Abstract
Globally, the proportion of new diagnoses of youth-onset diabetes represented by type 2 diabetes is increasing, and youth with type 2 diabetes commonly have complications and comorbidities, as well as a higher rate of mortality. In this review, we summarise what is known about the natural progression of youth-onset type 2 diabetes from published clinical trials and large-scale prospective epidemiological studies. It is important to note that the robust pathophysiological and treatment data specifically related to individuals with a diabetes onset at ≤20 years of age largely hails from the USA. Youth-onset type 2 diabetes is characterised by pathophysiological heterogeneity and inadequate glycaemic control, highlighting the need for new treatment approaches and innovative study designs in populations of varied genetic and cultural backgrounds.
Graphical abstract

Similar content being viewed by others

Background epidemiology
While there was a time that the term ‘juvenile diabetes’ was synonymous with type 1 diabetes, the global picture of youth-onset diabetes has changed significantly over the past 30 years. Overall, the incidence of paediatric-onset type 1 diabetes is still greater than paediatric type 2 diabetes. However, particularly among adolescents, the proportion of newly diagnosed youth-onset diabetes represented by type 2 diabetes is increasing, with estimated incidence rates ranging from 0.72/100,000 in the UK [1] to 2.56/100,000 in Kuwait [2] and 3.45/100,000 in Canada [3]. Moreover, in many countries, including Qatar [4], the USA [5], the UK [1], Canada [3] and China [6], the incidence is currently rising several percentage points per year. Youth-onset type 2 diabetes is a disease of adolescent onset occurring almost universally in youth who are overweight or obese, most of whom have a strong family history of type 2 diabetes and/or exposure to gestational diabetes in utero [7]. It should be noted that the global prevalence of being overweight and obese in adolescence is high, ranging from 8% to 40%, depending on the country [8], but youth-onset type 2 diabetes is still rare in comparison. The incidence of youth-onset type 2 diabetes also varies widely by genetic and cultural background (e.g., being more common in youth with Hispanic ethnicity and in indigenous populations in the USA and Canada). In the western world, youth-onset type 2 diabetes is almost twice as common in girls as in boys, whereas Asian countries report no differences in incidence by sex [9].
Diagnosis of type 2 diabetes at a young age has a significant impact on risk of mortality: data from Sweden demonstrate that mortality rates are three times higher in young adults with type 2 diabetes compared with the general population after a mean follow-up of 8 years [10]. Data from the SEARCH for Diabetes in Youth (SEARCH) study in the USA show similarly elevated mortality rates, 2.4 times higher than in the general population, for youth with type 2 diabetes after an average of only 5 years of follow-up [11]. Both of these studies, as well as data from Australia [12], demonstrate greater risk of mortality for youth with type 2 diabetes than with type 1 diabetes, despite a shorter disease duration. Because youth-onset type 2 diabetes is still a relatively new disease affecting a small proportion of the population, there is still much to be learned about its pathophysiology and effective treatments. The goal of this review is to summarise the lessons from the largest studies and clinical trials of youth-onset type 2 diabetes and to discuss challenges and future directions.
What do we know about treatment effects from current studies?
Clinical trials focusing on paediatric type 2 diabetes are limited. In 2007, Gottschalk et al. published results of a 26 week randomised trial of the sulfonylurea glipizide vs the biguanide metformin in 285 youth with type 2 diabetes [13]. They found a similar short-term reduction in HbA1c in both groups but significantly greater weight gain with glipizide. Other non-industry-sponsored clinical trials specific to youth-onset type 2 diabetes include the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study [14] and the paediatric arm of the Restoring Insulin SEcretion (RISE) study [15] (both of which were only in the USA and partly designed to study the pathophysiology of type 2 diabetes in youth), and an open-label study of metformin in Japan [16]. The Japanese metformin study did note improvements in HbA1c over 24 weeks without significant adverse effects; however, there was no comparison group in this study. While many industry-sponsored clinical trials are underway, only one study has published results to date. In this study of 135 US youth with type 2 diabetes, aged 10–16 years, the glucagon-like peptide-1 (GLP-1) agonist liraglutide resulted in a treatment difference for HbA1c of 11.6 mmol/mol (95% CI −18.0, −5.0) (−1.06% [95% CI −1.65, −0.46]), but no treatment effect on BMI at the end of the 26 week placebo-controlled phase [17]. However, the dose was titrated up only if fasting glucose was elevated, which limited the number of participants reaching a full 1.8 mg dose, possibly curtailing weight loss effects, and there was a reduction in BMI by −0.18 kg/m2 (95% CI −0.33, −0.03) after the 52 week open-label extension. The safety and efficacy data from this trial resulted in US Food and Drug Administration approval of liraglutide for treatment of youth-onset type 2 diabetes.
TODAY study
The TODAY study was a multi-site randomised clinical trial designed with the underlying hypothesis that early, aggressive intervention to improve insulin sensitivity would prolong glycaemic control, defined as persistent HbA1c ≥ 64 mmol/mol (≥8.0%) or failure to wean from insulin after starting due to metabolic decompensation. This study, which began before sodium–glucose cotransporter 2 (SGLT2) inhibitors or GLP-1 agonists were approved in the USA, compared treatment with metformin vs two add-on therapies (intensive lifestyle intervention and the thiazolidinedione rosiglitazone), with a primary outcome of loss of glycaemic control [18]. Participants (N = 699) were aged 10–17 years at baseline, had been diagnosed with type 2 diabetes <2 years and had BMI equal to or greater than the 85th percentile. The cohort was 64.7% female sex, 39.7% Hispanic, 32.5% non-Hispanic Black, 20.3% non-Hispanic White, 5.9% American Indian and 1.6% Asian. The run-in phase of the trial demonstrated that a large majority (~90%) of youth with recent onset of diabetes can be weaned from insulin and achieve glycaemic control on metformin monotherapy alone, regardless of presenting HbA1c [19, 20]. However, by the end of the trial, almost half (45.6%) of the participants reached the primary outcome, with a mean time to failure of 3.86 years [13]. The TODAY study demonstrated a beneficial add-on effect of rosiglitazone (failure rate 38.6% vs 51.7% with metformin alone), which was most notable in girls. Furthermore, it demonstrated that metformin monotherapy is particularly ineffective in non-Hispanic Black youth, with a 66.2% failure rate. Of note, rosiglitazone has since been withdrawn from the European market and only has very limited availability in the USA; pioglitazone, another thiazolidinedione, is still available. There was a decline in beta cell function over time in the TODAY study, regardless of treatment group, though there was a beneficial effect of rosiglitazone use during the first 6 months [21]. When compared with similar trials in adults, glycaemic control failure rates and decline in beta cell function were more than two times higher in the TODAY study, despite a shorter disease duration in youth [22]. For example, in the A Diabetes Outcome Progression Trial (ADOPT), failure rates in adults taking metformin alone were 12% as compared with 51.7% in participants who took metformin for the same duration in the TODAY study [23].
The incidence of glycaemic failure in the TODAY study plateaued over time, suggesting that there are subgroups of adolescents with type 2 diabetes who rapidly lose glycaemic control and others who can maintain good control over a longer period of time. In the TODAY study, residual beta cell function, not insulin sensitivity, was identified as the primary determinant of glycaemic control. However, of clinical relevance, a baseline (i.e. following run-in during which participants were treated with metformin monotherapy) HbA1c ≥ 45.4 mmol/mol (≥6.3%) predicted glycaemic failure over the first 48 months [24], suggesting that escalation of treatment may be needed in youth even earlier than current American Diabetes Association targets recommend (HbA1c ≥ 53.0 mmol/mol [≥7%]). Once participants reached the primary outcome in the TODAY study, metformin was continued, rosiglitazone (if present) discontinued and insulin initiated. Importantly, there was only a modest improvement in HbA1c (<5.5 mmol/mol [<0.5%]) 6 months after insulin initiation, and no significant improvement in 1 year, such that mean HbA1c was still 85.8 mmol/mol (10.0%) [25], highlighting the difficulty in achieving glycaemic control with only metformin and insulin once beta cell function has declined.
RISE study
Given the high rates of beta cell deterioration seen in the TODAY study, the RISE Consortium examined the effect of interventions designed to preserve or improve beta cell function in adults and children with prediabetes (impaired glucose tolerance and fasting glucose ≥5.0 mmol/l) or recently diagnosed type 2 diabetes [26]. The RISE paediatric clinical trial compared 3 months of insulin glargine followed by 9 months of metformin vs 12 months of metformin, with a primary outcome of beta cell function, assessed by the gold-standard hyperglycaemic clamp, 12 months after initiation of treatment and again at 15 months, after 3 months of washout. Participants (aged 10–19 years) had prediabetes (60%) or type 2 diabetes diagnosed within the last 6 months (40%). By 12 months, beta cell function declined significantly compared with baseline in both groups, without any significant treatment differences [15]. Transient reductions in HbA1c were observed in both groups, though HbA1c increased back to baseline by 12 months. There was no effect of treatment on fasting or 2 h OGTT glucose at 12 or 15 months. Thus, the RISE study extended the findings of the TODAY study to show that insulin and metformin were ineffective in preventing beta cell deterioration in youth with prediabetes or type 2 diabetes, even when initiated early in the disease course.
The RISE study protocols were the first to allow for direct phenotypic comparison of adults and youth with prediabetes or type 2 diabetes. At baseline, clamp-based insulin sensitivity was 46% lower in youth than in adults, and youth had higher acute, steady-state and maximal C-peptide and insulin responses. Despite initially robust beta cell response, youth showed beta cell decline during first 12 months, whereas adults treated with metformin or insulin showed improvement in beta cell response and HbA1c, as well as weight loss, during that time period. Taken together, the RISE study demonstrates a different phenotype in youth vs adults at type 2 diabetes onset, with greater insulin resistance and higher beta cell response; further, youth did not experience improvements with the only two US Food and Drug Administration-approved treatments at the time of the study.
SEARCH study
SEARCH is a large epidemiological study designed to describe the incidence and prevalence of diabetes in the USA. It also includes longitudinal cohorts to better characterise youth with type 1 and type 2 diabetes. Thus, results from the SEARCH study are more representative of real-world experience with youth-onset type 2 diabetes. In line with the TODAY study, baseline cross-sectional analysis in the SEARCH study (2002–2005) revealed that more than 50% of youth with a type 2 diabetes duration of at least 2 years had poor glycaemic control (HbA1c > 63.9 mmol/mol [>8%]) [27]. Most participants were treated with lifestyle intervention, metformin, insulin or a combination of these. After a mean of 7 years of follow-up, a significant proportion of participants changed their treatment regimen, adding or taking away metformin and/or insulin. A few took alternative glucose-lowering drugs, primarily thiazolidinediones or sulfonylureas, and only 35% were meeting glycaemic targets (HbA1c < 53.0 mmol/mol [<7%]) [28]. It is important to note that this follow-up time period occurred prior to publication of the TODAY study results. However, registry data from the US Pediatric Diabetes Consortium suggest that very few youth were treated with agents other than insulin and metformin in paediatric specialty centres in the years following the TODAY study, despite inadequate glycaemic control in >54% and failure of the TODAY study to show improvement in blood glucose levels after adding insulin. Together, the results of these studies demonstrate that new approaches and therapies are required for youth-onset type 2 diabetes, potentially including bariatric surgery, which appears to be more effective than the treatments used in the TODAY study [29].
Comorbidities and complications
Most of what is known about the prospective evolution of comorbidities and complications in youth-onset type 2 diabetes comes from the TODAY (and its observational extensions) and SEARCH studies. Both studies demonstrate that comorbidities and complications are common, even within 2 years of diagnosis. Data from the SEARCH study estimate that 72% of youth with type 2 diabetes experience at least one comorbidity or complication by early adulthood [30].
Microvascular complications
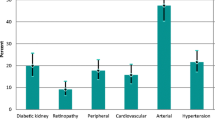
Microvascular complications, including diabetic kidney disease, retinopathy and neuropathy, have been described during adolescence in both the TODAY and SEARCH studies. These studies suggest a frequency of elevated albumin excretion in youth with recently diagnosed type 2 diabetes of 6.3–7.8%, increasing to 18.2–16.6% in early adulthood [31, 32]. Hyperfiltration, an early marker of risk for chronic kidney disease, increased in the TODAY study participants from 7% at baseline to 13.3% by 5 years [33]. In the TODAY and SEARCH studies, retinopathy was assessed using retinal photography and was found to be prevalent in 13.7% and 9.1%, respectively [30, 34]. In the TODAY study, retinopathy was associated with older age, longer duration of diabetes and poorer glycaemic control [34]. Early-onset retinopathy and nephropathy have also been described in Pima Indians, particularly compared with those with adult-onset type 2 diabetes [35]. Finally, peripheral neuropathy, as assessed by the Michigan Neuropathy Screening Inventory Examination, was present in 17.7% of SEARCH study participants [29]. Furthermore, data from the SEARCH study [30] and others [12, 36,37,38,39,40] show that these three microvascular complications are more common in youth with type 2 diabetes than in those with type 1 diabetes, despite a shorter disease duration on average in those with type 2 diabetes, even after adjusting for obesity, blood pressure and glycaemic control. It is important to note that these complication data were collected during the initial treatment phase of the TODAY study, whereas, in the SEARCH study, they were collected during the follow-up portion of the study, by which time participants were all late adolescents or young adults. Early-onset microvascular complications have also been described in population-based studies in relation to youth-onset diabetes in Manitoba (Canada) and India [41] and in young-onset diabetes in China [37] and Australia [42]. More information is needed on the early evolution of these complications as they relate to diabetes treatment in youth-onset type 2 diabetes in other cultural and genetic backgrounds.
Cardiovascular complications
Early cardiovascular disease risk markers also appear to be prevalent early in the disease course of paediatric type 2 diabetes, including hypertension, dyslipidaemia, vascular and cardiac dysfunction and cardiac structural abnormalities. At baseline, more than 25% of youth with type 2 diabetes had blood pressure above the 90th percentile in the TODAY [43] and SEARCH [44] studies. In addition, in the TODAY study, 11.6% of participants were hypertensive (blood pressure ≥ 130/80 or ≥95th percentile) at baseline, increasing to 33.8% after a mean of 3.9 years of follow-up [31]; male sex and higher BMI increased the risk of hypertension. Other cardiovascular disease risk markers were also common in youth-onset type 2 diabetes in the TODAY and SEARCH studies. Low HDL-cholesterol was most common, affecting 44–80% of youth, whereas 10–42% had elevated triacylglycerols and 5–14% had elevated LDL-cholesterol [43, 45]. During follow-up in the TODAY study, the prevalence of elevated LDL-cholesterol more than doubled over time (between baseline and end of study) [46]. In the TODAY study, echocardiograms were performed during the last year of the randomised trial, at a median of 4.5 years after diagnosis, and again several years later during observational follow-up. Initial findings included high/normal mean left ventricular mass and adverse left ventricular geometry in 16.2% [47]; higher left ventricular mass was positively associated with male sex, non-Hispanic Black race/ethnicity, BMI, systolic blood pressure, antihypertensive medications, blood glucose levels and smoking, and was inversely related to heart rate. The follow-up echocardiograms demonstrated reduced ejection fraction (<52%) in 11.7% of male TODAY study participants and a higher frequency of cardiac structural abnormalities, such as left ventricular hypertrophy, was seen in the TODAY participants (15.8%) as compared with age, race/ethnicity and sex-matched control individuals with similar BMI (5.7%) and participants with normal weight (0%) [48]. At a mean type 2 diabetes duration of 7.6 ± 1.5 years, arterial stiffness was identified in up to 50% of TODAY study participants, correlating with older age, race/ethnicity, female sex, higher HbA1c, blood pressure and BMI [49]. Moreover, 7 years after randomisation, the TODAY study participants showed, on average, reduced heart rate variability with parasympathetic loss and sympathetic overdrive vs control participants; cardiac autonomic dysfunction was present in 8% of the TODAY participants and correlated with higher HbA1c [50]. The SEARCH study additionally described a high prevalence of cardiovascular autonomic neuropathy (15.7%) and arterial stiffness (47.4%), after a mean diabetes duration of 7.9 years; arterial stiffness was more common than in youth with type 1 diabetes (11.6%, p < 0.001) [30]. Thus, cardiovascular disease risk is prevalent early in the course of youth-onset type 2 diabetes and appears to be worse than in peers with type 1 diabetes and peers with obesity but without diabetes. This is particularly foreboding in terms of early-onset cardiovascular events and the potential impact on healthcare costs and quality of life. Since these and other complications are directly related to glycaemic control and insulin resistance, it is critical to develop more effective treatments for diabetes and obesity in these youth.
Psychosocial comorbidities
Psychosocial comorbidities were also relatively common in the TODAY study, including binge eating behaviours (26%), clinically significant depressive symptoms (14.8%) and exposure to stressful life events (67%) [51, 52]. Furthermore, while little is known about diabetes distress (negative feelings that are specifically related to having and treating diabetes) in youth-onset type 2 diabetes, diabetes distress is known to be common in youth with type 1 diabetes [53] and in adults with type 2 diabetes [54]. These psychosocial comorbidities may substantially contribute to challenges in maintaining glycaemic control in youth-onset type 2 diabetes.
Future directions and clinical trial opportunities
Current data demonstrate that the therapies most widely used for treatment of youth-onset type 2 diabetes are inadequate for maintaining glycaemic control and preventing diabetes complications. Newer agents, such as SGLT2 inhibitors and GLP-1 agonists, have been shown to be clinically effective and safe, and to have additional benefits of providing modest weight loss and cardiorenal protection in adults; however, there are many barriers to completing trials of these agents in youth [22]. Furthermore, the fact that glycaemic control did not improve even after initiation of insulin in the TODAY study suggests that barriers to treatment and adherence also play a significant role in youth-onset diabetes. Future trials should also examine the effect of treating psychological comorbidities in youth with type 2 diabetes, who almost universally have undertreated psychosocial barriers to treatment [55], which can lead to non-adherence [51]. In the TODAY study, 14.8% of participants reported clinically significant depressive symptoms at baseline, although a recent review suggests that the prevalence of elevated depressive symptoms in youth-onset type 2 diabetes may be closer to 20% [56]. TODAY study participants with lower medication adherence were more likely to have clinically significant depression at baseline [57]; however, depressive symptoms were not related to glycaemic control [58]. Similarly, in SEARCH study participants with type 2 diabetes, baseline depression and changes in depressive symptoms were not associated with HbA1c; however, decreases in diabetes-specific quality of life predicted higher HbA1c, suggesting that youth with type 2 diabetes need effective coping and problem-solving skills [59]. Depression and diabetes distress may have effects on blood glucose through factors such as disordered eating and poor sleep behaviours, or through interactions with stress physiology, and these relationships may be bidirectional [56].
Treatment of youth-onset type 2 diabetes likely requires multicomponent, biobehavioural interventions, as well as the more recently available treatments for type 2 diabetes, which have been shown to be safe and effective in adults. Future trials need to individualise treatment randomisation, considering medical, demographic and psychosocial factors, in order to maximise potential benefit. For example, an individual with needle phobia may do better on a daily SGLT2 inhibitor than a weekly injectable GLP-1 agonist. On the other hand, a patient who struggles to have daily insulin injections and, thus, who is at greater risk for diabetic ketoacidosis (DKA) and less likely to monitor ketones, is probably not a good candidate for an SGLT2 inhibitor, given the potential adverse effect of euglycaemic DKA with these agents. Furthermore, there is evidence that race, ethnicity and genetic background influence response to treatment, but little is known about these factors in youth, particularly given the lack of clinical trials in youth-onset type 2 diabetes outside the USA. Finally, in addition to the impact of depression on diabetes care behaviours, there is also evidence that mood disorders may exacerbate insulin resistance and, thus, response to treatment [56]. Therefore, depressed mood needs to be addressed as a component of the treatment. Importantly, as was nicely outlined in the consensus statement by Nadeau et al. [22], the numbers of youth affected by type 2 diabetes are too limited to study each agent individually, as was done in adults with type 2 diabetes to obtain the clinical treatment indication for newer glucose-lowering agents.
For these reasons, the multiphase optimisation strategy (MOST) [60] and sequential multiple-assignment trials (SMARTs) [61] may be useful in developing and testing new treatment approaches. MOST is a framework for optimising and evaluating multicomponent, biobehavioural interventions, while a SMART is a trial aimed at building personalised, adaptive interventions that identify tailoring variables indicating the need for treatment intensification or modification. SMART designs are particularly useful in settings with pathophysiological heterogeneity, as is the case in youth-onset type 2 diabetes, allowing treatment decisions to be individualised. The factorial or fractional factorial design used in SMARTs is efficient, ideal for relatively uncommon diseases, can be used to evaluate interactions between treatments, and asks participants to implement fewer intervention components at the same time, thus improving adherence and retention. Particularly relevant to youth-onset type 2 diabetes, SMART designs are ideal when there is a need to balance efficacy with treatment burden, when adherence is challenging, and if comorbidities need to be considered in treatment algorithms. Figure 1 provides an example of a potential SMART design.
Schematic of a possible SMART design trial for youth-onset type 2 diabetes. The figure shows an example of a SMART design for a trial enrolling adolescents with type 2 diabetes treated with metformin ± insulin, positive for depressive or diabetes distress symptoms, and HbA1c > 47.5 mmol/mol (>6.5%). Interpersonal therapy (IPT) is effective for treatment of depression [62] and is particularly suitable for individuals from historically disadvantaged racial/ethnic groups [63]. Add-on pharmacological therapy (Add pharmaRx) involves the addition of a newer diabetes treatment agent (e.g., GLP-1 agonist, SGLT2 inhibitor), individualised based on patient characteristics. Mindfulness-based therapy (MBT) has been shown to decrease depression and may particularly target stress-related behaviours that undermine adherence and worsen outcomes in youth-onset type 2 diabetes [64, 65]. This figure is available as a downloadable slide
In summary, youth-onset diabetes is associated with inadequate glycaemic control and early-onset of complications in most individuals, resulting in early morbidity and mortality. Classic randomised trial designs, which fail to address the heterogeneity and psychosocial components of this disease, are unlikely to be effective. Thus, innovative clinical trials are needed to improve the treatment of youth-onset diabetes.
Change history
26 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00125-021-05518-z
Abbreviations
- ADOPT:
-
A Diabetes Outcome Progression Trial
- DKA:
-
Diabetic ketoacidosis
- GLP-1:
-
Glucagon-like peptide-1
- MOST:
-
Multiphase optimisation strategy
- RISE:
-
Restoring Insulin SEcretion
- SEARCH:
-
SEARCH for Diabetes in Youth
- SGLT2:
-
Sodium–glucose cotransporter 2
- SMART:
-
Sequential multiple-assignment trial
- TODAY:
-
Treatment Options for Type 2 Diabetes in Adolescents and Youth
References
Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH (2018) Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med 35(6):737–744. https://doi.org/10.1111/dme.13609
Al-Kandari H, Al-Abdulrazzaq D, Davidsson L et al (2019) Incidence of type 2 diabetes in Kuwaiti children and adolescents: results from the Childhood-Onset Diabetes Electronic Registry (CODeR). Front Endocrinol 10:836. https://doi.org/10.3389/fendo.2019.00836
Amed S, Islam N, Sutherland J, Reimer K (2018) Incidence and prevalence trends of youth-onset type 2 diabetes in a cohort of Canadian youth: 2002-2013. Pediatr Diabetes 19(4):630–636. https://doi.org/10.1111/pedi.12631
Alyafei F, Soliman A, Alkhalaf F et al (2018) Incidence of type 1 and type 2 diabetes, between 2012-2016, among children and adolescents in Qatar. Acta Biomed 89(S5):7–10. https://doi.org/10.23750/abm.v89iS4.7360
Mayer-Davis EJ, Lawrence JM, Dabelea D et al (2017) Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 376(15):1419–1429. https://doi.org/10.1056/NEJMoa1610187
Wu H, Zhong J, Yu M et al (2017) Incidence and time trends of type 2 diabetes mellitus in youth aged 5-19 years: a population-based registry in Zhejiang, China, 2007 to 2013. BMC Pediatr 17(1):85. https://doi.org/10.1186/s12887-017-0834-8
Shah AS, Nadeau KJ (2020) The changing face of paediatric diabetes. Diabetologia 63(4):683–691. https://doi.org/10.1007/s00125-019-05075-6
World Health Oranization (2021) Commission on Ending Childhood Obesity. Available from: www.who.int/end-childhood-obesity/en/. Accessed: 29 April 2021
Fazeli Farsani S, van der Aa MP, van der Vorst MM, Knibbe CA, de Boer A (2013) Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia 56(7):1471–1488. https://doi.org/10.1007/s00125-013-2915-z
Waernbaum I, Blohme G, Ostman J et al (2006) Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 49(4):653–659. https://doi.org/10.1007/s00125-005-0135-x
Constantino MI, Molyneaux L, Limacher-Gisler F et al (2013) Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 36(12):3863–3869. https://doi.org/10.2337/dc12-2455
Eppens MC, Craig ME, Cusumano J et al (2006) Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 29(6):1300–1306. https://doi.org/10.2337/dc05-2470
Gottschalk M, Danne T, Vlajnic A, Cara JF (2007) Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care 30(4):790–794. https://doi.org/10.2337/dc06-1554
Zeitler P, Hirst K, Pyle L et al (2012) A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366(24):2247–2256. https://doi.org/10.1056/NEJMoa1109333
RISE Consortium (2018) Impact of insulin and metformin versus metformin alone on beta-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 41(8):1717–1725. https://doi.org/10.2337/dc18-0787
Matsuura N, Amemiya S, Sugihara S et al (2019) Metformin monotherapy in children and adolescents with type 2 diabetes mellitus in Japan. Diabetol Int 10(1):51–57. https://doi.org/10.1007/s13340-018-0361-3
Tamborlane WV, Barrientos-Perez M, Fainberg U et al (2019) Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 381(7):637–646. https://doi.org/10.1056/NEJMoa1903822
Zeitler P, Epstein L, Grey M et al (2007) Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 8(2):74–87. https://doi.org/10.1111/j.1399-5448.2007.00237.x
Laffel L, Chang N, Grey M et al (2012) Metformin monotherapy in youth with recent onset type 2 diabetes:experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 13(5):369–375. https://doi.org/10.1111/j.1399-5448.2011.00846.x
Kelsey MM, Geffner ME, Guandalini C et al (2016) Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes 17(3):212–221. https://doi.org/10.1111/pedi.12264
TODAY Study Group (2013) Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care 36(6):1749–1757. https://doi.org/10.2337/dc12-2393
Nadeau KJ, Anderson BJ, Berg EG et al (2016) Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 39(9):1635–1642. https://doi.org/10.2337/dc16-1066
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355(23):2427–2443. https://doi.org/10.1056/NEJMoa066224
Zeitler P, Hirst K, Copeland KC et al (2015) HbA1c after a short period of monotherapy with metformin identifies durable glycemic control among adolescents with type 2 diabetes. Diabetes Care 38(12):2285–2292. https://doi.org/10.2337/dc15-0848
Bacha F, El Ghormli L, Arslanian S et al (2019) Predictors of response to insulin therapy in youth with poorly-controlled type 2 diabetes in the TODAY trial. Pediatr Diabetes 20(7):871–879. https://doi.org/10.1111/pedi.12906
RISE Consortium (2014) Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 37(3):780–788. https://doi.org/10.2337/dc13-1879
Badaru A, Klingensmith GJ, Dabelea D et al (2014) Correlates of treatment patterns among youth with type 2 diabetes. Diabetes Care 37(1):64–72. https://doi.org/10.2337/dc13-1124
Pinto CA, Stafford JM, Wang T et al (2018) Changes in diabetes medication regimens and glycemic control in adolescents and young adults with youth-onset type 2 diabetes: the SEARCH for diabetes in youth study. Pediatr Diabetes 19(6):1065–1072. https://doi.org/10.1111/pedi.12691
Inge TH, Laffel LM, Jenkins TM et al (2018) Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 172(5):452–460. https://doi.org/10.1001/jamapediatrics.2017.5763
Dabelea D, Stafford JM, Mayer-Davis EJ et al (2017) Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 317(8):825–835. https://doi.org/10.1001/jama.2017.0686
TODAY Study Group (2013) Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 36(6):1735–1741. https://doi.org/10.2337/dc12-2420
Kahkoska AR, Isom S, Divers J et al (2018) The early natural history of albuminuria in young adults with youth-onset type 1 and type 2 diabetes. J Diabetes Complicat 32(12):1160–1168. https://doi.org/10.1016/j.jdiacomp.2018.09.018
Bjornstad P, Nehus E, El Ghormli L et al (2018) Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis 71(1):65–74. https://doi.org/10.1053/j.ajkd.2017.07.015
TODAY Study Group (2013) Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care 36(6):1772–1774. https://doi.org/10.2337/dc12-2387
Krakoff J, Lindsay RS, Looker HC, Nelson RG, Hanson RL, Knowler WC (2003) Incidence of retinopathy and nephropathy in youth-onset compared with adult-onset type 2 diabetes. Diabetes Care 26(1):76–81. https://doi.org/10.2337/diacare.26.1.76
Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA (2014) Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 37(2):436–443. https://doi.org/10.2337/dc13-0954
Luk AO, Lau ES, So WY et al (2014) Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care 37(1):149–157. https://doi.org/10.2337/dc13-1336
McGrath NM, Parker GN, Dawson P (1999) Early presentation of type 2 diabetes mellitus in young New Zealand Maori. Diabetes Res Clin Pract 43(3):205–209. https://doi.org/10.1016/s0168-8227(99)00003-0
Scott A, Toomath R, Bouchier D et al (2006) First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J 119(1235):U2015
Yokoyama H, Okudaira M, Otani T et al (2000) Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int 58(1):302–311. https://doi.org/10.1046/j.1523-1755.2000.00166.x
Mohan V, Jaydip R, Deepa R (2007) Type 2 diabetes in Asian Indian youth. Pediatr Diabetes 8(Suppl 9):28–34. https://doi.org/10.1111/j.1399-5448.2007.00328.x
Wong J, Constantino M, Yue DK (2015) Morbidity and mortality in young-onset type 2 diabetes in comparison to type 1 diabetes: where are we now? Curr Diab Rep 15(1):566. https://doi.org/10.1007/s11892-014-0566-1
Copeland KC, Zeitler P, Geffner M et al (2011) Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 96(1):159–167. https://doi.org/10.1210/jc.2010-1642
Kim G, Divers J, Fino NF et al (2019) Trends in prevalence of cardiovascular risk factors from 2002 to 2012 among youth early in the course of type 1 and type 2 diabetes. The SEARCH for Diabetes in Youth Study. Pediatr Diabetes 20(6):693–701. https://doi.org/10.1111/pedi.12846
Kershnar AK, Daniels SR, Imperatore G et al (2006) Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 149(3):314–319. https://doi.org/10.1016/j.jpeds.2006.04.065
Tryggestad JB, Willi SM (2015) Complications and comorbidities of T2DM in adolescents: findings from the TODAY clinical trial. J Diabetes Complicat 29(2):307–312. https://doi.org/10.1016/j.jdiacomp.2014.10.009
Levitt Katz L, Gidding SS, Bacha F et al (2015) Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes 16(1):39–47. https://doi.org/10.1111/pedi.12119
TODAY Study Group (2020) Longitudinal changes in cardiac structure and function from adolescence to young adulthood in participants with type 2 diabetes mellitus: the TODAY follow-up study. Circ Heart Fail 13(6):e006685. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006685
Shah AS, El Ghormli L, Gidding SS et al (2018) Prevalence of arterial stiffness in adolescents with type 2 diabetes in the TODAY cohort: relationships to glycemic control and other risk factors. J Diabetes Complicat 32(8):740–745. https://doi.org/10.1016/j.jdiacomp.2018.05.013
Shah AS, El Ghormli L, Vajravelu ME et al (2019) Heart rate variability and cardiac autonomic dysfunction: prevalence, risk factors, and relationship to arterial stiffness in the treatment options for Type 2 Diabetes in Adolescents and Youth (TODAY) study. Diabetes Care 42(11):2143–2150. https://doi.org/10.2337/dc19-0993
Walders-Abramson N, Venditti EM, Ievers-Landis CE et al (2014) Relationships among stressful life events and physiological markers, treatment adherence, and psychosocial functioning among youth with type 2 diabetes. J Pediatr 165(3):504–508 e501. https://doi.org/10.1016/j.jpeds.2014.05.020
Wilfley D, Berkowitz R, Goebel-Fabbri A et al (2011) Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care 34(4):858–860. https://doi.org/10.2337/dc10-1704
Iturralde E, Rausch JR, Weissberg-Benchell J, Hood KK (2019) Diabetes-related emotional distress over time. Pediatrics 143(6):e20183011. https://doi.org/10.1542/peds.2018-3011
Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U (2008) A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med 25(9):1096–1101. https://doi.org/10.1111/j.1464-5491.2008.02533.x
Silverstein J, Cheng P, Ruedy KJ et al (2015) Depressive symptoms in youth with type 1 or type 2 diabetes: results of the pediatric diabetes consortium screening assessment of depression in diabetes study. Diabetes Care 38(12):2341–2343. https://doi.org/10.2337/dc15-0982
Gulley LD, Shomaker LB (2020) Depression in youth-onset type 2 diabetes. Curr Diab Rep 20(10):51. https://doi.org/10.1007/s11892-020-01334-8
Katz LL, Anderson BJ, McKay SV et al (2016) Correlates of medication adherence in the TODAY cohort of youth with type 2 diabetes. Diabetes Care 39(11):1956–1962. https://doi.org/10.2337/dc15-2296
Van Buren DJ, Wilfley DE, Marcus MD et al (2018) Depressive symptoms and glycemic control in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Res Clin Pract 135:85–87. https://doi.org/10.1016/j.diabres.2017.11.008
Hood KK, Beavers DP, Yi-Frazier J et al (2014) Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health 55(4):498–504. https://doi.org/10.1016/j.jadohealth.2014.03.011
Collins LM, Murphy SA, Nair VN, Strecher VJ (2005) A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med 30(1):65–73. https://doi.org/10.1207/s15324796abm3001_8
Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA (2014) Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med 4(3):260–274. https://doi.org/10.1007/s13142-014-0265-0
Eckshtain D, Kuppens S, Ugueto A et al (2020) Meta-analysis: 13-year follow-up of psychotherapy effects on youth depression. J Am Acad Child Adolesc Psychiatry 59(1):45–63. https://doi.org/10.1016/j.jaac.2019.04.002
Pu J, Zhou X, Liu L et al (2017) Efficacy and acceptability of interpersonal psychotherapy for depression in adolescents: a meta-analysis of randomized controlled trials. Psychiatry Res 253:226–232. https://doi.org/10.1016/j.psychres.2017.03.023
Chi X, Bo A, Liu T, Zhang P, Chi I (2018) Effects of mindfulness-based stress reduction on depression in adolescents and young adults: a systematic review and meta-analysis. Front Psychol 9:1034. https://doi.org/10.3389/fpsyg.2018.01034
Dunning DL, Griffiths K, Kuyken W et al (2019) Research review: The effects of mindfulness-based interventions on cognition and mental health in children and adolescents - a meta-analysis of randomized controlled trials. J Child Psychol Psychiatry 60(3):244–258. https://doi.org/10.1111/jcpp.12980
Acknowledgements
The authors would like to thank K. Nadeau (University of Colorado School of Medicine, Aurora, CO, USA) and L. Shomaker (Colorado State University, Fort Collins, CO, USA) for their input on the proposed clinical trial example.
Authors’ relationships and activities
MMK is currently a TODAY study investigator and LP was previously a TODAY study investigator. MMK is or has been a site investigator for clinical trials sponsored by Janssen, Merck, Daiichi-Sankyo and Boehringer-Ingelheim. She does not receive direct payments for this work and these trials are not discussed in the manuscript.
Funding
Work in the authors’ laboratory is supported by the America Diabetes Association (1-11-JF-23), the National Institutes of Health (NIH)/National Institute of Digestive, Diabetes, and Kidney Diseases (U01 DK061230-12, 1R01DK111-604-01A1, 1R01DK119450-01A1), and NIH/National Institute of Child Health and Human Development (5K12HD057022-04).
Author information
Authors and Affiliations
Contributions
Both authors were responsible for drafting the article and revising it critically for important intellectual content. Both authors approved the version to be published.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure slide
(PPTX 148 kb)
Rights and permissions
About this article
Cite this article
Pyle, L., Kelsey, M.M. Youth-onset type 2 diabetes: translating epidemiology into clinical trials. Diabetologia 64, 1709–1716 (2021). https://doi.org/10.1007/s00125-021-05480-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05480-w