, 75 metabolites were associated with incident CVD at FDR<0.05. A model comprising age, sex and three metabolites (albumin, triglycerides in large HDL and phospholipids in small LDL) performed comparably to conventional risk factors (C statistic 0.765 vs 0.762, p=0.893) and adding the three metabolites further improved CVD prediction (C statistic from 0.762 to 0.797, p=0.014) and improved discrimination and reclassification. The 3-metabolite score was validated in independent Chinese and Dutch cohorts.
Conclusions/interpretation
Altered metabolomic signatures in DKD are associated with incident CVD and improve CVD risk stratification.
Graphical Abstract

Similar content being viewed by others

Introduction
Chronic kidney disease (CKD) develops in approximately 40% of people with type 2 diabetes [1] and is associated with increased risk of CVD and mortality [2]. Diabetes is associated with two- to fourfold increased risk of CVD [3], whereas higher CVD incidence was reported in people with CKD than in diabetes [4], suggesting particularly high CVD risk in diabetic kidney disease (DKD). Despite multifactorial management and agents with pleiotropic cardiorenal benefits, DKD prognosis remains poor.
Type 2 diabetes is characterised by atherogenic dyslipidaemia: elevated triglyceride-rich lipoproteins (TRLs) and reduced HDL, contributing to substantial residual risk despite optimal LDL-cholesterol (LDL-C) levels [5]. In CKD, TRLs are increased owing to impaired lipoprotein lipase activities and diminished clearance caused by altered apolipoprotein C-3 (ApoC-3) metabolism [6]. The major structural protein of TRLs, apolipoprotein B (ApoB), can flux across endothelium and be trapped in the artery wall, initiating atherosclerosis by releasing cholesterol to macrophages [7]. Beyond lipids, the kidney also can regulate circulating metabolites via filtration, reabsorption, secretion, catabolism and anabolism [8]. With advances in technologies, metabolites can be quantified simultaneously in a high-throughput manner and multiple metabolites have been associated with DKD [8,9,10].
Higher TRLs, ApoB, phenylalanine, inflammation markers and lower HDL and apolipoprotein A-1 (ApoA-1) have been associated with decreased eGFR in people with type 2 diabetes [9], and replicated in a larger study [10], indicating that altered lipoprotein and metabolic profiles may reflect impaired kidney function in diabetes. Furthermore, TRLs, ApoB and phenylalanine have been associated with CVD in people with CKD or type 2 diabetes [11,12,13], suggesting that the altered metabolome in DKD may partly explain the increased CVD risk. Although the causal relation between the metabolites and CVD in people with DKD is not yet fully understood, Mendelian randomisation studies have suggested TRLs and ApoB are causally associated with CVD [14, 15]; phenylalanine has been associated with type 2 diabetes [16], impaired kidney function [9], heart failure [17] and CVD [18] in large cohort studies. Better understanding of the potential metabolic links between DKD and CVD is therefore warranted.
Herein, we investigated the metabolomic signature of DKD and examined its association with incident CVD in a well-characterised prospective cohort of individuals with type 2 diabetes. Metabolomic biomarkers were selected among metabolites associated with CVD and were evaluated for their prognostic value towards CVD prediction. External validation of the identified biomarkers for incident CVD was performed in Chinese [19] and Dutch cohorts [20].
Methods
Study population
The Hong Kong Diabetes Biobank
The Hong Kong Diabetes Biobank (HKDB) is a multicentre prospective cohort study, coordinated by the Chinese University of Hong Kong. The study design, recruitment methods, collection of baseline data and biochemical investigations have been published [21,22,23,24]. Briefly, HKDB used similar enrolment and assessment methods to that of the Hong Kong Diabetes Register (HKDR, based at the Prince of Wales Hospital [PWH], the teaching hospital of the Chinese University of Hong Kong), incorporating comprehensive and structured assessment of risk factors and diabetes complications [23]. At enrolment, participants consented for prospective follow-up (until death) and biobanking of blood samples for research. All participants provided written informed consent and the study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee and the Clinical Research Ethics Committee of each participating hospital.
HKDR and the Hoorn Diabetes Care System cohort
HKDR has been briefly described above [19]. The Hoorn Diabetes Care System (DCS) cohort provides diabetes care to people with type 2 diabetes living in the West-Friesland region in the Netherlands [20]. Medical assessment is performed during patients’ annual visit to the DCS research centre and individuals are invited to participate in the DCS research. We replicated the association of the identified metabolomic biomarkers with incident CVD in HKDR and the DCS cohort; the predictive value of biomarkers for CVD was also assessed in HKDR. A detailed description of the two cohorts and baseline characteristics across the three cohorts are available in electronic supplementary material (ESM) Methods and Results and ESM Tables 1–3.
Demographic and laboratory measurements
During recruitment, demographic data, medication and medical history were documented via face-to-face interview based on standardised questionnaires. Sex was determined as per self-reported by study participants. BP was measured in both arms after ≥5 min sitting and the mean value was used for analysis. BMI was calculated as weight in kilograms divided by height in metres squared. Blood samples after at least 8 h overnight fasting were measured for HbA1c, serum creatinine and lipid profile with certificated routine assays at local laboratories. Albumin was quantified in a random spot urine sample using immunoturbidimetry [22, 23]. Serum creatinine was measured by Jaffe’s kinetic method [22, 23] and eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Metabolomic profiling
Metabolomic profiles in fasting sera stored (−80°C, fresh from any freeze–thaw cycles) at PWH were quantified using targeted high-throughput NMR spectroscopy (Nightingale Health, Helsinki, Finland). A total of 170 metabolites were quantified simultaneously, including: absolute concentrations of lipoproteins and lipid contents within 14 lipoprotein subclasses, conventional lipids and low-molecular-weight metabolites (LMWMs), including amino acids, ketone bodies, glycolysis-related metabolites and glycoprotein acetyls (GlycA) as well as ratios of fatty acids to total fatty acids. The NMR platform has been extensively applied in large-scale epidemiological studies [18, 25, 26] and experimental details have been published [27]. Measures (phenylalanine, fatty acids, lipid traits, ApoA-1, ApoB, creatinine and albumin) were correlated between NMR and MS or clinical biochemistry [18, 25] and the median CV (IQR) was 5.0% (2.7–6.7%) [25].
Among the 2000 participants profiled from HKDB, 1991 participants were included in the analysis, after excluding two samples failing quality control and seven non-type 2 diabetes participants (ESM Fig. 1). Among the included samples, we assessed the measures compared with clinical biochemistry, including total cholesterol, HDL-cholesterol (HDL-C), LDL-C, triglycerides, fasting glucose and serum creatinine and the Pearson’s correlation coefficients ranged from 0.80 to 0.98 (ESM Fig. 2). ESM Table 4 summarises the measurement quality and distribution of each metabolite among the samples. No measures had ≥20% missing values and were all included in the analysis; zero values, indicating below the detection limit, were imputed with half of the minimum in each measurement.
Samples from HKDR (N=93) and the DCS cohort (N=1204) were also profiled using the same platform (ESM Methods and Results).
Outcome
Discharge codes based on ICD-9 (http://www.icd9data.com/2007/Volume1/default.htm) retrieved from electronic medical records were used to define CVD. CVD was defined as the first occurrence of cardiovascular death (ICD-10 [https://icd.who.int/browse10/2019/en]: I00-I99, retrieved from the Hong Kong Death Registry), coronary heart disease (myocardial infarction, ischaemic heart disease, or angina pectoris), stroke (ischaemic stroke except transient ischaemic attack, haemorrhagic stroke, or acute but ill-defined cerebrovascular disease), peripheral vascular disease (amputation, gangrene, or peripheral revascularisation), or hospitalisation for heart failure [21].
Statistical analysis
Continuous variables were presented as mean ± SD or median (IQR) and differences were compared by t test or Wilcoxon rank sum test as appropriate. Categorical variables were presented as number (%) and compared by χ2 test. To account for skewed distribution and facilitate interpretation, all metabolites were loge-transformed before being standardised to SD. The proportional hazards assumption was tested by scaled Schoenfeld residuals for all variables. False discovery rate (FDR) by the Benjamini–Hochberg procedure <0.05, which is more appropriate for ‘omics’ data, was considered significant to account for multiple testing of intercorrelated metabolites [28]. All analyses were performed in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Packages including survival, boot, prioritylasso, survIDINRI and nricens were used for the analysis.
The cross-sectional associations of metabolites with CKD (baseline eGFR<60 ml/min per 1.73 m2) or severely increased albuminuria (urinary albumin/creatinine ratio [UACR] >30 mg/mmol) were separately examined by linear regression, with the metabolite as dependent variable. Two models were considered: unadjusted and adjusted for age, male sex, ever smoking, diabetes duration, systolic BP (SBP), BMI, HbA1c, oral glucose-lowering drugs, insulin, antihypertensive drugs, renin–angiotensin-system (RAS) blockers, lipid-lowering drugs, statins, diabetic retinopathy and CVD history. Additionally, severely increased albuminuria (or CKD) was included as a covariate for the analysis of CKD (or severely increased albuminuria).
Among participants without prevalent CVD (N=1447), the crude association of CKD or severely increased albuminuria with incident CVD was assessed using Cox proportional hazards model. Given the established association between DKD and incident CVD, metabolites cross-sectionally associated with CKD or severely increased albuminuria were further examined for the prospective associations with incident CVD (N=1447) using Cox proportional hazards models, adjusting for the same covariates mentioned above, excluding history of CVD. To identify metabolomic biomarkers independent of conventional risk factors including DKD, metabolites remaining nominally significant (p<0.05) after further adjusting for CKD and severely increased albuminuria were assessed for the prognostic value. To account for multicollinearity and data dimensionality, priority least absolute shrinkage and selection operator (priority-Lasso) Cox regression was used to retain metabolites with non-zero coefficients [29]. Two blocks were defined: the first block was unpenalised and included 14 covariates, CKD and severely increased albuminuria; the second block comprised all metabolites associated with incident CVD. The optimal penalisation parameter λ in the second block was determined by the one with minimal cross-validated error as determined by tenfold cross-validation [29]. To account for overfitting, the process was repeated by 1000-times bootstrapping.
To assess the predictive value of the identified metabolomic biomarkers, a risk score comprising the selected metabolites, age and sex was compared with the risk score containing conventional risk factors (original model) and an established prediction model (RECODe model) [30]. The incremental predictive value of the metabolomic biomarkers was also assessed over the original and RECODe models. The predictive value was assessed using C statistic, integrated discrimination improvement (IDI), categorical and continuous net reclassification improvement (NRI). The calculation of IDI and NRI was based on 5-year risk and the risk categories for categorical NRI were <5%, 5–10% and >10%. The 95% CI was estimated by 1000-times bootstrapping. As missing values in each covariate were small (≤2.0%), multiple imputation was not performed. Key R codes are provided in the ESM Methods and Results.
Sensitivity analyses
We examined cross-sectional associations of metabolites with eGFR and UACR and included eGFR and UACR as covariates in the prospective analysis. We further adjusted for sodium–glucose cotransporter-2 inhibitors (SGLT2i) use during follow-up (N=384) in the prospective analysis, as none of the participants were on SGLT2i at baseline. To assess the robustness of variable selection, backward elimination based on Akaike’s information criterion with 1000-times bootstrapping was also performed.
Results
Baseline characteristics (N=1991)
Table 1 summarises the baseline characteristics of participants. Briefly, mean age was 61.1 years, 59.7% were male, mean diabetes duration was 11.4 years and 27.3% had prevalent CVD. The mean eGFR was 75.8 ml/min per 1.73 m2 and 545 participants had prevalent CKD; the median UACR was 2.7 mg/mmol and 399 participants had prevalent severely increased albuminuria. Characteristics of participants with or without CKD or severely increased albuminuria are summarised in Table 1.
Metabolites cross-sectionally associated with DKD (N=1991)
At FDR<0.05, 151 metabolites were associated with CKD (Fig. 1). Cholesterol, phospholipids and total lipids in ApoB-containing lipoproteins were positively, while lipids in HDL were inversely, associated with CKD; concentrations of lipoprotein particles exhibited similar patterns. Triglycerides across all lipoproteins were positively associated with CKD, except triglycerides in large HDL. ApoB and ApoB/ApoA-1 were positively, while ApoA-1 was negatively, associated with CKD. Other lipids, including phosphoglycerides, total cholines, phosphatidylcholines and sphingomyelins, were positively associated with CKD. Relative concentrations of n-6 fatty acids, polyunsaturated fatty acids (PUFAs), docosahexaenoic acid (DHA) and PUFAs/monounsaturated fatty acids (MUFAs) were inversely, while relative concentration of MUFAs was positively, associated with CKD. For LMWMs, glycine, phenylalanine, citrate and GlycA were positively, while leucine, valine, tyrosine, glucose, lactate and albumin were negatively, associated with CKD.
Metabolites associated with CKD (a) or severely increased albuminuria (b). Estimated by linear regression adjusted for age, male sex, ever smoking, diabetes duration, SBP, BMI, HbA1c, oral glucose-lowering drugs, insulin, antihypertensive drugs, lipid-lowering drugs, RAS blockers, statins, diabetic retinopathy and severely increased albuminuria (for the association with severely increased albuminuria, CKD was included instead). Metabolites were loge-transformed and scaled to SD. The top 20 most significant metabolites were named
At FDR<0.05, 128 metabolites were associated with severely increased albuminuria (Fig. 1). Cholesterol, phospholipids and total lipids in VLDL and LDL were positively, while lipids in larger HDL were negatively, associated with severely increased albuminuria; concentrations of lipoprotein particles exhibited similar patterns. Triglycerides in non-HDL and medium and small HDL were associated with severely increased albuminuria. Other traits including ApoB and ApoB/ApoA-1, MUFAs, isoleucine, phenylalanine, glucose, citrate, 3-hydroxybutyrate, creatinine and GlycA were positively, while PUFAs, DHA, PUFAs/MUFAs, tyrosine and albumin were negatively, associated with severely increased albuminuria.
When eGFR or UACR was assessed as the dependent variable, 148 overlapping metabolites were associated with CKD and 125 overlapping metabolites were associated with severely increased albuminuria at FDR<0.05.
Associations between DKD-related metabolites and incident CVD (N=1447)
Of metabolites associated with CKD (N=151) or severely increased albuminuria (N=128), 123 metabolites were associated with both CKD and severely increased albuminuria, 28 metabolites only associated with CKD, and five metabolites only associated with severely increased albuminuria. Consequently, 156 metabolites associated with DKD were included in the prospective analysis.
Among 1447 participants without prevalent CVD, 125 (8.6%) participants developed CVD over median (IQR) 5.2 (5.0–5.4) years of follow-up, corresponding to an incidence rate (95% CI) of 17.5 (14.6, 20.9) per 1000-person-years. Both CKD and severely increased albuminuria were associated with incident CVD (HR 3.77 [95% CI 2.65, 5.37] and 3.96 [2.77, 5.67] for CKD and severely increased albuminuria, respectively). At FDR<0.05, 116 metabolites were associated with incident CVD in the unadjusted model and 75 metabolites remained significant after accounting for confounders (Fig. 2). Briefly, triglycerides in all lipoproteins were positively associated with incident CVD. Other lipid components in TRLs and LDL were positively, while lipid components in medium and small HDL were inversely, associated with incident CVD with lipoprotein concentrations exhibiting similar patterns. ApoB, ApoB/ApoA-1, glycine, phenylalanine and GlycA were associated with higher risk of CVD, while DHA, leucine, valine, tyrosine and albumin were inversely associated with CVD.
Associations between DKD-related metabolites and incident CVD. Estimated by Cox regression adjusted for age, male sex, ever smoking, diabetes duration, SBP, BMI, HbA1c, oral glucose-lowering drugs, insulin, antihypertensive drugs, lipid-lowering drugs, RAS blockers, statins and diabetic retinopathy. Metabolites were loge-transformed and scaled to SD. *p<0.05; **p<0.01
Prognostic value of selected metabolites for incident CVD (N=1447)
Among the 75 metabolites associated with incident CVD, 22 remained nominally significant after further adjusting for CKD and severely increased albuminuria (ESM Fig. 3, ESM Table 5), including concentrations of very small VLDL and small HDL and their lipid components, triglycerides in intermediate-density lipoprotein (IDL), LDL and larger HDL, leucine and albumin. Albumin, triglycerides in large HDL and phospholipids in small LDL were most consistently selected by priority-Lasso (ESM Table 6). The key selection by priority-Lasso was well-represented in backward elimination.
A metabolite score (triglycerides in large HDL plus phospholipids in small LDL minus albumin) was strongly associated with incident CVD (HR 1.43 per SD [95% CI 1.28, 1.59] and 3.31 [1.93, 5.70] for tertile 3 vs tertile 1; ESM Table 7). The three metabolites reached a C statistic (95% CI) of 0.725 (0.672, 0.778) and C statistic of the metabolomic model comprising age, sex and the three metabolites (0.765 [0.718, 0.812]) was comparable with that of traditional risk factors (0.762 [0.717, 0.807], P=0.893) and RECODe model (0.765 [0.719, 0.812], P=0.994; ESM Fig. 4) [30]. The metabolites further improved CVD risk prediction (improvement in C statistic=0.035 and 0.030, P=0.014 and 0.007 over the fully adjusted model and RECODe model, respectively) as well as IDI, continuous NRI and correct reclassification of non-cases for incident CVD (Table 2).
The metabolite score was also associated with CVD in HKDR (HR 1.76 per SD [95% CI 1.34, 2.31] and 5.72 [2.24, 14.61] for tertile 3 vs tertile 1; ESM Table 8) and remained significant after adjusting for some confounders (HR 1.74 per SD [95% CI 1.33, 2.28] and 11.12 [3.68, 33.61] for tertile 3 vs tertile 1); further adjusting for CKD and severely increased albuminuria did not change the association. The selected metabolites reached a C statistic of 0.734 (0.621, 0.846) and further improved CVD risk prediction over RECODe model (improvement in C statistic=0.042, P=0.043; ESM Fig. 5).
In the DCS cohort, all three metabolites have been associated with impaired kidney function [9]. Over a mean 8.3±3.2 years of follow-up, 141 (11.7%) participants developed incident CVD. The metabolite score was associated with incident CVD (HR 1.16 per SD [95% CI 1.06, 1.27], and 1.99 [1.30, 3.03] for tertile 3 vs tertile 1; ESM Table 9). Adjustment for the same covariates as in the HKDB analysis attenuated the association.
Sensitivity analyses
Of 75 metabolites associated with incident CVD, 13 remained significant after further adjusting for eGFR and UACR, including two selected metabolites (albumin and triglycerides in large HDL) (ESM Table 10). All metabolites were associated with CVD after accounting for SGLT2i use during follow-up (ESM Table 11).
Discussion
Applying NMR metabolomics in a well-characterised type 2 diabetes cohort, we comprehensively examined the cross-sectional associations of lipoproteins, lipids and LMWMs with DKD and prospective associations of DKD-related metabolites with incident CVD, identified and assessed metabolomic biomarkers for incident CVD prediction. We found that: (1) TRLs associated with both DKD and incident CVD; (2) HDL inversely associated with DKD and the inverse association with incident CVD appeared mainly driven by smaller (medium and small) HDL; (3) triglycerides across all lipoproteins associated with CVD; and (4) replicated in both Chinese and Europeans, metabolomic biomarkers performed comparably to conventional risk factors and improved CVD risk stratification beyond established prediction models. The results demonstrate profound metabolomic alterations in DKD and close relation with development of CVD, highlighting potential molecular links between DKD and CVD and potential application of metabolomics for diabetes complication prediction.
Some metabolic alterations associated with decreased eGFR are common across different populations and we further identified metabolites associated with severely increased albuminuria in Chinese (ESM Tables 12–13). Consistently, TRLs were associated with decreased eGFR [9, 10] and also with severely increased albuminuria in our study. TRLs have been associated with CVD [31]; larger differences in TRLs in decreased eGFR were found in people with vs without diabetes [10], suggesting a potential role of TRLs for residual CVD risk in people with DKD. In our prospective analysis, TRLs were associated with incident CVD, with VLDL exhibiting the strongest association, although VLDL, IDL and ApoB were all associated with CVD. Hepatic VLDL production and secretion is increased by insulin resistance [32] and altered metabolism of ApoC-3 in CKD further elevates TRLs by overproduction and impaired clearance [6]. All ApoB-containing lipoproteins, including TRLs, can enter the arterial intima leading to cholesterol deposition [7]. In contrast to LDL for which oxidative modification is usually required before phagocytosis, larger TRLs can be trapped more easily and can be directly phagocytised by macrophages to form foam cells [33]. Moreover, hydrolysis of triglycerides in TRLs by lipoprotein lipase can liberate NEFA, inducing inflammation, promoting atherosclerosis [34].
CKD modifies HDL structure and composition, which may partly explain the increased CVD risk in CKD [35]. Consistent with previous findings [9], we found that HDL was negatively associated with CKD and severely increased albuminuria; the association with CKD was stronger. HDL was inversely associated with CVD in our prospective analysis and the association appeared limited to medium and small HDL. However, in previous population-based studies the inverse association between HDL and CVD was limited to large and medium HDL [18, 25]. HDL’s potential modification by diabetes [36] and CKD [35] may partly explain the contrasting results. Furthermore, a recent MR analysis found that medium and small HDL were CVD-protective [37]. Our observed association of small HDL appeared independent of DKD, which is consistent with findings that small HDL has greater atheroprotective capacities via reverse cholesterol transport, anti-inflammatory, antioxidant and endothelial protection [8, 24]. Further studies are warranted to investigate whether detailed HDL composition (proteins, lipids or enzymes) or HDL function may be potential modulators [35]. We replicated previous findings that LDL was associated with DKD [9, 10] and that small LDL was associated with higher CVD risk.
Triglycerides across all lipoproteins were associated with DKD and incident CVD, including TRLs, LDL and HDL. Despite the fact that 67% of participants were on statins, and cholesterol in LDL was not associated with incident CVD, triglycerides in LDL were associated with CVD in our analysis. In people with prediabetes (impaired glucose tolerance and/or impaired fasting glucose) or diabetes and stable coronary artery disease (73.9% on statins), LDL triglycerides were associated with CVD and improved CVD risk prediction, indicating the prognostic value of LDL triglycerides for residual risk [38].
Lower albumin has been associated with DKD [10] and frailty in older people with type 2 diabetes [39] and albumin levels are inversely associated with CVD or mortality in people with CKD [40], suggesting that as a marker linked with malnutrition, liver and kidney dysfunction and inflammation, albumin may partly capture the integrated altered metabolic signature in diabetes and thus associates with adverse outcomes. As a validated marker for systemic inflammation, GlycA was associated with DKD [9, 10] and incident CVD [18, 25], although further adjustment for DKD attenuated the association with CVD. Taken together, our findings suggest that low-grade inflammation in diabetes may be one of the pathogenetic pathways for diabetes complications.
Other lipids, including sphingomyelins, were also associated with DKD [9, 10], however, none were associated with incident CVD, consistent with previous findings that sphingomyelins were associated with DKD but not CVD [41]. In line with previous studies, MUFAs were positively associated with DKD [10], however, PUFAs were negatively linked with DKD in our analysis and the inverse association was mainly driven by DHA. DHA was inversely associated with macrovascular events in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study [42], which supports our result that DHA as a marker negatively linked with DKD was associated with lower risk of CVD. In CKD, dysfunctional activity of phenylalanine hydroxylase impairs the conversion of phenylalanine to tyrosine [43]. Accordingly, we found that higher phenylalanine and lower tyrosine were associated with DKD [9, 10] and were both associated with risk of CVD. Similar to findings from ADVANCE [11], further adjustment for kidney function attenuated associations with CVD, suggesting that the link between dysregulated phenylalanine or tyrosine and CVD may be mediated by kidney dysfunction. Leucine and isoleucine have been associated with decreased eGFR [9], and branched-chain amino acids (BCAAs) have been negatively associated with CKD in a larger study [10]. We found that leucine and valine were negatively associated with CKD and isoleucine was positively associated with severely increased albuminuria. Leucine and valine were also inversely associated with CVD, in line with the inverse association of leucine and valine with all-cause mortality in ADVANCE [11]. The different associations between BCAAs and DKD across studies might be attributed to participant characteristics, dietary intake, medications or analytical strategies.
Integrating information from gene expression and environmental factors and interacting with the microbiome, metabolites may carry molecular information that is not captured by traditional risk factors [8]. Among metabolites associated with CVD independent of conventional risk factors, three metabolites, albumin, triglycerides in large HDL and phospholipids in small LDL, were identified to be most informative for CVD prediction by machine learning method. The metabolite score comprising these three metabolites was strongly associated with CVD, which was validated independently in both Chinese and European cohorts. The selected metabolites performed comparably to conventional risk factors for CVD prediction and improved risk stratification beyond well-established prediction models, highlighting the prognostic value of metabolomic biomarkers for diabetes complications.
Extending the cross-sectional associations between metabolites and DKD, we found some DKD-related metabolites were associated with incident CVD. We further replicated the association between the identified metabolites and incident CVD in HKDR and the Dutch DCS cohort. Other strengths include the extensively phenotyped data and complete follow-up, well-established metabolomics platform with stringent quality control and consistent results across sensitivity analyses. Nevertheless, there are limitations. Only Chinese individuals were included in the discovery analysis, which might limit generalisability of our findings, however, most metabolites associated with DKD in previous studies were replicated in our study and the selected metabolomic biomarkers were validated in two independent cohorts. Around 70% of participants were on lipid-lowering drugs and we could not account for their potential influence on lipoprotein metabolism, although medication use was accounted for and our findings were consistent with a study in people not on lipid therapies [10]. UACR was based on single measurement and to account for intra-individual variability, we used severely increased albuminuria to define albuminuria. Among metabolites ranked by 1000-times bootstrapping priority-Lasso, an arbitrary cut-off (>70%) was applied to select prognostic metabolomic biomarkers. Although fasting samples were profiled, dietary intake and physical activity that may modulate the metabolome [8] were not captured in our cohort. Given the observational design, residual confounding cannot be ruled out and causal inference is not feasible. Although the study population included slightly more men than women, analyses have been adjusted for the sex of the study participants, and the findings should be applicable to both men and women with diabetes.
In conclusion, DKD is linked with alterations in multiple metabolites, including TRLs, HDL, fatty acids, amino acids, albumin and inflammation. Some DKD-related metabolites (TRLs, smaller HDL, leucine and albumin) are also associated with incident CVD. Metabolomic biomarkers provided comparable predictive utility to traditional risk factors and improved CVD risk stratification over established prediction models. Further investigations on pathophysiology and disease prediction of metabolites are warranted.
Abbreviations
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- ApoA-1:
-
Apolipoprotein A-1
- ApoB:
-
Apolipoprotein B
- ApoC-3:
-
Apolipoprotein C-3
- CKD:
-
Chronic kidney disease
- DCS:
-
Hoorn Diabetes Care System
- DHA:
-
Docosahexaenoic acid
- DKD:
-
Diabetic kidney disease
- FDR:
-
False discovery rate
- GlycA:
-
Glycoprotein acetyls
- HDL-C:
-
HDL-cholesterol
- HKDB:
-
Hong Kong Diabetes Biobank
- HKDR:
-
Hong Kong Diabetes Register
- IDI:
-
Integrated discrimination improvement
- IDL:
-
Intermediate-density lipoprotein
- LDL-C:
-
LDL-cholesterol
- LMWMs:
-
Low-molecular-weight metabolites
- MR:
-
Mendelian randomisation
- MUFAs:
-
Monounsaturated fatty acids
- NRI:
-
Net reclassification improvement
- Priority-Lasso:
-
Priority least absolute shrinkage and selection operator
- PUFAs:
-
Polyunsaturated fatty acids
- PWH:
-
Prince of Wales Hospital
- RAS:
-
Renin–angiotensin system
- SBP:
-
Systolic BP
- SGLT2i:
-
Sodium–glucose cotransporter-2 inhibitors
- TRLs:
-
Triglyceride-rich lipoproteins
- UACR:
-
Urinary albumin/creatinine ratio
References
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12(12):2032–2045. https://doi.org/10.2215/cjn.11491116
Matsushita K, Coresh J, Sang Y et al (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3(7):514–525. https://doi.org/10.1016/s2213-8587(15)00040-6
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376(15):1407–1418. https://doi.org/10.1056/NEJMoa1608664
Tonelli M, Muntner P, Lloyd A et al (2012) Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 380(9844):807–814. https://doi.org/10.1016/s0140-6736(12)60572-8
Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF (2016) Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 65(7):1767–1778. https://doi.org/10.2337/db16-0046
Chan DT, Dogra GK, Irish AB et al (2009) Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J Lipid Res 50(12):2524–2531. https://doi.org/10.1194/jlr.P900003-JLR200
Ference BA, Kastelein JJP, Catapano AL (2020) Lipids and Lipoproteins in 2020. JAMA 324(6):595–596. https://doi.org/10.1001/jama.2020.5685
Jin Q, Ma RCW (2021) Metabolomics in diabetes and diabetic complications: insights from epidemiological studies. Cells 10(11):2832. https://doi.org/10.3390/cells10112832
Tofte N, Vogelzangs N, Mook-Kanamori D et al (2020) Plasma metabolomics identifies markers of impaired renal function: a meta-analysis of 3089 persons with type 2 diabetes. J Clin Endocrinol Metab 105(7):dgaa173. https://doi.org/10.1210/clinem/dgaa173
Aguilar-Ramirez D, Alegre-Díaz J, Herrington WG et al (2021) Association of kidney function with NMR-quantified lipids, lipoproteins, and metabolic measures in Mexican adults. J Clin Endocrinol Metab 106(10):2828–2839. https://doi.org/10.1210/clinem/dgab497
Welsh P, Rankin N, Li Q et al (2018) Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE trial. Diabetologia 61(7):1581–1591. https://doi.org/10.1007/s00125-018-4619-x
Bajaj A, Xie D, Cedillo-Couvert E et al (2019) Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am J Kidney Dis 73(6):827–836. https://doi.org/10.1053/j.ajkd.2018.11.010
Lamprea-Montealegre JA, Staplin N, Herrington WG et al (2020) Apolipoprotein B, triglyceride-rich lipoproteins, and risk of cardiovascular events in persons with CKD. Clin J Am Soc Nephrol 15(1):47–60. https://doi.org/10.2215/cjn.07320619
Ference BA, Kastelein JJP, Ray KK et al (2019) Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 321(4):364–373. https://doi.org/10.1001/jama.2018.20045
Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A (2013) Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 34(24):1826–1833. https://doi.org/10.1093/eurheartj/ehs431
Ahola-Olli AV, Mustelin L, Kalimeri M et al (2019) Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 62(12):2298–2309. https://doi.org/10.1007/s00125-019-05001-w
Delles C, Rankin NJ, Boachie C et al (2018) Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: results from PROSPER and FINRISK 1997. Eur J Heart Fail 20(4):663–673. https://doi.org/10.1002/ejhf.1076
Würtz P, Havulinna AS, Soininen P et al (2015) Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation 131(9):774–785. https://doi.org/10.1161/circulationaha.114.013116
Yang XL, So WY, Kong AP et al (2006) End-stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia 49(10):2299–2308. https://doi.org/10.1007/s00125-006-0376-3
van der Heijden AA, Rauh SP, Dekker JM et al (2017) The hoorn Diabetes Care System (DCS) cohort. A prospective cohort of persons with type 2 diabetes treated in primary care in the Netherlands. BMJ Open 7(5):e015599. https://doi.org/10.1136/bmjopen-2016-015599
Jin Q, Lau ESH, Luk AOY et al (2021) Skin autofluorescence is associated with higher risk of cardiovascular events in Chinese adults with type 2 diabetes: a prospective cohort study from the Hong Kong Diabetes Biobank. J Diabetes Complications 35(10):108015. https://doi.org/10.1016/j.jdiacomp.2021.108015
Jin Q, Lau ES, Luk AO et al (2022) Skin autofluorescence is associated with progression of kidney disease in type 2 diabetes: a prospective cohort study from the Hong Kong diabetes biobank. Nutr Metab Cardiovasc Dis 32(2):436–446. https://doi.org/10.1016/j.numecd.2021.10.007
Jin Q, Luk AO, Lau ESH et al (2022) Nonalbuminuric diabetic kidney disease and risk of all-cause mortality and cardiovascular and kidney outcomes in type 2 diabetes: findings from the Hong Kong diabetes biobank. Am J Kidney Dis 80(2):196-206.e191. https://doi.org/10.1053/j.ajkd.2021.11.011
Jin Q, Lau ESH, Luk AO et al (2022) High-density lipoprotein subclasses and cardiovascular disease and mortality in type 2 diabetes: analysis from the Hong Kong Diabetes Biobank. Cardiovasc Diabetol 21(1):293. https://doi.org/10.1186/s12933-022-01726-y
Holmes MV, Millwood IY, Kartsonaki C et al (2018) Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol 71(6):620–632. https://doi.org/10.1016/j.jacc.2017.12.006
Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M (2017) Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -omic technologies. Am J Epidemiol 186(9):1084–1096. https://doi.org/10.1093/aje/kwx016
Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M (2015) Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8(1):192–206. https://doi.org/10.1161/circgenetics.114.000216
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125(1–2):279–284. https://doi.org/10.1016/s0166-4328(01)00297-2
Klau S, Jurinovic V, Hornung R, Herold T, Boulesteix AL (2018) Priority-Lasso: a simple hierarchical approach to the prediction of clinical outcome using multi-omics data. BMC Bioinformatics 19(1):322. https://doi.org/10.1186/s12859-018-2344-6
Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS (2017) Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol 5(10):788–798. https://doi.org/10.1016/s2213-8587(17)30221-8
Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD (2020) Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol 75(17):2122–2135. https://doi.org/10.1016/j.jacc.2020.02.059
Santamarina-Fojo S, Haudenschild C, Amar M (1998) The role of hepatic lipase in lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol 9(3):211–219. https://doi.org/10.1097/00041433-199806000-00005
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10):709–721. https://doi.org/10.1038/nri3520
Nordestgaard BG, Varbo A (2014) Triglycerides and cardiovascular disease. Lancet 384(9943):626–635. https://doi.org/10.1016/s0140-6736(14)61177-6
Noels H, Lehrke M, Vanholder R, Jankowski J (2021) Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol 17(8):528–542. https://doi.org/10.1038/s41581-021-00423-5
Xepapadaki E, Nikdima I, Sagiadinou EC, Zvintzou E, Kypreos KE (2021) HDL and type 2 diabetes: the chicken or the egg? Diabetologia 64(9):1917–1926. https://doi.org/10.1007/s00125-021-05509-0
Zhao Q, Wang J, Miao Z et al (2021) A Mendelian randomization study of the role of lipoprotein subfractions in coronary artery disease. Elife 10:e58361. https://doi.org/10.7554/eLife.58361
Jin JL, Zhang HW, Cao YX et al (2020) Long-term prognostic utility of low-density lipoprotein (LDL) triglyceride in real-world patients with coronary artery disease and diabetes or prediabetes. Cardiovasc Diabetol 19(1):152. https://doi.org/10.1186/s12933-020-01125-1
Fung E, Lui L-T, Huang L et al (2021) Characterising frailty, metrics of continuous glucose monitoring, and mortality hazards in older adults with type 2 diabetes on insulin therapy (HARE): a prospective, observational cohort study. Lancet Healthy Longevity 2(11):e724–e735. https://doi.org/10.1016/S2666-7568(21)00251-8
Amdur RL, Feldman HI, Dominic EA et al (2019) Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis 73(3):344–353. https://doi.org/10.1053/j.ajkd.2018.09.012
Pongrac Barlovic D, Harjutsalo V, Sandholm N, Forsblom C, Groop PH (2020) Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 63(9):1847–1856. https://doi.org/10.1007/s00125-020-05201-9
Harris K, Oshima M, Sattar N et al (2020) Plasma fatty acids and the risk of vascular disease and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE study. Diabetologia 63(8):1637–1647. https://doi.org/10.1007/s00125-020-05162-z
Kopple JD (2007) Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr 137(6 Suppl 1): 1586S-1590S; discussion 1597S-1598S. https://doi.org/10.1093/jn/137.6.1586S
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Acknowledgements
The authors thank all the participants and investigators of HKDB for their contributions, and the medical and nursing staff of the participating hospitals for their professional input and support.
Data availability
The datasets used during the current study are available from the corresponding author (RCWM) upon reasonable request.
Funding
This work was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region (CU R4012-18), Research Grants Council Theme-based Research Scheme (T12-402/13N), the Focused Innovation Scheme, the University Grants Matching Scheme, and a Croucher Foundation Senior Medical Research Fellowship. The metabolomic studies in the DCS cohort were performed within the framework of the Biobanking and Biomolecular Resources Research Infrastructure (BBMRI) Metabolomics Consortium funded by BBMRI-NL, a research infrastructure financed by the Dutch Research Council, NWO (grant numbers 184021.007 and 184033.111). It was also funded by ZonMW Priority Medicines Elderly (grant number 113102006) and the Parelsnoer Initiative (PSI). PSI is part of and is funded by the Dutch Federation of University Medical Centres and, from 2007 to 2011, received initial funding from the Dutch government. The funding sources did not have any role in the design, interpretation of the study or the decision to publish the results.
Authors’ relationships and activities
RCWM is a member of the editorial board of Diabetologia. JCNC received consultancy fees or speaker honoraria from Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Celltrion, Eli Lilly, Hua Medicine, Lee Power Pharmaceuticals, Merck, MSD, Pfizer, Roche, Sanofi, Servier, Viatris Pharmaceutical and Zuelling Pharma. JCNC is the Chief Executive Officer (pro bono) of the Asian Diabetes Foundation. APSK received research grants from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Kyowa Kirin, and Merck Serono, and honoraria for consultancy or giving lectures from Nestlé, Novo Nordisk, Pfizer and Sanofi. EYKC received speaker fees from Sanofi and Novartis, and institutional research funding from Sanofi, Medtronic Diabetes and Powder Pharmaceuticals Inc. RCWM received research funding from AstraZeneca, Bayer, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Roche Diagnostics and Tricida Inc. for carrying out clinical trials or studies, and from AstraZeneca, Bayer, Boehringer Ingelheim and Merck for speaker honoraria or consultancy in advisory boards. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. The remaining authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
QJ, AOL, AJJ, JCNC and RCWM conceptualised and designed the study. QJ, ESHL, AOL, CHTT, RO, CKPL, HW, EYKC, APSK, HML, BF, ACWN, GJ, KFL, SCS, GH, CCT, KPL, JYL, MT, EYNC, GK, ITL, JKL, VTFY, EL, SL, SF, YLC, CCC, WY, SKWT, BT, YH, H-yL, CCS, EF, WYS, JCNC and RCWM contributed to data acquisition. QJ conducted statistical analysis. QJ, AJJ, EF and RCWM were involved in data interpretation. MTB, LMH and MM conducted data acquisition and replication analyses in DCS. QJ and RCWM drafted the manuscript. All authors contributed to the editing, review and critical revision of the manuscript. WYS, JCNC and RCWM contributed to funding acquisition for the study. All authors read and approved the final version to be published. RCWM is the guarantor of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, Q., Lau, E.S.H., Luk, A.O. et al. Circulating metabolomic markers linking diabetic kidney disease and incident cardiovascular disease in type 2 diabetes: analyses from the Hong Kong Diabetes Biobank. Diabetologia 67, 837–849 (2024). https://doi.org/10.1007/s00125-024-06108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06108-5