Abstract
Left ventricular diastolic dysfunction (LVDD) without symptoms, and heart failure (HF) with preserved ejection fraction (HFpEF) represent the most common phenotypes of HF in individuals with type 2 diabetes mellitus, and are more common than HF with reduced ejection fraction (HFrEF), HF with mildly reduced ejection fraction (HFmrEF) and left ventricular systolic dysfunction (LVSD) in these individuals. However, diagnostic criteria for HF have changed over the years, resulting in heterogeneity in the prevalence/incidence rates reported in different studies. We aimed to give an overview of the diagnosis and epidemiology of HF in type 2 diabetes, using both a narrative and systematic review approach; we focus narratively on diagnosing (using the 2021 European Society of Cardiology [ESC] guidelines) and screening for HF in type 2 diabetes. We performed an updated (2016–October 2022) systematic review and meta-analysis of studies reporting the prevalence and incidence of HF subtypes in adults ≥18 years with type 2 diabetes, using echocardiographic data. Embase and MEDLINE databases were searched and data were assessed using random-effects meta-analyses, with findings presented as forest plots. From the 5015 studies found, 209 were screened using the full-text article. In total, 57 studies were included, together with 29 studies that were identified in a prior meta-analysis; these studies reported on the prevalence of LVSD (n=25 studies, 24,460 individuals), LVDD (n=65 studies, 25,729 individuals), HFrEF (n=4 studies, 4090 individuals), HFmrEF (n=2 studies, 2442 individuals) and/or HFpEF (n=8 studies, 5292 individuals), and on HF incidence (n=7 studies, 17,935 individuals). Using Hoy et al’s risk-of-bias tool, we found that the studies included generally had a high risk of bias. They showed a prevalence of 43% (95% CI 37%, 50%) for LVDD, 17% (95% CI 7%, 35%) for HFpEF, 6% (95% CI 3%, 10%) for LVSD, 7% (95% CI 3%, 15%) for HFrEF, and 12% (95% CI 7%, 22%) for HFmrEF. For LVDD, grade I was found to be most prevalent. Additionally, we reported a higher incidence rate of HFpEF (7% [95% CI 4%, 11%]) than HFrEF 4% [95% CI 3%, 7%]). The evidence is limited by the heterogeneity of the diagnostic criteria over the years. The systematic section of this review provides new insights on the prevalence/incidence of HF in type 2 diabetes, unveiling a large pre-clinical target group with LVDD/HFpEF in which disease progression could be halted by early recognition and treatment.
Registration PROSPERO ID CRD42022368035.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) and type 2 diabetes are two highly intertwined diseases that exist in a vicious circle; people with type 2 diabetes are approximately two times more likely to develop HF than those without [1,2,3,4]. Furthermore, 30–40% of people with HF suffer from type 2 diabetes or show signs of impaired glucose tolerance, with the rate increasing to up to 50% in patients hospitalised for HF [5, 6]. Given an estimated prevalence of 537 million cases for diabetes [7] and 64.3 million cases for HF [8] worldwide, the risks of hospitalisation, CVD-attributable mortality and all-cause mortality in people with both of these diseases represent an increasing burden on healthcare, including healthcare-related costs [9, 10].
Rather than being an encapsulated disease, HF should be viewed as a heterogeneous syndrome, consisting of multiple clinical entities and different stages. HF can be categorised as HF with reduced ejection fraction (HFrEF; left ventricle ejection fraction [LVEF], ≤40%), HF with mildly reduced ejection fraction (HFmrEF; LVEF, 41–49%) and HF with preserved ejection fraction (HFpEF; LVEF, ≥50%). Furthermore, echocardiographically distinct phenotypes of ventricular dysfunction in systole (left ventricular systolic dysfunction [LVSD]) and in diastole (left ventricular diastolic dysfunction [LVDD]) can be identified; these reflect ventricular dysfunction without clinical symptomatology of HF [11]. Out of these categories, LVDD and HFpEF currently represent the most common phenotypes of HF in type 2 diabetes, although there is no consensus on the exact prevalence of the HF subtypes [12, 13].
Since pathophysiology, treatment and prognosis differ depending on the subtype of HF [14], a timely and accurate diagnosis of HF (subtype) and identification of people at risk for HF is important. This is even more true for people with type 2 diabetes, since sodium−glucose cotransporter 2 (SGLT2) inhibitors provide both glucose lowering and cardiovascular protection, showing promising effects on cardiovascular outcomes [15, 16]. Knowledge about the exact prevalence and incidence of HF and its pre-clinical stages is a key factor in the process of accurately diagnosing HF.
In this review, we provide an overview of the epidemiology and diagnostic process of HF in individuals with type 2 diabetes, covering diagnosis, screening and prognosis in a narrative way. Furthermore, we report the findings from an updated systematic review and meta-analysis of the study by Bouthoorn et al [12, 13] on the prevalence of LVSD, LVDD, HFpEF, HFrEF and HFmrEF, including studies published from 2016 onwards. We also present results from a novel systematic review and meta-analysis on the incidence of HF subtypes in type 2 diabetes. In doing so, we aim to provide the most updated numbers on prevalence and incidence of HF in type 2 diabetes.
Diagnosis of HF in type 2 diabetes
Over the years, many algorithms and guidelines have been proposed to ease the process of clinically diagnosing and categorising HF. Nevertheless, much controversy remains, especially about the diagnosis of LVDD/HFpEF. In 2021, the European Society of Cardiology (ESC) published guidelines for the diagnosis and treatment of HF [17]. In this section, we aim to give an overview of the diagnostic process based on these guidelines.
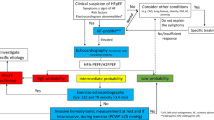
For diagnosing HF, the presence of cardinal symptoms (e.g., breathlessness, ankle swelling and/or fatigue) are obligatory and might, in more advanced clinical stages, be accompanied with signs of HF (e.g., elevated jugular venous pressure, pulmonary crackles, peripheral oedema) (Fig. 1). Questionnaires, such as the Kansas City Cardiomyopathy questionnaire [18] and the Minnesota Living with Heart Failure Questionnaire [19], can be used to assess symptoms in a validated manner. Furthermore, in this stage, non-cardiac diseases (that can coexist with HF and exacerbate the HF syndrome) such as anaemia, and pulmonary, renal, thyroid or hepatic disease should be excluded.
Schematic overview of diagnostic pathway for HF in people with type 2 diabetes, based on the 2021 ESC guidelines for the diagnosis and treatment of HF. HF can be categorised as HFrEF, HFmrEF or HFpEF. The diagnosis of HF requires the presence of cardinal symptoms (ia) and, occasionally, signs (ib). Supporting investigations (ii) include ECG, risk factor assessment (risk factors include medical history of cardiovascular events, older age [>70 years], sex and obesity) and analysis of NP levels (note that in individuals with atrial fibrillation [AF], diagnostic values are ≥365 pg/ml N-terminal pro–B-type NP [NT-proBNP] and ≥105 pg/ml brain NP [BNP]). Echocardiography (iii) allows for differentiation into different categories of HF. A reduced LVEF is needed to diagnose HFrEF and HFmrEF (≤40% for HFrEF and 41–49% for HFmrEF), and a preserved LVEF (≥50%) combined with echocardiographic functional and/or structural abnormalities and/or serological abnormalities is required for the diagnosis of HFpEF. Secondary diagnostic tests may include use of diagnostic algorithms, which are non-invasive. More invasive tests (e.g., exercise echocardiography, [exercise] right heart catheterisation and/or pulmonary artery wedge pressure) may be used if HF is suspected despite normal results for tests of echocardiographic functional and/or structural abnormalities and serological abnormalities, and if other comorbidities do not sufficiently explain symptoms/signs. F, female; M, male; SR, sinus rhythm. This figure is available as part of a downloadable slideset
Additionally, the determination of risk factors for HF (e.g. a medical history of cardiovascular events, older age [>70 years], sex and obesity) and an abnormal ECG can support clinical suspicion of HF. Natriuretic peptides (NPs) play a key role as initial diagnostic markers, and the ESC guidelines state that elevated NP concentrations (N-terminal pro–B-type NP [NT-proBNP] ≥125 pg/ml [≥365 pg/ml in individuals with atrial fibrillation]; brain NP [BNP] ≥35 pg/ml [≥105 pg/ml in atrial fibrillation]) support a diagnosis of HF (Fig. 1) [17]. For HFrEF and LVSD, the sensitivity and negative predictive value of ECG and NP analysis to detect cardiac disease are high [17, 20, 21], but they appear less reliable for diagnosing HFpEF [22,23,24]. A meta-analysis reported low sensitivity and specificity for the detection of LVDD and HFpEF based on ECG and NP analysis (sensitivity: 65% [95% CI 51%, 85%]; specificity: 80% [95% CI 70%, 90%]), accompanied by a reasonable ability to rule out LVDD (negative predictive value: 85% [95% CI 78%, 93%]) but poor positive-predictive value (60% [95% CI 30%, 90%]) [23]. Furthermore, NPs tend to be increased in the older population, relate inversely to BMI, are affected by kidney function and can be falsely elevated. Therefore, even though NP levels can be good indicators for HF, diagnosis of HF cannot be made or omitted based on NP measurements alone.
Echocardiography is key in the initial diagnostic work-up as it provides information about LVEF and the underlying aetiology (e.g., ischaemic, valvular) [17]. The diagnosis of HFrEF and HFmrEF requires the presence of symptoms (and, optionally, signs) of HF, as well as a reduced LVEF (≤40% for HFrEF and 41–49% for HFmrEF). However, the diagnosis of HFpEF remains challenging. Before 2021, several diagnostic guidelines/algorithms had been proposed to diagnose HFpEF, of which the H2FPEF algorithm and the Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final etiology (HFA-PEFF) score, together with the 2016 American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) recommendation guidelines [25], are the most well-known [26, 27]. The ASE/EACVI guidelines use echocardiographic factors only, whilst the H2FPEF and HFA-PEFF algorithms use a combination of echocardiographic factors and, clinical factors/patient characteristics, and differentiate between low, intermediate or high probability of having HFpEF. However, the use of these algorithms is subject to interpretation and is reported in a heterogeneous way [28]. Furthermore, when the H2FPEF and HFA-PEFF algorithms were applied to the same population, a significant fraction of individuals were classified discordantly, with 41% of participants being placed in different likelihood categories by each of the two scores [29,30,31]. Validation studies show that the H2FPEF score has a superior diagnostic performance compared with the HFA-PEFF score [32]; nevertheless, neither are perfect discriminators.
In 2021, the ESC published guidelines that recommend a simplified pragmatic approach for HF diagnosis, using the common major elements from earlier algorithms but in a more accessible and clinician-friendly way [17]. This approach became the preferred diagnostic tool to use. It is based on clinical symptoms (and, optionally, signs), and the presence of either structural and/or functional abnormalities in people with a preserved ejection fraction (≥50%), which are assessed using echocardiographic variables that represent signs of LVDD and are relatively easily to access (Fig. 1). By use of this algorithm, HFpEF can be diagnosed in a relatively non-invasive way. Nevertheless, if HF is suspected despite normal results, and other comorbidities do not sufficiently explain symptoms/signs, diastolic stress tests [33], such as exercise echocardiography [34] and/or (exercise) right heart catheterisation/assessment of pulmonary capillary wedge pressure [33, 35, 36], are recommended.
The prevalence and incidence of HF in type 2 diabetes: an updated systematic review
Knowledge on the prevalence of HF in people with type 2 diabetes is essential to identify a population at high risk. Nevertheless, a consensus has not been reached on the precise prevalence of (undiagnosed) HF and its subtypes in the type 2 diabetes population. In 2016–2018, Bouthoorn et al performed two meta-analyses on the prevalence of HF and left ventricular dysfunction [12, 13]; these analyses included a total of 29 studies. For LVDD, they found a prevalence of 35% (95% CI 24%, 46%) in the general population and 48% (95% CI 38%, 59%) in the hospital population [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], whilst for HFpEF, they reported a prevalence of 25% (95% CI 21%, 28%) in the general population [48] and 8% (95% CI 5%, 14%) in the hospital population [61]. For LVSD, they reported a prevalence of 2% (95% CI 2%, 3%) in the general population and 18% (95% CI 17%, 19%) in the hospital population [38,39,40,41,42, 44, 47,48,49, 52, 56, 57, 59, 62,63,64,65] and, finally, for HFrEF, they found a prevalence of 5.8% (95% CI 3.9%, 7.6%) in the general population (based on one study) [48]. Since the publication of the meta-analyses by Bouthoorn and colleagues, new diagnostic guidelines have become available, allowing for more precise prevalence estimates. Therefore, we have updated the search from Bouthoorn et al including studies from 2016 to 20 October 2022. We used the same search strategy as Bouthoorn et al with terms for HF (e.g., HFpEF, HFrEF, systolic, diastolic), echocardiography and diabetes/type 2 diabetes (see electronic supplementary material [ESM] Methods, ‘Search strategies’ section), and we included studies reporting on prevalence and incidence of cardiac dysfunction/HF based on echocardiographic measurements. Additionally, we meta-analysed the prevalence of LVDD categorically (grade I, II, III and/or indeterminate/definitive LVDD) when this information was available, and we performed a sensitivity analysis on the prevalence of LVDD, which only included studies that used a cut-off of LVEF ≥50%, to adhere to the most recent guidelines. Methodological quality assessment of the included studies was performed; this was based on Hoy et al’s risk-of-bias tool [66]. A detailed description of the methods used can be found in the ‘Systematic review and meta-analysis’ section of the ESM Methods. Initial screening was done by three authors (AGH, JWJB and EW) and selection was done by two authors (AGH, and JWJB). Data extraction/risk-of-bias assessment was done by AGH and was performed in twofold for 25% of the extracted papers (JWJB and EDC), with an excellent agreement for data extraction (absolute agreement: 98%) and a good agreement for risk of bias (absolute agreement on final score: 74%; note that Hoy et al reported an agreement value of 72% in the validation process in their study [66]). Screening and selection was done independently and consensus was used to resolve disagreement. There were no automation tools used. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed [67], and the completed checklists can be found in ESM Tables 1 and 2. The protocol for this review was registered in the International Prospective Register of Systematic Reviews, the PROSPERO database, under number: CRD42022368035.
Of the 5015 unique studies identified, 209 were screened using the full-text article and, in total, 50 studies reported on prevalence of LVSD (n=8) [68,69,70,71,72,73,74,75], LVDD (n=41) [68, 70, 71, 74, 76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112], HFrEF (n=3) [113,114,115], HFmrEF (n=2) [113, 114] and HFpEF (n=6) [73, 106, 113, 115,116,117] were included in this updated review and meta-analysis (Table 1). A PRISMA flow diagram of the process for selection of relevant articles is presented in Fig. 2. Eight studies included participants derived from the general population or a primary-care population [68, 69, 89, 92, 93, 102, 103, 111], three studies did not specify where the participants with type 2 diabetes were selected from [94, 104, 105] and the remaining studies all included patients with type 2 diabetes from a hospital setting (cardiology/endocrinology departments) or specialised outpatients clinics that focused on either cardiology/endocrinology [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88, 90, 91, 95,96,97,98,99,100,101,102, 104,105,106,107,108,109,110, 113,114,115]. All but three [94, 98, 104] studies reported the mean age of their participants, ranging from 44.5 years to 76.2 years (Table 1). Diabetes duration was reported in 30 studies and ranged from a mean of 3 years to 14.8 years (or 17.9 years in a subgroup of the study by Zoppini et al [87]) (Table 1). Due to a lack of consensus on how to diagnose LVDD and HFpEF in the past, the method used for diagnosing these conditions varied largely between studies; an overview of the criteria used to diagnose LVDD and HFpEF in each study is given in ESM Table 3 and ESM Table 4, respectively.
PRISMA flow chart showing the process for selection of relevant articles included in the systematic review and meta-analysis. This figure is available as part of a downloadable slideset
LVSD, HFmrEF and HFrEF
The prevalence of LVSD (reported in n=8 studies; based on a total of 16,918 individuals), yielded a summary prevalence of 12% (95% CI 9%, 17%) for individuals from the general population (n=2 studies, with a medium risk of bias) and 3% (95% CI 1%, 12%) for a hospital population (n=6 studies, 5/6 of which had a medium risk of bias and 1/6 had a high risk of bias). Overall, studies showed a high level of heterogeneity (I2=77–94%). Our findings are in contrast to the estimates reported by Bouthoorn et al [12], who found that, on average, 2% (95% CI 2%, 3%) of the general population and 18% (95% CI 17%, 19%) of the hospital population had LVSD. Compared to Bouthoorn et al, we used a different method to statistically handle the occurrence of 0% prevalences in individual studies (Freeman-Turkey transformation in the paper by Bouthoorn et al vs continuity correction in our analysis [‘Systematic review and meta-analysis’ section of the ESM Methods]). Both methods lead to different summary estimates: 18% (95% CI 17%, 19%) found by Bouthoorn et al vs 8% (95% CI 3%, 19%) in our analysis for the hospital population (Fig. 3, section Hospital population [Bouthoorn]), and 2% (95% CI 2%, 3%) found by Bouthoorn et al vs 3% (95% CI 1%, 7%) in our analysis for the general population (Fig. 3, section General population [Bouthoorn]). The use of different, albeit valid, methods can partly explain the difference in found prevalence estimates. Furthermore, our updated analysis in the general population included two studies only, both reporting a relatively high prevalence of 10% and 16%. The overall meta-analysis of studies identified by the current review and the meta-analysis by Bouthoorn et al (based on a total of 24,460 individuals from n=25 studies) yielded a combined prevalence of 6% (95% CI 3%, 10%) (Fig. 3).
Prevalence of LVSD in individuals with type 2 diabetes in the hospital and general population, categorised by new studies included in this updated meta-analysis and studies in the original meta-analysis by Bouthoorn et al. The combined prevalence of our and Bouthoorn et al’s meta-analyses is shown in bold black font. This figure is available as part of a downloadable slideset
The prevalence of HFmrEF and HFrEF (reported in n=2 and n=3 studies, respectively; based on a total of 2442–3485 individuals), yielded a summary prevalence for the hospital population of 12% (95% CI 7%, 22%) for HFmrEF and 7% (95% CI 2%, 20%) for HFrEF (Fig. 4a,b). Overall, the included studies showed a high level of study heterogeneity (I2=98% for HFmrEF and 98% for HFrEF) and a low–medium level of bias. In the meta-analysis by Bouthoorn et al [12], only one study was included that reported on HFrEF prevalence in the general population (stated as 5.8% [95% CI 3.9%, 7.6%]), which is comparable to our findings. No studies reporting on HFmrEF were identified by the meta-analysis by Bouthoorn et al [12]. For HFrEF, when combining both our and Bouthoorn et al’s meta-analyses (based on a total of 4090 individuals from n=4 studies), a prevalence of 7% (95% CI 3%, 15%) was found in the overall population (Fig. 4b).
Prevalence of (a) HFmrEF and (b) HFrEF in individuals with type 2 diabetes in the hospital and general population. For HFrEF results are categorised by new studies included in this updated meta-analysis and studies in the original meta-analysis by Bouthoorn et al. The combined prevalence of our and Bouthoorn et al’s meta-analyses is shown in bold black font. NA, not applicable. This figure is available as part of a downloadable slideset
LVDD and HFpEF
The analysis of the prevalence of LVDD included 65 studies, and was based on a total of 25,729 individuals. Studies were categorised based on their method of reporting LVDD (binary or categorical). In studies that reported LVDD as a binary variable (yes/no, based on ≥2 echocardiographic parameters), we found an overall prevalence of 38% (95% CI 28%, 49%), of which 39% (95% CI 27%, 52%) were in the hospital population and 35% (95% CI 16%, 60%) were in the general population (Fig. 5). Heterogeneity was high (I2=97-99%), as was the risk of bias for most studies (10/21), with 9/21 studies scoring a medium risk, one study scoring a low risk of bias, and one for which risk of bias could not be assessed due to unavailability of the supplementary materials. A sensitivity analysis only including studies with an LVEF ≥50% (n=10) showed a similar, hence slightly lower prevalence (nominal difference; total population: 27% [95% CI 13%, 47%], I2=99%; hospital population: 29% [95% CI 13%, 53%], I2=99%), corresponding to the stricter cut-off value used (ESM Fig. 1). Our findings are comparable with the findings of Bouthoorn et al [13] who reported an LVDD prevalence of 48% (95% CI 38%, 59%) in the hospital population and 35% (95% CI 24%, 46%) in the general population. When all studies were combined (based on a total of 21,795 individuals from n=46 studies), an LVDD prevalence of 43% (95% CI 37%, 50%) was found in the total population (Fig. 5).
Prevalence of LVDD in individuals with type 2 diabetes in the hospital population, general population and in populations that were not specified. Outcomes are categorised by new studies included in this updated meta-analysis and studies in the original meta-analysis by Bouthoorn et al. The combined prevalence of our and Bouthoorn et al’s meta-analyses is shown in bold black font. This figure is available as part of a downloadable slideset
Additionally, we included a large number of studies that reported grade of LVDD (grade I, II or III) (Fig. 6a–c) and/or categories of indeterminate LVDD and definitive LVDD (Fig. 7a,b) according to the ASC/EACVI recommendations [25]. We found that, on average, 40% (95% CI 27%, 53%) of the type 2 diabetes population had grade I LVDD (43% [95% CI 27%, 61%] in a hospital-specific population) based on a total of 2264 individuals, 14% (95% CI 9%, 21%) had grade II LVDD (10% [95% CI 7%, 15%] in a hospital-specific population) based on a total of 2202 individuals, and 3% (95% CI 2%, 7%) had grade III LVDD (3% [95% CI 1%, 6%] in a hospital-specific population) based on a total of 1480 individuals. Heterogeneity was the same for grade I and grade II LVDD (I2=96%) but appeared better for grade III LVDD (I2=84%), and most studies showed a low level of bias (7/12), with 2/12 studies showing a medium level of bias and 3/12 studies showing a high level of bias. Sensitivity analysis including studies with an LVEF ≥50% showed similar results (ESM figure 2a–c). Furthermore, when categorised according to indeterminate LVDD vs definitive LVDD, 9% (95% CI 6%, 12%) had indeterminate LVDD (9% [95% CI 6%, 13%] in the hospital population) and 11% (95%CI 5%, 21%) had definitive LVDD (12% [95% CI 5%, 24%] in the hospital population) (Fig. 7), both based on a total of 1670 individuals. Heterogeneity was moderate–high for both the analysis on indeterminate LVDD (I2=77%) and definitive LVDD (I2=96%). Sensitivity analysis only including studies with an LVEF ≥50% showed similar results (ESM Fig. 3a,b).
Prevalence of LVDD in individuals with type 2 diabetes in the hospital, general population and in populations that were not specified, categorised as (a) grade I, (b) grade II and (c) grade III based on ASE/EACVI recommendations. This figure is available as part of a downloadable slideset
Prevalence of LVDD in individuals with type 2 diabetes in the hospital and general population, categorised as (a) indeterminate or (b) definitive based on ASE/EACVI recommendations. This figure is available as part of a downloadable slideset
The analysis of the prevalence of HFpEF included six studies, and was based on a total of 4527 individuals, all of whom were in a hospital population, yielding a summary prevalence of 18% (95% CI 6%, 44%). Overall, studies showed a high level of heterogeneity (I2=99%). Two studies had a low level of bias and four had a medium level of bias. In the meta-analysis by Bouthoorn et al [13], only two studies were included that reported on HFpEF prevalence, with values ranging from 8% to 25%, which are comparable to our findings. When both our and Bouthoorn et al’s meta-analyses were combined (based on a total of 5292 individuals from n=8 studies), a prevalence of 17% (95% CI 7%, 35%) was found for HFpEF in the total population (Fig. 8).
Prevalence of HFpEF in individuals with type 2 diabetes in the hospital and general population. Results are categorised by new studies included in this updated meta-analysis and studies in the original meta-analysis by Bouthoorn et al. The combined prevalence of our and Bouthoorn et al’s meta-analyses is shown in bold black font. This figure is available as part of a downloadable slideset
To our knowledge, our review is the first to provide summary estimates of LVDD subcategories among individuals with type 2 diabetes, which give a more accurate reflection of the degree to which this population is affected by LVDD. This is especially important since grade I LVDD is often seen as part of ageing and is not considered clinically relevant. Knowledge on the prevalence of grade II and III LVDD gives more insight into the clinically relevant group of individuals with LVDD, and brings nuance to the high prevalence reported for LVDD in studies that do not report categories of LVDD.
Discussion of systematic review findings
In general, our findings are in agreement with the findings presented by Bouthoorn et al [12, 13] in their meta-analyses. When both our results and those of Bouthoorn et al were combined, they showed an overall prevalence of 43% for LVDD and 17% for HFpEF, which was much more than the 6% prevalence found for LVSD and 7% prevalence found for HFrEF. In addition, we were able to analyse the prevalence of the different categories of LVDD, bringing more insight into the clinically relevant groups (stage II and III) of individuals with LVDD. Nevertheless, it needs to be acknowledged that moderate–high heterogeneity was present in all analyses. Multiple explanations for the observed heterogeneity can be given: first, slight differences in study design between studies, such as population (e.g., general population vs general hospital population [outpatient and hospital-ward population] vs endocrinology ward population vs cardiology ward population), as well as variation in inclusion and exclusion criteria (e.g., the inclusion or exclusion of people with known ischaemic disease) can result in different estimates. Second, heterogeneity can be introduced due to factors that are inherently connected to the pathophysiology of HF, for example mean age, male/female ratio, diabetes duration and the number of comorbidities for individuals in a subpopulation, since all of these factors are also confounders or mediators in the pathophysiology of HF. Finally, an important cause of heterogeneity is induced by the lack of consensus on methods for diagnosing HF and left ventricular dysfunction (especially for diagnosing LVDD and HFpEF). As mentioned previously, over the past decades, heterogeneous methods and criteria have been used to diagnose LVDD and HFpEF, resulting in heterogeneity in the way in which they have been diagnosed between studies (ESM Tables 3 and 4), which can be seen as a limitation of this analysis. Future studies focusing on subgroups of people with type 2 diabetes, for example female participants only or people with a history of CVD, are needed to map the prevalence of HF in these subpopulations to a further extent. As a final limitation of this study, it should be noted that data extraction and risk-of-bias assessment was performed in twofold for 25% of the studies included (the remaining 75% was done by one author [AGH]); however, good agreement was observed.
Screening for ventricular dysfunction and HF
Different sets of comorbidities and risk factors have been associated with the development of HFrEF and HFpEF. Along with the direct detrimental effects of hyperinsulinaemia and hyperglycaemia [118], HFrEF most often occurs secondary to comorbidities such as coronary artery disease, chronic kidney disease and hypertension [119,120,121]. On the other hand, HFpEF is associated with arterial and pulmonary hypertension, obesity and atrial fibrillation, together with multiple pathophysiological mechanisms related to hyperglycaemia, insulin resistance and hyperinsulinaemia [122]. Given that individuals with HF can reside in a pre-clinical phase for years, the question can be raised as to whether screening for HF in a subpopulation can be beneficial.
In a study by van Giessen et al [123], the cost-effectiveness of five screening strategies was investigated; these methods varied from screening medical records to allowing individuals to undergo full echocardiographic screening. The authors found that screening for HF by checking electronic medical records for patient characteristics and medical history plus the assessment of symptoms in patients with type 2 diabetes who were 60 years or older was cost-effective at the commonly used willingness-to-pay threshold of €20,000/quality-adjusted life year (QALY). These findings had a sensitivity of up to 85% and 92% for individuals in the New York Heart Association (NYHA) grading of symptoms for heart failure classifications 2 and 3, respectively, and a specificity of 61% [123]. Echocardiographic screening showed a high effectiveness but at the cost of a higher willingness-to-pay value. To our knowledge, this is the only study of its kind investigating a population with type 2 diabetes. However, screening of patient records and symptoms is invasive, and avoiders of care will likely be missed in this approach. Nevertheless, the authors state that cost-effectiveness increases with increasing effectiveness of therapies [123]. Since this study was conducted before use of SGLT2 inhibitors became standard practice in type 2 diabetes care (which, to date, is the only effective therapy with proven glucose-lowering and cardiovascular-protective effects [15, 16]) screening is likely to be even more beneficial in the present day. Unfortunately, a minimally invasive but sensitive screening tool is lacking. Even though the usefulness and significance of biomarkers for HFrEF in the general population has been established, only NPs and urine albumin/creatinine ratio have been associated with the presence of HFpEF [23, 124, 125] and, as discussed above, these have low specificity and a poor positive-predictive value. Studies investigating the use of NPs to screen for HF have been able to identify over a third of true HF patients; however, they did not make a distinction between HF subtypes in the diagnosis [126, 127]. Furthermore, as aforementioned, NPs tend to increase in the older population, relate inversely to BMI and are affected by kidney function. Overall, even though they are helpful, NPs are not the ideal biomarker where screening is concerned. The majority of studies investigating (novel) diagnostic HF biomarkers, especially those for HFpEF, show a high risk of bias, reducing their reproducibility and the potential for application of their findings in clinical care [128]. A previous review identified emerging biomarkers, including high-sensitivity C-reactive protein (hs-CRP), high-sensitive cardiac troponin T (hs-cTnT) and galectin-3 as possible screening tools for HF [129], and a recently published study found that circulating endotrophin levels are increased in patients with HFpEF and are independently associated with worse outcomes [130]. Nevertheless, more research is needed on the sensitivity on these biomarkers before they can be implemented in clinical practice.
Incidence of HF in type 2 diabetes
To our knowledge, seven studies (including n=17,935 individuals) have separately reported on the incidences of HFpEF [131,132,133,134,135,136,137] and HFrEF [131,132,133,134] (Table 2), with a follow-up range of 3 to 12.4 years. For HFpEF, cumulative incidences ranged from 2.5% to 20.8% (2.0 to 69.4 cases/1000 person-years) in the hospital population and from 4.2% to 8.9% (4.5 to 7.8 cases/1000 person-years) in the general population. For HFrEF, reported cumulative incidences ranged from 2.0% to 5.3% (1.6 to 7.4 cases/1000 person-years) in the hospital population and from 4.0% to 7.5% (4.3 to 6.6 cases/1000 person-years) in the general population (Table 2). When meta-analysed, a combined overall incidence of 7% (95% CI 4%, 11%) (6% [95% CI 3%, 10%] in the hospital population) and 4% (95% CI 3%, 7%) (3% [95% CI 2%, 6%] in the hospital population) was found for HFpEF and HFrEF, respectively (Fig. 9a,b). Similar to the studies that were included in the meta-analysis for HF prevalence, there was a large variety of methods used to diagnose the HF entities in the studies reporting HF incidence (with the exception that all studies included clinical symptoms as a diagnostic criteria) (see Table 2, ‘Outcome’ column). No studies reporting on the incidence of HFmrEF were found. Our outcomes are comparable with a meta-analysis investigating the overall incidence of HF (not reporting subtypes) in people with type 2 diabetes [138], which found a mean cumulative incidence of overall HF of 10.7% (range: 1.4–39%).
Incidence of (a) HFpEF and (b) HFrEF in individuals with type 2 diabetes in the hospital population, general population and in populations that were not specified. This figure is available as part of a downloadable slideset
Prognosis of ventricular dysfunction and HF in type 2 diabetes
A substantially increased risk for all-cause mortality, CVD-attributable mortality and first hospitalisation for worsening of HF are observed in individuals with concomitant type 2 diabetes and HF [139,140,141]. Moreover, for both HFrEF and HFpEF, patients with type 2 diabetes represent a specific clinical phenotype with worse outcomes as compared with patients without type 2 diabetes [142,143,144]. Individuals with HFpEF and concomitant diabetes who were enrolled in the RELAX trial had a higher risk of hospitalisation for HF compared with those without diabetes (47% vs 28%), as well as a higher risk of hospitalisation for cardiac or renal causes (23.7% vs 4.9%) at 6 months after enrolment [142]. Using data from the Get With the Guidelines Heart Failure registry for patients with HFpEF hospitalised for new or worsening HF, type 2 diabetes was associated with a significantly longer length of stay (OR 1.27 [95% CI 1.23, 1.31]), a lower likelihood of home discharge (OR 0.83 [95% CI 0.81, 0.86]) and an increased likelihood of all-cause 30-day readmission (HR 1.10 [95% CI 1.05, 1.15]) [143]. Clinical outcomes in the long term were also poorer for these individuals as type 2 diabetes was a significant predictor of risk of all-cause mortality and risk of hospitalisation for HFpEF (HR 1.72 [95% CI 1.1, 2.6]) over a 25±11 month period; this finding was independent of age, BMI, kidney function and functional class [144]. Similar results have been found for HFrEF, whereby, in a number of consecutive trials, individuals with both type 2 diabetes and HFrEF had higher risk of all-cause mortality (HR 1.3–2.0) and CVD-attributable mortality (HR 1.5–1.8) compared with those without type 2 diabetes [145,146,147,148,149,150].
Limited data are available on the prognosis of HFpEF vs HFrEF in people with type 2 diabetes. A large meta-analysis in the general population showed that the risk of all-cause mortality was significantly lower in individuals with HFmrEF (37.5%) than those with HFrEF (43.7%) and HFpEF (47.3%), and that individuals with HFrEF had a lower risk of all‐cause mortality compared with those with HFpEF (HFpEF vs HFrEF: OR 1.0 [95% CI 1.0, 1.1]; p=0.01). CVD-attributable mortality was lowest in individuals with HFpEF (11.4%), and highest in those with HFrEF (21.1%), mainly owing to HF-associated death and sudden cardiac death. In comparison, a subgroup analysis in individuals with type 2 diabetes showed that the risk of all‐cause mortality in this population followed a contrasting pattern to that in the general population, with the highest risk of mortality being found for HFpEF and the lowest risk for HFrEF [151]. However, only two studies with contrasting results were included in this subgroup analysis and statistical significance was not reached [151]. Therefore, no conclusions can be made regarding the risk of all-cause and CVD-attributable mortality in individuals with HFpEF vs those with HFrEF.
Conclusion and future directions
HF and type 2 diabetes are two highly intertwined diseases and concomitantly pose an increased risk of morbidity and mortality. Early diagnosis and treatment might delay clinical progression and, therefore, a timely diagnosis of HF and identification of people at risk for HF is important, with epidemiological knowledge being an essential tool in this process. In this review, we aimed to shed a light on these aspects of HF in individuals with type 2 diabetes.
This updated meta-analysis and the studies by Bouthoorn et al [12, 13] showed an overall prevalence of 43% (95% CI 37%, 50%) and 17% (95% CI 7%, 35%) for LVDD and HFpEF respectively, and a prevalence of 6% (95% CI 3%, 10%) and 7% (95% CI 3%, 15%) for LVSD and HFrEF, respectively, hereby establishing that LVDD and HFpEF are more prevalent in type 2 diabetes than the other forms of HF and LVSD. Furthermore, we reported a higher incidence rate of HFpEF than HFrEF (7% [95% CI 4%, 11%] vs 4% [95% CI 3%, 7%]). In an additional analysis, for LVDD, we found that grade I and/or indeterminate function were highly prevalent and likely to be responsible for the high overall LVDD prevalence rates reported. It must be noted, however, that mild diastolic abnormalities (that place people in grade I/indeterminate function categories of LVDD) are often seen as part of ageing. Overall, these findings suggest that there is a large pre-clinical target group that has early LVDD in which disease progression could be halted by early recognition and adequate treatment, thereby reducing disease burden.
Moving forward, we believe there is need for easily accessible and reliable tools for diagnosing HF. Even though NPs are widely incorporated in practice, they are not optimal for screening and diagnosing HFpEF. The cost–benefit of screening is proven, and the cost-effectiveness increases with increasing effectiveness of therapies, such as SGLT2 inhibitors, therefore thorough clinical investigation is recommended when HF is suspected. The large heterogeneity shown in the studies included in this review can be minimalised by having one uniform way of diagnosing (pre-)clinical entities of HF. With the development of new, uniform and clinically accessible ESC 2021 guidelines for diagnosing HF (especially LVDD/HFpEF), we hope that a consensus is reached about the best way to report subtypes of HF, leading to less heterogeneity in future studies. This will lead to more accurate HF diagnosis, more reliable data and more reliable tools to measure change/progression of HF. When combined, this will ultimately lead to more knowledge and better care for patients with type 2 diabetes and HF.
Abbreviations
- ASE:
-
American Society of Echocardiography
- EACVI:
-
European Association of Cardiovascular Imaging
- ESC:
-
European Society of Cardiology
- HF:
-
Heart failure
- HFA-PEFF:
-
Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final etiology
- HFmrEF:
-
Heart failure with mildly reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LVDD:
-
Left ventricular diastolic dysfunction
- LVEF:
-
Left ventricle ejection fraction
- LVSD:
-
Left ventricular systolic dysfunction
- NP:
-
Natriuretic peptides
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SGLT2:
-
sodium−glucose cotransporter 2
References
Giugliano D, Bellastella G, Longo M et al (2020) Relationship between improvement of glycaemic control and reduction of major cardiovascular events in 15 cardiovascular outcome trials: a meta-analysis with meta-regression. Diabetes Obes Metab 22(8):1397–405. https://doi.org/10.1111/dom.14047
American Diabetes Association (2021) 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2021. Diabetes Care 44(Suppl 1):S125–S150. https://doi.org/10.2337/dc21-S010
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB (2004) The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27(8):1879–84. https://doi.org/10.2337/diacare.27.8.1879
Kodama S, Fujihara K, Horikawa C et al (2020) Diabetes mellitus and risk of new-onset and recurrent heart failure: a systematic review and meta-analysis. ESC Heart Fail 7(5):2146–74. https://doi.org/10.1002/ehf2.12782
Adams KF Jr, Fonarow GC, Emerman CL et al (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149(2):209–16. https://doi.org/10.1016/j.ahj.2004.08.005
Dei Cas A, Khan SS, Butler J et al (2015) Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 3(2):136–45. https://doi.org/10.1016/j.jchf.2014.08.004
Sun H, Saeedi P, Karuranga S et al (2022) IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–858. https://doi.org/10.1016/S0140-6736(18)32279-7
Cosentino F, Grant PJ, Aboyans V et al (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats A (2023) Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 118(17):3272–3287. https://doi.org/10.1093/cvr/cvac013
Bozkurt B, Coats AJS, Tsutsui H et al (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 23(3):352–80. https://doi.org/10.1002/ejhf.2115
Bouthoorn S, Gohar A, Valstar G et al (2018) Prevalence of left ventricular systolic dysfunction and heart failure with reduced ejection fraction in men and women with type 2 diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 17(1):58. https://doi.org/10.1186/s12933-018-0690-3
Bouthoorn S, Valstar GB, Gohar A et al (2018) The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: a systematic review and meta-analysis. Diab Vasc Dis Res 15(6):477–93. https://doi.org/10.1177/1479164118787415
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–71. https://doi.org/10.1016/j.jacc.2013.02.092
Dunlay SM, Givertz MM, Aguilar D et al (2019) Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 140(7):e294–e324. https://doi.org/10.1161/CIR.0000000000000691
Packer M, Anker SD, Butler J et al (2021) Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced Trial. Circulation 143(4):326–36. https://doi.org/10.1161/CIRCULATIONAHA.120.051783
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368
Spertus JA, Jones PG, Sandhu AT, Arnold SV (2020) Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol 76(20):2379–90. https://doi.org/10.1016/j.jacc.2020.09.542
Garin O, Herdman M, Vilagut G et al (2014) Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev 19(3):359–67. https://doi.org/10.1007/s10741-013-9394-7
Cowie MR, Struthers AD, Wood DA et al (1997) Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 350(9088):1349–53. https://doi.org/10.1016/S0140-6736(97)06031-5
Khan NK, Goode KM, Cleland JG et al (2007) Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail 9(5):491–501. https://doi.org/10.1016/j.ejheart.2006.11.003
Dal Canto E, Remmelzwaal S, van Ballegooijen AJ et al (2022) Diagnostic value of echocardiographic markers for diastolic dysfunction and heart failure with preserved ejection fraction. Heart Fail Rev 27(1):207–18. https://doi.org/10.1007/s10741-020-09985-1
Remmelzwaal S, van Ballegooijen AJ, Schoonmade LJ et al (2020) Natriuretic peptides for the detection of diastolic dysfunction and heart failure with preserved ejection fraction-a systematic review and meta-analysis. BMC Med 18(1):290. https://doi.org/10.1186/s12916-020-01764-x
van de Bovenkamp AA, Enait V, de Man FS et al (2021) Validation of the 2016 ASE/EACVI Guideline for diastolic dysfunction in patients with unexplained dyspnea and a preserved left ventricular ejection fraction. J Am Heart Assoc 10(18):e021165. https://doi.org/10.1161/JAHA.121.021165
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138(9):861–70. https://doi.org/10.1161/CIRCULATIONAHA.118.034646
Pieske B, Tschope C, de Boer RA et al (2020) How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 22(3):391–412. https://doi.org/10.1002/ejhf.1741
Oh JK, Miranda WR, Bird JG, Kane GC, Nagueh SF (2020) The 2016 Diastolic Function Guideline: is it already time to revisit or revise them? JACC Cardiovasc Imaging 13(1 Pt 2):327–35. https://doi.org/10.1016/j.jcmg.2019.12.004
Nikorowitsch J, Bei der Kellen R, Kirchhof P et al (2021) Applying the ESC 2016, H2 FPEF, and HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail 8(5):3603–12. https://doi.org/10.1002/ehf2.13532
Selvaraj S, Myhre PL, Vaduganathan M et al (2020) Application of diagnostic algorithms for heart failure with preserved ejection fraction to the community. JACC Heart Fail 8(8):640–53. https://doi.org/10.1016/j.jchf.2020.03.013
Sanders-van Wijk S, Barandiaran Aizpurua A, Brunner-La Rocca HP et al (2021) The HFA-PEFF and H2FPEF scores largely disagree in classifying patients with suspected heart failure with preserved ejection fraction. Eur J Heart Fail 23(5):838–40. https://doi.org/10.1002/ejhf.2019
Reddy YNV, Kaye DM, Handoko ML et al (2022) Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol 7(9):891–9. https://doi.org/10.1001/jamacardio.2022.1916
Huis In ’t Veld AE, de Man FS, van Rossum AC, Handoko ML (2016) How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J 24(4):244–51. https://doi.org/10.1007/s12471-016-0811-0
Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA (2017) Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 135(9):825–38. https://doi.org/10.1161/CIRCULATIONAHA.116.024822
van de Bovenkamp AA, Wijkstra N, Oosterveer FPT et al (2022) The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail 15(4):e008935. https://doi.org/10.1161/CIRCHEARTFAILURE.121.008935
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM (2010) Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3(5):588–95. https://doi.org/10.1161/CIRCHEARTFAILURE.109.930701
Bajraktari G, Qirko S, Rexhepaj N et al (2005) Non-insulin dependent diabetes as an independent predictor of asymptomatic left ventricular diastolic dysfunction. Croat Med J 46(2):225–31
Annonu AK, Fattah AA, Mokhtar MS, Ghareeb S, Elhendy A (2001) Left ventricular systolic and diastolic functional abnormalities in asymptomatic patients with non-insulin-dependent diabetes mellitus. J Am Soc Echocardiogr 14(9):885–91. https://doi.org/10.1067/mje.2001.112892
Dodiyi-Manuel ST, Akpa MR, Odia OJ (2013) Left ventricular dysfunction in normotensive type II diabetic patients in Port Harcourt, Nigeria. Vasc Health Risk Manag 9:529–33. https://doi.org/10.2147/VHRM.S44540
Albertini JP, Cohen R, Valensi P, Sachs RN, Charniot JC (2008) B-type natriuretic peptide, a marker of asymptomatic left ventricular dysfunction in type 2 diabetic patients. Diabetes Metab 34(4 Pt 1):355–62. https://doi.org/10.1016/j.diabet.2008.02.004
Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH (2005) Screening for heart disease in diabetic subjects. Am Heart J 149(2):349–54. https://doi.org/10.1016/j.ahj.2004.06.021
Dawson A, Morris AD, Struthers AD (2005) The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 48(10):1971–9. https://doi.org/10.1007/s00125-005-1896-y
Ernande L, Bergerot C, Rietzschel ER et al (2011) Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 24(11):1268–75 e1. https://doi.org/10.1016/j.echo.2011.07.017
Faden G, Faganello G, De Feo S et al (2013) The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pract 101(3):309–16. https://doi.org/10.1016/j.diabres.2013.07.004
Kazlauskaite R, Doukky R, Evans A et al (2010) Predictors of diastolic dysfunction among minority patients with newly diagnosed type 2 diabetes. Diabetes Res Clin Pract 88(2):189–95. https://doi.org/10.1016/j.diabres.2009.12.007
Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P (2011) Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res 2(4):213–22. https://doi.org/10.4103/0975-3583.89805
Poulsen MK, Henriksen JE, Dahl J et al (2010) Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging 3(1):24–31. https://doi.org/10.1161/CIRCIMAGING.109.855510
Boonman-de Winter LJ, Rutten FH, Cramer MJ et al (2012) High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 55(8):2154–62. https://doi.org/10.1007/s00125-012-2579-0
Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, Chen HH (2014) The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J Card Fail 20(5):304–9. https://doi.org/10.1016/j.cardfail.2014.02.007
From AM, Scott CG, Chen HH (2010) The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 55(4):300–5. https://doi.org/10.1016/j.jacc.2009.12.003
Pareek M, Nielsen ML, Gerke O et al (2015) Worsening diastolic function is associated with elevated fasting plasma glucose and increased left ventricular mass in a supra-additive fashion in an elderly, healthy, Swedish population. Int J Cardiol 184:466–72. https://doi.org/10.1016/j.ijcard.2015.03.037
Srivastava PM, Calafiore P, Macisaac RJ et al (2008) Prevalence and predictors of cardiac hypertrophy and dysfunction in patients with type 2 diabetes. Clin Sci 114(4):313–20. https://doi.org/10.1042/CS20070261
Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG (2001) Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24(1):5–10. https://doi.org/10.2337/diacare.24.1.5
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87(3):320–3. https://doi.org/10.1016/S0002-9149(00)01366-7
Akiyama T, Eto Y, Matsuda H, Kimura Y, Yanagawa T (2014) Albuminuria and left ventricular mass index are associated with left ventricular diastolic dysfunction in type 2 diabetes mellitus patients. Diabetol Int 5(2):129–33. https://doi.org/10.1007/s13340-013-0146-7
Chaudhary AK, Aneja GK, Shukla S, Razi SM (2015) Study on diastolic dysfunction in newly diagnosed type 2 diabetes mellitus and its correlation with glycosylated haemoglobin (HbA1C). J Clin Diagn Res 9(8):OC20-2. https://doi.org/10.7860/JCDR/2015/13348.6376
Chen Y, Zhao CT, Zhen Z, Wong A, Tse HF, Yiu KH (2014) Association of myocardial dysfunction with vitamin D deficiency in patients with type 2 diabetes mellitus. J Diabetes Complications 28(3):286–90. https://doi.org/10.1016/j.jdiacomp.2014.01.003
Chillo P, Rieck AE, Lwakatare J, Lutale J, Gerdts E (2013) Left atrial volume index as a marker of left ventricular diastolic dysfunction in asymptomatic Tanzanian diabetic patients. Blood Press 22(2):86–93. https://doi.org/10.3109/08037051.2012.707351
Cioffi G, Giorda CB, Chinali M et al (2012) Analysis of midwall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: the DYDA study. Eur J Prev Cardiol 19(5):935–43. https://doi.org/10.1177/1741826711417759
Boyer JK, Thanigaraj S, Schechtman KB, Perez JE (2004) Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93(7):870–5. https://doi.org/10.1016/j.amjcard.2003.12.026
Utrera-Lagunas M, Orea-Tejeda A, Castillo-Martinez L et al (2013) Abnormal myocardial perfusion and risk of heart failure in patients with type 2 diabetes mellitus. Exp Clin Cardiol 18(1):e44–e46
Aigbe IF, Kolo PM, Omotoso AB (2012) Left ventricular structure and function in black normotensive type 2 diabetes mellitus patients. Ann Afr Med 11(2):84–90. https://doi.org/10.4103/1596-3519.93530
Chaowalit N, Arruda AL, McCully RB, Bailey KR, Pellikka PA (2006) Dobutamine stress echocardiography in patients with diabetes mellitus: enhanced prognostic prediction using a simple risk score. J Am Coll Cardiol 47(5):1029–36. https://doi.org/10.1016/j.jacc.2005.10.048
Jørgensen PG, Jensen MT, Mogelvang R et al (2016) Abnormal echocardiography in patients with type 2 diabetes and relation to symptoms and clinical characteristics. Diab Vasc Dis Res 13(5):321–30. https://doi.org/10.1177/1479164116645583
Xanthakis V, Sung JH, Samdarshi TE et al (2015) Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson Heart Study. Diabetes Care 38(6):1082–8. https://doi.org/10.2337/dc14-2460
Hoy D, Brooks P, Woolf A et al (2012) Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65(9):934–9. https://doi.org/10.1016/j.jclinepi.2011.11.014
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Alizadehasl A, Bakhshandeh H, Sadeghipour P et al (2021) Normal values of echocardiographic parameters and incidence of valvular heart findings in 2229 Iranian peaple: results from the 'HAMRAH survey'. Res Cardiovasc Med 10(2):45–53. https://doi.org/10.4103/rcm.rcm_18_21
Kishi S, Gidding SS, Reis JP et al (2017) Association of insulin resistance and glycemic metabolic abnormalities with LV structure and function in middle age: the CARDIA study. JACC Cardiovasc Imaging 10(2):105–14. https://doi.org/10.1016/j.jcmg.2016.02.033
Antakly-Hanon Y, Ben Hamou A, Garçon P et al (2021) Asymptomatic left ventricular dysfunction in patients with type 2 diabetes free of cardiovascular disease and its relationship with clinical characteristics: the DIACAR cohort study. Diabetes Obes Metab 23(2):434–43. https://doi.org/10.1111/dom.14236
Chee KH, Tan KL, Luqman I et al (2021) Prevalence and predictors of left ventricular diastolic dysfunction in Malaysian patients with type 2 diabetes mellitus without prior known cardiovascular disease. Front Cardiovasc Med 8:676862. https://doi.org/10.3389/fcvm.2021.676862
Fudim M, White J, Pagidipati NJ et al (2019) Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL trial. Circulation 140(20):1613–22. https://doi.org/10.1161/CIRCULATIONAHA.119.041659
Jensen J, Schou M, Kistorp C et al (2019) Prevalence of heart failure and the diagnostic value of MR-proANP in outpatients with type 2 diabetes. Diabetes Obes Metab 21(3):736–40. https://doi.org/10.1111/dom.13583
Patro PK, Dash BK, Choudhury S, Sethy RC (2021) Study of microalbuminuria in type 2 diabetes mellitus as a predictor of left ventricular dysfunction- a cohort study. J Clin Diag Res 15:OC05-OC9. https://doi.org/10.7860/JCDR/2021/51306.15501
Wu PY, Huang JC, Chen SC, Chen LI (2018) Type 2 diabetes mellitus-related changes in left ventricular structure and function in patients with chronic kidney disease. Oncotarget 9(18):14661–8. https://doi.org/10.18632/oncotarget.24482
Alhibaly HA, Al-Jameel HH, Bdair BWH, Algraittee SJR (2021) Evaluation of clinically significant cardiac abnormalities in patients with normal electrocardiogram using transthoracic echocardiography. Acta Medica Iranica 59:97–107. https://doi.org/10.18502/acta.v59i2.5576
Bayat FK, Khani M, Saffarian F, Shahrbaf MA (2020) Correlation between type II diabetes mellitus and left atrial function as assessed by 2D speckle-tracking echocardiography in patients without coronary artery disease. Iran Heart J 21(1):82–93
Cioffi G, Giorda CB, Lucci D et al (2021) Effects of linagliptin on left ventricular DYsfunction in patients with type 2 DiAbetes and concentric left ventricular geometry: results of the DYDA 2 trial. Eur J Prev Cardiol 28(1):8–17. https://doi.org/10.1177/2047487320939217
Huang Z, Zhong J, Zhang S et al (2022) Association between serum cystatin C and early impairment of cardiac function and structure in type 2 diabetes patients with normal renal function. Clin Cardiol 45(12):1287–1296. https://doi.org/10.1002/clc.23920
Ibrahim IM, Abdel-Kareem A-RO, Mahfouz RA, Shehata IE (2021) The association between morning blood pressure surge and cardiovascular disease in normotensive type 2 diabetic patients: observational analytical study in the form of a cross-sectional study. SN Compr Clin Med 3(4):955–63. https://doi.org/10.1007/s42399-021-00797-4
Liu Y, Ma J, Guo J, Lu H, Zhang Y, Chen Y (2021) Characteristics of myocardial perfusion in type 2 diabetes mellitus and its association with left ventricular diastolic dysfunction: a study of myocardial contrast echocardiography. Int J Gen Med 14:7533–43. https://doi.org/10.2147/IJGM.S340642
Lu Z, Lense L, Sharma M et al (2017) Prevalence of QT prolongation and associated LVEF changes in diabetic patients over a four-year retrospective time period. J Community Hosp Intern Med Perspect 7(2):87–94. https://doi.org/10.1080/20009666.2017.1320203
Maiello M, Zito A, Carbonara S, Ciccone MM, Palmiero P (2017) Left ventricular mass, geometry and function in diabetic patients affected by coronary artery disease. J Diabetes Complications 31(10):1533–7. https://doi.org/10.1016/j.jdiacomp.2017.06.014
Qureshi SA, Pirzada SAR, Naeem HAG, Qazi MA (2016) Frequency of left ventricular diastolic dysfunction among type-II diabetics with non-alcoholic fatty liver disease. Pakistan J Med Health Sci 10(1):200–2
Shahapure P, Sharma S (2020) Cardiovascular morbidity in diabetic non-alcoholic fatty liver disease (NAFLD) using NAFLD fibrosis score as an early indicator. Turk J Endocrinol Metab 24:314–20. https://doi.org/10.25179/tjem.2020-77679
Wang Y, Cui S, You C (2022) Application value of four-dimensional left atrial strain in evaluating the left ventricular diastolic function of T2DM. Acta Medica Mediterranea 38:979–84
Zoppini G, Bergamini C, Bonapace S et al (2018) Left ventricular chamber dilation and filling pressure may help to categorise patients with type 2 diabetes. BMJ Open Diabetes Res Care 6(1):e000529. https://doi.org/10.1136/bmjdrc-2018-000529
Zuo X, Liu X, Chen R et al (2019) An in-depth analysis of glycosylated haemoglobin level, body mass index and left ventricular diastolic dysfunction in patients with type 2 diabetes. BMC Endocr Disord 19(1):88. https://doi.org/10.1186/s12902-019-0419-7
Kim SH, Sung KC, Lee SK et al (2019) Longitudinal changes in left ventricular structure and function in patients with type 2 diabetes: normal weight versus overweight/obesity. Diab Vasc Dis Res 16(5):450–7. https://doi.org/10.1177/1479164119843760
Lee H, Kim G, Choi YJ et al (2020) Association between non-alcoholic steatohepatitis and left ventricular diastolic dysfunction in type 2 diabetes mellitus. Diabetes Metab J 44(2):267–76. https://doi.org/10.4093/dmj.2019.0001
Lee KA, Kim YJ, Park TS, Lee JH, Jin HY (2020) The association between cardiac autonomic neuropathy and heart function in type 2 diabetic patients. Somatosens Mot Res 37(3):149–56. https://doi.org/10.1080/08990220.2020.1756245
Segar MW, Khan MS, Patel KV et al (2021) Prevalence and prognostic implications of diabetes with cardiomyopathy in community-dwelling adults. J Am Coll Cardiol 78(16):1587–98. https://doi.org/10.1016/j.jacc.2021.08.020
Wang Y, Yang H, Huynh Q, Nolan M, Negishi K, Marwick TH (2018) Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. JACC Cardiovasc Imaging 11(10):1390–400. https://doi.org/10.1016/j.jcmg.2018.03.015
Shaker A (2019) Exercise electrocardiogram testing in asymptomatic patient with type 2 diabetes and left ventricular diastolic dysfunction. J Indian Coll Cardiol 9:205–10. https://doi.org/10.4103/JICC.JICC_21_19
Bergerot C, Davidsen ES, Amaz C et al (2018) Diastolic function deterioration in type 2 diabetes mellitus: predictive factors over a 3-year follow-up. Eur Heart J Cardiovasc Imaging 19(1):67–73. https://doi.org/10.1093/ehjci/jew331
Joseph TP, Kotecha NS, Kumar HBC et al (2020) Coronary artery calcification, carotid intima-media thickness and cardiac dysfunction in young adults with type 2 diabetes mellitus. J Diabetes Complications 34(8):107609. https://doi.org/10.1016/j.jdiacomp.2020.107609
Lumori BAE, Nuwagira E, Abeya FC et al (2022) Association of body mass index with left ventricular diastolic dysfunction among ambulatory individuals with diabetes mellitus in rural Uganda: a cross-sectional study. BMC Cardiovasc Disord 22(1):279. https://doi.org/10.1186/s12872-022-02718-2
Nasir M, Hyder SN, Hassan A (2016) Frequency of left ventricle diastolic dysfunction in asymptomatic type II diabetic patients. Pakistan J Med Health Sci 905-8
Shogade TT, Essien IO, Ekrikpo UE et al (2018) Association of microalbuminuria with left ventricular dysfunction in Nigerian normotensive type 2 diabetes patients. Cardiovasc J Afr 29(5):283–8. https://doi.org/10.5830/CVJA-2018-026
Tremamunno S, De Vita A, Villano A et al (2022) Relation of endothelial and cardiac autonomic function with left ventricle diastolic function in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 38(2):e3484. https://doi.org/10.1002/dmrr.3484
Zhen Z, Chen Y, Liu JH et al (2016) Increased T-wave alternans is associated with subclinical myocardial structural and functional abnormalities in patients with type 2 diabetes. J Cardiol 68(4):329–34. https://doi.org/10.1016/j.jjcc.2015.10.010
Demmer RT, Allison MA, Cai J et al (2016) Association of impaired glucose regulation and insulin resistance with cardiac structure and function: results from ECHO-SOL (Echocardiographic Study of Latinos). Circ Cardiovasc Imaging 9(10):e005032. https://doi.org/10.1161/CIRCIMAGING.116.005032
Klajda MD, Scott CG, Rodeheffer RJ, Chen HH (2020) Diabetes mellitus is an independent predictor for the development of heart failure: a population study. Mayo Clin Proc 95(1):124–33. https://doi.org/10.1016/j.mayocp.2019.07.008
Kasha A, Mallem B, Noorul Ameen KH (2017) Study to detect the prevalence of LV diastolic dysfunction in diabetic patients aged less than or equal to 50 years in a tertiary care center. Res J Pharm Biol Chem Sci 8(4):26–32
Yang H, Wang Y, Negishi K, Nolan M, Marwick TH (2016) Pathophysiological effects of different risk factors for heart failure. Open Heart 3(1):e000339. https://doi.org/10.1136/openhrt-2015-000339
Oo MM, Tan Chung Zhen I, Ng KS et al (2021) Observational study investigating the prevalence of asymptomatic stage B heart failure in patients with type 2 diabetes who are not known to have coronary artery disease. BMJ Open 11(1):e039869. https://doi.org/10.1136/bmjopen-2020-039869
Raghothama S, Rao A (2021) Revelation of subclinical left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus using 2016 ASE/ EACVI guidelines. Caspian J Intern Med 12:586–92. https://doi.org/10.22088/cjim.12.4.586
Sunil Kumar S, Desai N, Gona OJ, Vinay Kumar K, Madhu B (2021) Impact of updated 2016 ASE/EACVI VISÀ-VIS 2009 ASE recommendation on the prevalence of diastolic dysfunction and Lv filling pressures in patients with preserved ejection fraction. J Cardiovasc Imaging 29:31–43. https://doi.org/10.4250/jcvi.2020.0117
Yang SY, Hwang HJ (2022) Does diabetes increase the risk of cardiovascular events in patients with negative treadmill stress echocardiography? Endocr J 69(7):785–96. https://doi.org/10.1507/endocrj.EJ21-0693
Wu MZ, Chen Y, Yu YJ et al (2021) Sex-specific pattern of left ventricular hypertrophy and diastolic function in patients with type 2 diabetes mellitus. Eur Heart J Cardiovasc Imaging 22(8):930–40. https://doi.org/10.1093/ehjci/jeaa079
Wan SH, Pumerantz AS, Dong F, Ochoa C, Chen HH (2019) Comparing the influence of 2009 versus 2016 ASE/EACVI diastolic function guidelines on the prevalence and echocardiographic characteristics of preclinical diastolic dysfunction (stage B heart failure) in a Hispanic population with type 2 diabetes mellitus. J Diabetes Complications 33(8):579–84. https://doi.org/10.1016/j.jdiacomp.2019.04.015
Wang Z, Wang C, Xie Z et al (2022) Echocardiographic phenotypes of Chinese patients with type 2 diabetes may indicate early diabetic myocardial disease. ESC Heart Failure 9(5):3327–3344. https://doi.org/10.1002/ehf2.14062
Gimeno-Orna JA, Rodríguez-Padial L, Anguita-Sánchez M, Barrios V, Muñiz J, Pérez A (2021) Association of the KDIGO risk classification with the prevalence of heart failure in patients with type 2 diabetes. J Clin Med 10(20):4634. https://doi.org/10.3390/jcm10204634
McGuire DK, Alexander JH, Johansen OE et al (2019) Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation 139(3):351–61. https://doi.org/10.1161/CIRCULATIONAHA.118.038352
Zhou M, Huang D, Cheng Y et al (2022) Opportunistic screening for asymptomatic left ventricular dysfunction in type 2 diabetes mellitus. Postgrad Med J 99(1171):476–483. https://doi.org/10.1136/postgradmedj-2022-141548
Ianoș RD, Pop C, Iancu M, Rahaian R, Cozma A, Procopciuc LM (2021) Diagnostic performance of serum biomarkers fibroblast growth factor 21, galectin-3 and copeptin for heart failure with preserved ejection fraction in a sample of patients with type 2 diabetes mellitus. Diagnostics 11(9):1577. https://doi.org/10.3390/diagnostics11091577
Li J, Wu N, Dai W et al (2016) Association of serum calcium and heart failure with preserved ejection fraction in patients with type 2 diabetes. Cardiovasc Diabetol 15(1):140. https://doi.org/10.1186/s12933-016-0458-6
Packer M (2021) Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail 9(8):535–49. https://doi.org/10.1016/j.jchf.2021.05.019
Nichols GA, Hillier TA, Erbey JR, Brown JB (2001) Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 24(9):1614–9. https://doi.org/10.2337/diacare.24.9.1614
Adler AI, Stratton IM, Neil HA et al (2000) Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321(7258):412–9. https://doi.org/10.1136/bmj.321.7258.412
Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr (2004) Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27(3):699–703. https://doi.org/10.2337/diacare.27.3.699
Marwick TH, Ritchie R, Shaw JE, Kaye D (2018) Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol 71(3):339–51. https://doi.org/10.1016/j.jacc.2017.11.019
van Giessen A, Boonman-de Winter LJ, Rutten FH et al (2016) Cost-effectiveness of screening strategies to detect heart failure in patients with type 2 diabetes. Cardiovasc Diabetol 15:48. https://doi.org/10.1186/s12933-016-0363-z
Anjan VY, Loftus TM, Burke MA et al (2012) Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 110(6):870–6. https://doi.org/10.1016/j.amjcard.2012.05.014
de Boer RA, Nayor M, deFilippi CR et al (2018) Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol 3(3):215–24. https://doi.org/10.1001/jamacardio.2017.4987
Pandey A, Vaduganathan M, Patel KV et al (2021) Biomarker-based risk prediction of incident heart failure in pre-diabetes and diabetes. JACC Heart Fail 9(3):215–23. https://doi.org/10.1016/j.jchf.2020.10.013
Ceriello A, Lalic N, Montanya E et al (2023) NT-proBNP point-of-care measurement as a screening tool for heart failure and CVD risk in type 2 diabetes with hypertension. J Diabetes Complications 37(3):108410. https://doi.org/10.1016/j.jdiacomp.2023.108410
Henkens M, Remmelzwaal S, Robinson EL et al (2020) Risk of bias in studies investigating novel diagnostic biomarkers for heart failure with preserved ejection fraction. A systematic review. Eur J Heart Fail 22(9):1586–97. https://doi.org/10.1002/ejhf.1944
Chhor M, Law W, Pavlovic M, Aksentijevic D, McGrath K, McClements L (2023) Diagnostic and prognostic biomarkers reflective of cardiac remodelling in diabetes mellitus: a scoping review. Diabet Med 40:e15064. https://doi.org/10.1111/dme.15064
Chirinos JA, Zhao L, Reese-Petersen AL et al (2022) Endotrophin, a collagen VI formation-derived peptide, in heart failure. N Engl J Med 1(10):10.1056/evidoa2200091. https://doi.org/10.1056/EVIDx2200258
Liu JJ, Liu S, Wang J et al (2022) Risk of incident heart failure in individuals with early-onset type 2 diabetes. J Clin Endocrinol Metab 107(1):e178–e87. https://doi.org/10.1210/clinem/dgab620
Patel KV, Bahnson JL, Gaussoin SA et al (2020) Association of baseline and longitudinal changes in body composition measures with risk of heart failure and myocardial infarction in type 2 diabetes: findings from the Look AHEAD trial. Circulation 142(25):2420–30. https://doi.org/10.1161/CIRCULATIONAHA.120.050941
Khan H, Anker SD, Januzzi JL Jr et al (2019) Heart failure epidemiology in patients with diabetes mellitus without coronary heart disease. J Card Fail 25(2):78–86. https://doi.org/10.1016/j.cardfail.2018.10.015
Mordi IR, Tee A, Palmer CN, McCrimmon RJ, Doney ASF, Lang CC (2020) Microvascular disease and heart failure with reduced and preserved ejection fraction in type 2 diabetes. ESC Heart Fail 7(3):1168–77. https://doi.org/10.1002/ehf2.12669
Fan L, Pan JA, Lin H, Wang CQ, Zhang JF, Gu J (2022) Optimal management of blood glucose, blood pressure and atrial fibrillation to reduce the risk of heart failure with preserved ejection fraction. Intern Med J 52(2):301–9. https://doi.org/10.1111/imj.15006
Gu J, Fan YQ, Zhang JF, Wang CQ (2018) Association of hemoglobin A1c variability and the incidence of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and arterial hypertension. Hellenic J Cardiol 59(2):91–7. https://doi.org/10.1016/j.hjc.2017.08.001
Lebedev DA, Lyasnikova EA, Vasilyeva EY, Likhonosov NP, Sitnikova MY, Babenko AY (2021) Association between markers of fibrosis and heart failure incidence in patients with type 2 diabetes mellitus. J Diabetes Res 2021:9589185. https://doi.org/10.1155/2021/9589185
Wang Y, Negishi T, Negishi K, Marwick TH (2015) Prediction of heart failure in patients with type 2 diabetes mellitus- a systematic review and meta-analysis. Diabetes Res Clin Pract 108(1):55–66. https://doi.org/10.1016/j.diabres.2015.01.011
Dauriz M, Targher G, Laroche C et al (2017) Association between diabetes and 1-year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Diabetes Care 40(5):671–8. https://doi.org/10.2337/dc16-2016
Dauriz M, Targher G, Temporelli PL et al (2017) Prognostic impact of diabetes and prediabetes on survival outcomes in patients with chronic heart failure: a post-hoc analysis of the GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nella Insufficienza Cardiaca-Heart Failure) Trial. J Am Heart Assoc 6(7):e005156. https://doi.org/10.1161/JAHA.116.005156
Cavender MA, Steg PG, Smith SC Jr et al (2015) Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 132(10):923–31. https://doi.org/10.1161/CIRCULATIONAHA.114.014796
Lindman BR, Davila-Roman VG, Mann DL et al (2014) Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 64(6):541–9. https://doi.org/10.1016/j.jacc.2014.05.030
McHugh K, DeVore AD, Wu J et al (2019) Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol 73(5):602–11. https://doi.org/10.1016/j.jacc.2018.11.033
Lejeune S, Roy C, Slimani A et al (2021) Diabetic phenotype and prognosis of patients with heart failure and preserved ejection fraction in a real life cohort. Cardiovasc Diabetol 20(1):48. https://doi.org/10.1186/s12933-021-01242-5
Sarma S, Mentz RJ, Kwasny MJ et al (2013) Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail 15(2):194–202. https://doi.org/10.1093/eurjhf/hfs153
MacDonald MR, Petrie MC, Varyani F et al (2008) Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the candesartan in heart failure: assessment of Reduction in Mortality and Morbidity (CHARM) programme. Eur Heart J 29(11):1377–85. https://doi.org/10.1093/eurheartj/ehn153
Ruschitzka F, Abraham WT, Singh JP et al (2013) Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 369(15):1395–405. https://doi.org/10.1056/NEJMoa1306687
Shindler DM, Kostis JB, Yusuf S et al (1996) Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) trials and Registry. Am J Cardiol 77(11):1017–20. https://doi.org/10.1016/S0002-9149(97)89163-1
McMurray JJ, Packer M, Desai AS et al (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077
Kristensen SL, Preiss D, Jhund PS et al (2016) Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 9(1):e002560. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002560
Liang M, Bian B, Yang Q (2022) Characteristics and long-term prognosis of patients with reduced, mid-range, and preserved ejection fraction: a systemic review and meta-analysis. Clin Cardiol 45(1):5–17. https://doi.org/10.1002/clc.23754
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
We gratefully acknowledge the authors and participants of all of the individual studies from which we used summary data in this systematic review and meta-analysis.
Data availability
The data that support the findings of this study were extracted from the cited papers and can be found in Table 1. R scripts and raw study material are available from the corresponding author upon reasonable request.
Funding
Work in JWJB’s laboratories is supported by a ZorgOnderzoek Nederland (ZON) en het gebied Medische Wetenschappen (MW), Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) Vidi grant (91 71 8304).
Authors’ relationships and activities
MLH received financial support from Novartis, Boehringer Ingelheim, ViforPharma, AstraZeneca, Bayer, MSD and Abbot.
Contribution statement
AGH performed the study selection, data extraction and quality assessment, drafted the manuscript, made major revisions to the manuscript, performed the analyses, and drafted tables and figures. EW performed the literature search and study selection and critically revised the manuscript. PJME, EDC, MLH and NB provided support in the design and execution of the review and meta-analyses and made major revisions to the manuscript. EDC also participated in the data extraction and quality assessment. JWJB provided support in the design and execution of the review and meta-analyses, performed the literature search, study selection, data extraction and quality assessment and made major revisions to the manuscript. All authors gave final approval of the version to be published. Both AGH and JWJB are responsible for the integrity of the work as a whole.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoek, A.G., Dal Canto, E., Wenker, E. et al. Epidemiology of heart failure in diabetes: a disease in disguise. Diabetologia 67, 574–601 (2024). https://doi.org/10.1007/s00125-023-06068-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-06068-2