Abstract
Aims/hypothesis
Angiotensin II is well-recognised to be a key mediator in driving the pathological events of diabetes-associated atherosclerosis via signalling through its angiotensin II type 1 receptor (AT1R) subtype. However, its actions via the angiotensin II type 2 receptor (AT2R) subtype are still poorly understood. This study is the first to investigate the role of the novel selective AT2R agonist, Compound 21 (C21) in an experimental model of diabetes-associated atherosclerosis (DAA).
Methods
Streptozotocin-induced diabetic Apoe-knockout mice were treated with vehicle (0.1 mol/l citrate buffer), C21 (1 mg/kg per day), candesartan cilexetil (4 mg/kg per day) or C21 + candesartan cilexetil over a 20 week period. In vitro models of DAA using human aortic endothelial cells and monocyte cultures treated with C21 were also performed. At the end of the experiments, assessment of plaque content and markers of oxidative stress, inflammation and fibrosis were conducted.
Results
C21 treatment significantly attenuated aortic plaque deposition in a mouse model of DAA in vivo, in association with a decreased infiltration of macrophages and mediators of inflammation, oxidative stress and fibrosis. On the other hand, combination therapy with C21 and candesartan (AT1R antagonist) appeared to have a limited additive effect in attenuating the pathology of DAA when compared with either treatment alone. Similarly, C21 was found to confer profound anti-atherosclerotic actions at the in vitro level, particularly in the setting of hyperglycaemia. Strikingly, these atheroprotective actions of C21 were completely blocked by the AT2R antagonist PD123319.
Conclusions/interpretation
Taken together, these findings provide novel mechanistic and potential therapeutic insights into C21 as a monotherapy agent against DAA.
Similar content being viewed by others
Introduction
Overactivation of the renin–angiotensin system (RAS) is well-recognised to be a major underlying pathological mechanism that drives the development of diabetic vascular disease, with its effector peptide, angiotensin II (Ang II), being the main mediator [1]. Pharmacological inhibition of the RAS by targeting either Ang II production with an angiotensin-converting enzyme inhibitor or the activity of Ang II with an angiotensin receptor blocker (ARB) remains first-line treatment for patients with diabetes and vascular disease [2]. However, it has been proposed that the beneficial effects of these treatments are out of proportion with the magnitude of blood pressure reduction, suggesting more direct effects on end organs, including the macrovasculature.
Although Ang II binds with similar affinity to both the AT1 and AT2 receptor subtypes [3], the relative importance of these receptors in modulating vascular injury remains poorly understood. Angiotensin II type 1 receptors (AT1Rs) are primarily involved in mediating the pathophysiological actions of Ang II, including its ability to regulate blood pressure, vasoconstriction and cellular differentiation and promote hypertrophy [4]. Strikingly, Ang II negatively regulates its classical actions through the angiotensin II type 2 receptors (AT2Rs) [5], with this receptor subtype being markedly upregulated in numerous diseased states, including atherosclerosis [6, 7], possibly to counterbalance the adverse effects mediated by the AT1R. Taken together, these actions highlight the potential of the AT2R as a key therapeutic target. With numerous studies focusing on the role of the AT2R in the non-diabetes setting [8–10], the vascular actions of this receptor in the context of diabetes still remains unclear. Given our previous findings that selective activation of the AT2R by a novel non-peptide agonist, Compound 21 (C21), significantly ameliorated the progression of nephropathy in an insulin-deficient model [11], we aimed to further investigate the protective role of C21 on macrovascular disease in this diabetes model. C21 is a highly specific AT2R agonist, with a Ki value of 0.4 nmol/l and >1000 nmol/l for the AT2R and AT1R, respectively [12]. This study is the first to delineate the vasoprotective role of C21 in an experimental model of streptozotocin (STZ)-induced insulin-deficient diabetes in vivo, followed by extension of the findings to explore potential underlying mechanisms in vitro.
Methods
Animals
Male apolipoprotein E-knockout (Apoe −/−) mice on a C57BL6/J background (Jackson Laboratory, Sacramento, CA, USA) were used throughout the study based on previous findings that the mice in this model develop accelerated DAA upon the induction of diabetes and so constitute an appropriate model of DAA [13]. Mice were housed and maintained on a 12 h light–dark cycle in a pathogen-free environment and had free access to water and rodent lab chow (Specialty Feeds, Glen Forrest, WA, Australia). Experiments were conducted in accordance with the Australian code of practice for the care and use of laboratory animals for scientific purposes.
In vivo experimental procedure
Seven-week-old Apoe −/− mice were rendered diabetic via a daily intraperitoneal injection of STZ (Sigma-Aldrich, St Louis, MO, USA) at a dose of 55 mg/kg per day over a period of 5 days. Animals with blood glucose and HbA1c levels greater than 15 mmol/l and 10% (85.8 mmol/mol), respectively, after 10 days post-STZ-administration, were included in the study as diabetic. Diabetic mice were randomised and treated with vehicle (0.1 mol/l citrate buffer), C21 (1 mg/kg per day; Vicore Pharma, Göteborg, Sweden), the AT1R antagonist candesartan cilexetil (4 mg/kg per day; AstraZeneca Södertälje, Sweden) or C21 + candesartan treatment, via daily gavaging, over a 20 week period. These concentrations of C21 and candesartan had previously been shown to successfully ameliorate diabetic nephropathy [11, 14]. Non-diabetic mice treated in the absence or presence of C21 were also studied. At the end of the study, mice were killed and aortic tissues were excised for analysis. For assessment of body weight, metabolic variables and blood pressure, mice were placed in individual metabolic cages (Iffa Credo, L’Arbresele, France) for a period of 24 h and performed as described [11].
Cell culture
The atherosclerotic properties of the AT2R were further investigated in human aortic endothelial cells (HAEC) and monocyte cultures (THP-1) (obtained from ATCC, Manassas, VA, USA). Experiments were performed three or four separate times in duplicate. Cells were regularly tested for mycoplasma contamination by the media services (Baker IDI Heart and Diabetes Institute, Australia).
HAECs were cultured in endothelial growth media supplemented with EGM-2 Bulletkit (Lonza, Allendale, NJ, USA). In all experiments, EGM-2 media containing 5.6 mmol/l glucose was used as the normal-glucose condition, while EGM-2 media containing 25 mmol/l glucose (Sigma-Aldrich) was used as high-glucose condition.
THP-1 cells were maintained in RPMI supplemented with 10% fetal calf serum, penicillin (50 U/ml) and streptomycin (50 μg/ml). To induce monocyte–macrophage differentiation, cells were treated with phorbol-12-myristate-13-acetate (1 μmol/l; Sigma-Aldrich) 3 days before subjecting the cells to the various treatments.
In vitro experimental procedure
To determine the optimal dose of C21, HAEC and THP-1 cultures (tested negative for mycoplasma contamination) were seeded into 12-well plates at equal density (1 × 105 to 1.5 × 105 cells/well) and treated with C21 (0.1–1.0 μmol/l) in both normal- (5 mmol/l) and high-glucose (25 mmol/l) conditions for 72 h. Additional studies were also conducted to determine the following: (1) whether the optimal dose at which C21 inhibited high-glucose-stimulated inflammatory and fibrotic markers was able to inhibit Ang II-mediated activity; (2) whether the ability of C21 to mediate this activity occurred specifically through the AT2R, by ascertaining whether C21-induced effects in these cells were blocked by the AT2R antagonist PD123319 (5 μmol/l; Cayman Chemical, Ann Arbour, MI, USA), (3) whether C21 as a combination therapy with an AT1R blocker, candesartan cilexetil (10 μmol/l) had any additive effect in attenuating DAA and (4) whether the candesartan cilexetil-mediated effects were altered by PD123319. At the end of each experiment, RNA and protein were extracted from the cell layer with Trizol reagent (Life Technologies, Rockville, MD, USA) for subsequent analysis.
Evaluation of atherosclerotic plaque size and content
Plaque area was quantified in an en face manner, as described previously [15]. Total and segmental plaque areas were quantified as a percentage area of aorta stained (Adobe Photoshop, version 7.0; Adobe Systems, Chatswood, NSW, Australia).
Frozen sections of aortic sinus were stained with Oil Red O solution (Sigma-Aldrich) for 1 h followed by counterstaining with haematoxylin. Measurement of necrotic cores was subsequently quantified as described previously [16]. Results were expressed as area of necrotic core per lesion.
Immunohistochemistry
Paraffin sections of aorta were stained for vascular cell adhesion molecule-1 (VCAM-1; 1:250 dilution; BD Pharmingen, San Diego, CA, USA), as described previously [17]. Positive-stained sections were quantified using Image-Pro Analyser 7.0 (Media Cybernetics, Bethesda, MD, USA) and stained areas were expressed as either stained area (μm2) or percentage of the total plaque area.
Quantitative real-time PCR analysis
Expression of genes encoding several markers of DAA were analysed by quantitative real-time PCR (qPCR) using the Taqman System on an ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA, USA) as described previously [11]. Gene expression was normalised to 18S mRNA and reported as relative ratio to the control group (either the non-diabetic mice or normal-glucose-treated cells), which was assigned an arbitrary value of 1.
Western blotting
Proteins (10 μg) were electrophoresed on 10.5% acrylamide gels under reducing conditions. Western blot analyses were performed with primary antibodies to either AT2R (H-143, 1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or AT1R (N-10, 1:1,000 dilution; Santa Cruz) and assessed with appropriate secondary antibodies. The housekeeping protein, α-tubulin (Sigma-Aldrich) was included to demonstrate equal loading of protein samples. Blots were detected using an enhanced chemiluminescent detection kit (Sigma-Aldrich) followed by quantification by densitometry using Quantity-One 4.5.2 software (Bio-Rad Laboratories, Richmond, CA, USA).
Statistical analysis
Data were analysed by one-way ANOVA using SPSS 20.0 software (IBM, St Leonards, NSW, Australia). Post hoc comparisons were performed among the various groups using Fisher’s least significant difference method. Data are expressed as mean ± SEM, with p < 0.05 considered as statistically significant.
Results
Metabolic variables
STZ-treated Apoe −/− mice developed sustained hyperglycaemia over the 20 week experimental period with elevated blood glucose and HbA1c levels (p < 0.01 vs non-diabetic mice). Diabetic mice showed reduced body weight and elevated levels of total plasma cholesterol and triacylglycerol (Table 1). Neither treatment with C21 alone nor candesartan alone affected any of these variables in either non-diabetic (control) or diabetic mice. While C21 did not have any systemic haemodynamic effect in either normoglycaemic or hyperglycaemic groups, administration of candesartan alone and in combination with C21 significantly reduced blood pressure in diabetic mice.
Atherosclerotic plaque area
En face analysis of the whole aorta revealed a twofold increase in plaque area in diabetic mice compared with their non-diabetic counterparts (p < 0.05) (Fig. 1). Similar findings were also observed in the aortic arch and thoracic and abdominal segments. C21-treatment significantly reduced atherosclerotic lesion area in diabetic mice (p < 0.05 vs diabetic mice). While a similar reduction was seen with candesartan treatment alone, there was no further decrease seen in diabetic mice co-treated with C21 and candesartan. Importantly, C21 had no effect in modulating plaque lesion in non-diabetic mice.
Atherosclerotic lesions in mouse aorta stained with Oil Red O and counterstained with haematoxylin (a) and total atherosclerotic plaque area (as percentage area of aorta stained) in the whole aorta (b) and within the arch (c) and thoracic (d) and abdominal (e) aortic segments. Magnification ×4.2. Data are expressed as the mean ± SEM of n = 6–13 mice per group. **p < 0.01 vs vehicle-treated control (non-diabetic) group; † p < 0.05 and †† p < 0.01 vs diabetic group. Cand, candesartan; CTL, control; Diab, diabetic
Sinus plaque content
Diabetic mice demonstrated a twofold increase in lipid deposition compared with their non-diabetic counterparts (Fig. 2a; p < 0.01). This accumulation of lipid within the atherosclerotic plaque was ameliorated by treatment with either C21 or candesartan alone (both p < 0.05 vs diabetic mice) and by co-treatment with C21 and candesartan when compared with diabetic mice (p < 0.01 vs diabetic mice). The combination treatment showed a trend towards a further modest reduction in plaque content when compared with either treatment alone (p = 0.39 vs diabetic mice treated with C21; p = 0.05 vs diabetic mice treated with candesartan alone). However, C21 had no effect in modulating lipid content within the intimal layer of the aortic sinus in non-diabetic mice.
(a) Lipid content within the atherosclerotic plaque in mouse aorta. (b) CD68 staining. Magnification ×4.2. Data are expressed as the mean ± SEM of 5–11 mice per group. **p < 0.01 vs vehicle-treated control (non-diabetic) group; † p < 0.05 and †† p < 0.01 vs diabetic group. Cand, candesartan; CTL, control; Diab, diabetic
Macrophage infiltration
Immunohistochemically stained aortic sections from diabetic mice demonstrated a significant increase in CD68 protein expression (Fig. 2b; p < 0.01 vs non-diabetic mice). Neither C21 nor candesartan treatment inhibited diabetes-induced macrophage infiltration.
Aortic expression of AT2R and AT1R
qPCR analysis performed on mouse aorta confirmed the expression of both AT2Rs and AT1Rs. mRNA levels of At 2 r (also known as Agtr2) were significantly upregulated in diabetic mice (p < 0.05 vs non-diabetic mice; Fig. 3a) but were not modulated by C21, candesartan or C21 + candesartan treatment. In contrast, no significant difference in At 1 r (also known as Agtr1) gene expression was observed when comparing the non-diabetic and diabetic mouse groups (Fig. 3b). Moreover, At 1 r expression remained unchanged in all treatment groups.
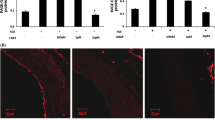
(a–e) At 2 r (a), At 1 r (b) and Vcam-1 (c) mRNA levels in mouse aorta; also shown are the representative photomicrographs (magnification ×45) of VCAM-1-stained aortic sinus (d). (e–i) Aortic mRNA levels of p47phox (e), Nox1 (f), Nox4 (g), Mcp-1 (h) and Tgf-β (i). Data are shown as the mean ± SEM of 5–12 mice per group. mRNA levels were normalised to 18S mRNA and reported as relative ratio to the control group. *p < 0.05 and **p < 0.01 vs vehicle-treated control (non-diabetic); † p < 0.05 and †† p < 0.01 vs diabetic group. Cand, candesartan; CTL, control; Diab, diabetic
Aortic expression of the adhesion marker VCAM-1
With the adhesion of monocytes to endothelial cells considered a critical step in the initiation and development of DAA, VCAM-1 expression was examined at both the gene and protein level. qPCR analysis revealed a significant upregulation in Vcam-1 (also known as Vcam1) gene expression in diabetic mice (p < 0.01 vs non-diabetic mice, Fig. 3c). While C21 treatment appeared to induce a trend towards a reduction in Vcam-1 gene expression in diabetic mice, this AT2R agonist significantly inhibited diabetes-induced VCAM-1 expression at the protein level (p < 0.05 vs diabetic mice; Fig. 3d). Conversely, a significant reduction in Vcam-1 mRNA and VCAM-1 protein expression was observed in diabetic mice treated with candesartan alone and in combination with C21. Nonetheless, both qPCR and immunohistochemical analyses demonstrated that combination therapy with C21 and candesartan produced no additive effect in further reducing diabetes-induced VCAM-1 expression, when compared with either treatment alone. Importantly, C21 had no effect in modulating VCAM-1 expression at the gene or protein level in non-diabetic mice.
Aortic expression of markers of oxidative stress, inflammation and fibrosis
Expression of p47phox, NADPH oxidase (NOX)-1 and NOX-4 was determined as part of the assessment of oxidative stress, while that of monocyte-chemoattractant protein-1 (MCP-1) and TGF-β1 was examined to assess inflammatory and fibrotic pathways. Treatment with either C21 or candesartan significantly ameliorated diabetes-induced p47phox (also known as Ncf1), Nox1, Mcp-1 (Ccl2) and Tgf-β1 (Tgfb1) mRNA levels (p < 0.05 vs diabetic mice; Fig. 3e, f, h, i); with Nox4 expression this amelioration was produced by only C21 and not candesartan (Fig. 3g). Co-administration of C21 and candesartan markedly inhibited the expression of all the analysed markers in diabetic mice (p < 0.05 vs diabetic mice) while again no synergistic effect was seen when compared with either therapy alone. Importantly, C21 did not influence any of the analysed markers in non-diabetic mice.
In vitro effect of C21 on high-glucose- and Ang II-stimulated cells
Consistent with our in vivo data, qPCR analysis of mRNA showed that markers of inflammation (ICAM-1 [ICAM1]), oxidative stress (NOX-4) and fibrosis (TGF-β, CTGF) were upregulated in hyperglycaemic conditions in both HAEC and THP-1 cells (p < 0.05 vs normal-glucose-treated cells) (Fig. 4). Administration of C21 significantly inhibited the upregulation of these high-glucose-induced markers dose dependently, being most effective at a concentration of 1 μmol/l in both cell populations. Moreover, C21 at this dose significantly inhibited Ang II-induced effects (Figs 5, 6). Strikingly, these protective effects of C21 were completely abolished by the AT2R antagonist, PD123319 in both cell types (Figs 5, 6). While C21 (1 μmol/l) had similar efficacy to that of candesartan (10 μmol/l), co-administration of C21 and candesartan produced no additive effect in inhibiting high-glucose- and Ang-II-induced ICAM-1, NOX-4, TGF-β and Ctgf expression (Figs 5, 6). Based on the similar efficacy of candesartan and C21 in attenuating the pathology of DAA, further delineation of the involvement of the AT2R with respect to the effects of the ARB were further studied in HAEC. Interestingly, candesartan-mediated protective effects were blocked by the AT2R antagonist, PD123319 (Fig. 5).
ICAM-1, NOX4, TGF-β and CTGF mRNA levels from HAEC (a) and THP-1 cells (b) cultured in either normal (5 mmol/l) or high (25 mmol/l) glucose conditions. Cells were treated with C21 (0.1–1.0 μmol/l); mannitol (25 mmol/l)-treated cells served as osmotic control. Data are shown as the mean ± SEM; n = 3 or 4. mRNA levels were normalised to 18S mRNA and reported as relative ratio to the normal glucose group. *p < 0.05 and **p < 0.01 vs normal-glucose-treated cells; † p < 0.05 and †† p < 0.01 vs high-glucose-treated cells
ICAM-1, NOX4, TGF-β and CTGF mRNA levels from HAEC cells cultured in either normal (5 mmol/l) or high (25 mmol/l) glucose conditions stimulated in the absence (a) or presence (b) of AngII (3 μmol/l) and treated with either C21 (1 μmol/l), PD123319 (5 μmol/l), candesartan (10 μmol/l), candesartan+PD123319, C21+PD123319 or C21+candesartan. Cells treated with mannitol (25 mmol/l) served as osmotic control. Data are shown as the mean ± SEM; n = 3–10. mRNA levels were normalised to 18S mRNA and reported as relative ratio to the normal glucose group. *p < 0.05 and **p < 0.01 vs normal glucose (NG); † p < 0.05 and †† p < 0.01 vs high glucose (HG); ‡‡ p < 0.01 vs HG+C21; § p < 0.05 and §§ p < 0.01 vs HG+candesartan; ¶¶ p < 0.01 vs NG+AngII; Φ p < 0.05 and ΦΦ p < 0.01 vs NG+AngII+C21; Ψ p < 0.05 and ΨΨ p < 0.01 HG+AngII, ∂ p < 0.05 and ∂∂ p < 0.01 vs HG+AngII+C21, Ω p < 0.05 vs HG+AngII+candesartan
ICAM-1, NOX4, TGF-β and CTGF mRNA levels from THP-1 cells cultured in normal (5 mmol/l) or high (25 mmol/l) glucose conditions stimulated in the absence (a) or presence (b) of AngII (3 μmol/l) and treated with either C21 (1 μmol/l), PD123319 (5 μmol/l), candesartan (10 μmol/l), C21 + PD123319 or C21+candesartan. Cells treated with mannitol (25 mmol/l) served as osmotic control. Data are shown as the mean ± SEM; n = 3–10. mRNA levels were normalised to 18S mRNA and reported as relative ratio to the normal glucose group. **p < 0.01 vs normal glucose (NG); † p < 0.05 and †† p < 0.01 vs high glucose (HG); ‡‡ p < 0.01 vs HG+C21; ¶ p < 0.05 and ¶¶ p < 0.01 vs NG+AngII; ΦΦ p < 0.01 vs NG+AngII+C21; ΨΨ p < 0.01 HG+AngII; ∂∂ p < 0.01 vs HG+AngII+C21
In vitro expression of AT2R and AT1R
Western blot analysis confirmed the expression of both AT2R and AT1R in HAEC and THP-1 cells (Fig. 7). Although AT2R protein expression was significantly elevated in cells cultured in the high-glucose condition, neither C21 nor the AT2R and AT1R inhibitors (PD123319 and candesartan, respectively) had any effect in modulating AT2R expression. In contrast, AT1R expression remained unchanged in all conditions (Fig. 7). Importantly, both AT2R and AT1R protein expression remained unchanged in normal-glucose conditions (data not shown). Similar results were also observed at the gene level (electronic supplementary material [ESM] Fig. 1).
AT2R and AT1R protein levels from HAEC (a) and THP-1 cells (b) cultured in normal (5 mmol/l) and high (25 mmol/l) glucose conditions treated in the absence or presence of C21 (1 μmol/l), C21(1 μmol/l) + PD123319 (PD) (5 μmol/l), candesartan (10 μmol/l) or C21 + candesartan. α-Tubulin blots were used to demonstrate equivalent loading of protein samples. Data are the mean ± SEM absorbance (Abs) levels of AT2R and AT1R and are expressed relative to that of the normal glucose (NG)-treated cells; n = 3 or 4. **p < 0.01 vs NG
Discussion
AT2R activation is increasingly recognised to confer protective effects in numerous disease states including atherosclerosis [18, 19], which is considered to be the major contributor to premature mortality and morbidity. However, the biological effects mediated by the AT2R with respect to DAA have not been extensively delineated. This study is the first to exploit the novel non-peptide AT2R agonist C21 in investigating the role of AT2Rs in an experimental model of type 1 DAA.
At the in vivo level in mice, C21 was found to significantly reduce aortic plaque deposition and plaque size, in association with its ability to reduce expression of key mediators of inflammation, oxidative stress and fibrosis in the aorta. Indeed, C21 at a relatively low dose was found to retard the progression of DAA with similar efficacy to that afforded by the AT1R antagonist candesartan, when continuously administered over a 20 week treatment period. Interestingly, co-administration of C21 and candesartan had no clear-cut additive effect in attenuating DAA when compared with either treatment alone. Nevertheless, these findings support the potential role for C21 in monotherapy for providing protection against the progression of DAA by inhibiting inflammation, oxidative stress and fibrosis, with the AT2R agonist affording similar vasoprotective properties to the AT1R antagonist. Consistent with our findings, in an experimental model of myocardial infarction, C21 was demonstrated to improve cardiac function and reduce infarct size with similar efficacy to that observed with candesartan alone [20]. Our additional findings that C21 had no effect in influencing metabolic control or blood pressure in diabetic mice suggest that the beneficial effects seen with this agent do not occur via effects on glycaemic control or via a direct systemic haemodynamic action.
These protective effects of C21 were further explored in a more mechanistic manner at the in vitro level, in two human cell lines considered to be involved in the development of atherosclerosis—endothelial cells and macrophages [21, 22]. C21 was found to significantly ameliorate markers of oxidative stress, inflammation and fibrosis in both high-glucose- and Ang II-stimulated HAEC and monocytes cultures, with similar efficacy to that of candesartan treatment. Strikingly, these protective effects of C21 were completely abolished by the AT2R antagonist PD123319, thereby confirming the protective actions of C21 occur via the AT2R. Nevertheless, with limited understanding and knowledge of the signalling pathways of the AT2R, it remains to be fully elucidated as to how C21 confers its benefits in diabetes. Our in vitro and in vivo studies have demonstrated that these effects of C21 are not dependent on cell type or species and emphasise the central role of C21 as an anti-inflammatory, antioxidant and antifibrotic agent. This is consistent with previous studies demonstrating the efficacy of C21 as a reno-protective agent in halting the progression of insulin-deficient diabetic nephropathy [11, 23]. Thus, these observations strengthen the notion that the AT2R agonist C21 may represent a novel therapy for the treatment of diabetes-associated complications, including both micro- and macrovascular complications.
Given the wide tissue distribution of the AT2R [24], AT2R activation has been shown to be protective against numerous disease states including renal [25, 26], neuronal [27] and pancreatic injury [28]. However, the role of this receptor in the cardiovascular system still remains poorly understood. Previous studies have variously demonstrated that the AT2R could exert a protective [6, 29–33], detrimental [34] or neutral [35, 36] role in atherosclerosis. These conflicting reports on the atherosclerotic role of the AT2R may suggest that its actions are likely to be dependent on a number of factors. These include the severity of the disease, where the efficacy at which AT2R mediates its protective effects may be lost in severe chronic conditions [37], the absence or presence of hyperglycaemia [34, 38] and the particular animal strain examined in the study [5]. More recently, the diverse cardiac response associated with the AT2R has also been shown to be dependent on the transcription factors with which it interacts. The ability of the AT2R to bind and facilitate the translocation of promyelocytic leukaemia zinc finger transcription factor to the nucleus has been shown to be implicated in driving its hypertrophic activity in response to Ang II [39].
Nonetheless, findings in this study have demonstrated that C21-mediated AT2R activation is beneficial against DAA. Consistent with these end-organ protective effects, albeit in the non-diabetic setting, C21 has been shown previously to reduce myocardial fibrosis and vascular injury [8], improve ventricular function [20] and promote vasodilatation [40] in experimental models of cardiovascular disease. Although both the AT1R and AT2R were found to be expressed in the human monocytes and aortic endothelial cells used in this study, C21 did not have any marked effects on expression of either receptor. This is consistent with the view that the agonist most likely mediates its actions by influencing post-receptor signalling pathways. Indeed, recent studies have demonstrated that AT2R inhibits AT1R-mediated effects by targeting its downstream effectors, including TGF-β1 [41] and NOX [29], as well as its ability to activate vasoconstriction inhibiting factor, an endogenous co-factor of Ang II [42]. Moreover, with previous studies demonstrating that AT2R forms a heterodimer receptor complex with AT1R to block the signalling and mediated functions of the latter [43], it is possible that some of the effects of the AT2R agonist involves the complex interaction between the two Ang II receptor subtypes.
Based on our findings that C21 exhibits similar anti-atherogenic efficacy as candesartan and that the effects of candesartan could be inhibited in the presence of the AT2R antagonist PD123319, this could indicate that candesartan-mediated actions occur at least in part as a result of the unopposed action of the AT2R. Consistent with these findings are studies, albeit in the non-diabetic setting, where candesartan-mediated reduction of infarct size in pigs [44] and inhibition of cardiac remodelling in rats [45] were found to be dependent on AT2R activation. Furthermore, it is possible that AT1R blockade and AT2R agonism are acting through similar signalling pathways. Indeed, several studies have demonstrated that candesartan and C21 inhibit the mitogen-activated protein kinase pathway and signal through a nitric oxide-dependent pathway to mediate their cardioprotective effects [30, 46, 47].
Pilot studies have suggested that C21 can antagonise the thromboxane receptor (TxR) [48]. Since a TxR antagonist has been shown to reduce atherosclerosis in a similar diabetic apolipoprotein E model [49], one cannot exclude the possibility that the anti-atherosclerotic actions of C21 could involve the TxR. It remains to be determined whether this is a direct effect of C21 or involves an indirect action via the AT2R.
Future studies are warranted to further elucidate interactions between AT1R and AT2R, particularly in cells/organs that co-express the two Ang II receptors, and to identify potential effectors that are involved in mediating the activity of the AT2R. Our additional finding that AT2Rs were upregulated during diabetes could explain why the agonist did not affect aortic function under normal physiological conditions and only displayed its protective effects in DAA. Moreover, recent studies demonstrate that the AT1R undergoes desensitisation in response to prolonged exposure to its ligand and that the AT2R is unable to recruit β-arrestins, thereby contributing to the receptor subtype not being desensitised in the same manner as AT1R [50]. Hence, this allows C21 to have comparable long-term effects (compared with ARB) in exerting its protective effects, presumably via the AT2R. Taken together, these findings further highlight the advantage of C21 as a potential alternative therapy for DAA.
In conclusion, this study has clearly demonstrated that diabetes-related atherosclerosis can be influenced by approaches that target the two angiotensin receptor subtypes. These findings have important clinical significance in providing insight into potential therapeutic agents targeting the RAS that can be developed to halt the progression of DAA. Treatment with C21 as a monotherapy has been shown to retard the development of aortic atherosclerosis, particularly since it leads to suppression of the underlying oxidative stress, inflammation and fibrosis associated with insulin-deficient DAA. Furthermore, C21 was found to inhibit DAA-induced pathology to an extent similar to that produced by an ARB, suggesting that C21 may provide an alternative therapy for retarding DAA. Further elucidation of the signalling pathways that are activated by C21 would represent a crucial step in determining the strengths and limitations of this AT2R agonist as a therapeutic agent in treating the vascular complications of both type 1 and type 2 diabetes.
Abbreviations
- Ang II:
-
Angiotensin II
- ARB:
-
Angiotensin receptor blocker
- AT1R:
-
Angiotensin II type 1 receptor
- AT2R:
-
Angiotensin II type 2 receptor
- C21:
-
Compound 21
- DAA:
-
Diabetes-associated atherosclerosis
- HAEC:
-
Human aortic endothelial cells
- MCP:
-
Monocyte-chemoattractant protein
- NOX:
-
NADPH oxidase
- RAS:
-
Renin–angiotensin system
- STZ:
-
Streptozotocin
- TxR:
-
Thromboxane receptor
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Ma TK, Kam KK, Yan BP, Lam YY (2010) Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 160:1273–1292
Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME (2005) Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens 23:463–473
Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE (2011) Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121:297–303
Mehta PK, Griendling KK (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292:C82–C97
Johren O, Dendorfer A, Dominiak P (2004) Cardiovascular and renal function of angiotensin II type-2 receptors. Cardiovasc Res 62:460–467
Sales VL, Sukhova GK, Lopez-Ilasaca MA, Libby P, Dzau VJ, Pratt RE (2005) Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation 112:3328–3336
Johansson ME, Fagerberg B, Bergstrom G (2008) Angiotensin type 2 receptor is expressed in human atherosclerotic lesions. J Renin Angiotensin Aldosterone Syst 9:17–21
Rehman A, Leibowitz A, Yamamoto N et al (2012) Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 59:291–299
Matavelli LC, Huang J, Siragy HM (2011) Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 57:308–313
Dhande I, Ali Q, Hussain T (2013) Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: role in renoprotection in obese rats. Hypertension 61:1218–1226
Koulis C, Chow BS, McKelvey M et al (2015) AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension 65:1073–1081
Wan Y, Wallinder C, Plouffe B et al (2004) Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem 47:5995–6008
Hsueh W, Abel ED, Breslow JL et al (2007) Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100:1415–1427
Fan Q, Liao J, Kobayashi M et al (2004) Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant 19:3012–3020
Candido R, Jandeleit-Dahm KA, Cao Z et al (2002) Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation 106:246–253
Koulis C, Kanellakis P, Pickering RJ et al (2014) Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond) 127:485–497
Candido R, Allen TJ, Lassila M et al (2004) Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation 109:1536–1542
Li Y, Li XH, Yuan H (2012) Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovasc Diagn Ther 2:56–62
Kljajic ST, Widdop RE, Vinh A et al (2013) Direct AT2 receptor stimulation is athero-protective and stabilizes plaque in apolipoprotein E-deficient mice. Int J Cardiol 169:281–287
Kaschina E, Grzesiak A, Li J et al (2008) Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118:2523–2532
Jaipersad AS, Lip GY, Silverman S, Shantsila E (2014) The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63:1–11
Tabas I, Garcia-Cardena G, Owens GK (2015) Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209:13–22
Matavelli LC, Zatz R, Siragy HM (2015) A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharmacol 65:371–376
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472
Ma J, Nishimura H, Fogo A, Kon V, Inagami T, Ichikawa I (1998) Accelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstruction. Kidney Int 53:937–944
Chang SY, Chen YW, Chenier I, Tran Sle M, Zhang SL (2011) Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diabetes Res 2011:521076
Li J, Culman J, Hortnagl H et al (2005) Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J 19:617–619
Ulmasov B, Xu Z, Tetri LH, Inagami T, Neuschwander-Tetri BA (2009) Protective role of angiotensin II type 2 receptor signaling in a mouse model of pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol 296:G284–G294
Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J et al (2005) Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation 112:1636–1643
Takata H, Yamada H, Kawahito H et al (2015) Vascular angiotensin II type 2 receptor attenuates atherosclerosis via a kinin/NO-dependent mechanism. J Renin Angiotensin Aldosterone Syst 16:311–320
Daugherty A, Manning MW, Cassis LA (2001) Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol 134:865–870
Hu C, Dandapat A, Chen J, Liu Y, Hermonat PL, Carey RM, Mehta JL (2008) Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis 199:288–294
Kato T, Kawahito H, Kishida S et al (2015) Bone marrow angiotensin AT2 receptor deficiency aggravates atherosclerosis development by eliminating macrophage liver X receptor-mediated anti-atherogenic actions. J Renin Angiotensin Aldosterone Syst 16:936–946
Koitka A, Cao Z, Koh P et al (2010) Angiotensin II subtype 2 receptor blockade and deficiency attenuate the development of atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes. Diabetologia 53:584–592
Daugherty A, Rateri DL, Howatt DA, Charnigo R, Cassis LA (2013) PD123319 augments angiotensin II-induced abdominal aortic aneurysms through an AT2 receptor-independent mechanism. PLoS One 8:e61849
Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA (2004) Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation 110:3849–3857
Oishi Y, Ozono R, Yano Y et al (2003) Cardioprotective role of AT2 receptor in postinfarction left ventricular remodeling. Hypertension 41:814–818
Falcon BL, Stewart JM, Bourassa E et al (2004) Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol Genomics 19:255–261
Wang N, Frank GD, Ding R et al (2012) Promyelocytic leukemia zinc finger protein activates GATA4 transcription and mediates cardiac hypertrophic signaling from angiotensin II receptor 2. PLoS One 7:e35632
Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES (2010) Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol 159:709–716
Chow BS, Kocan M, Bosnyak S et al (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86:75–85
Salem S, Jankowski V, Asare Y et al (2015) Identification of the vasoconstriction-inhibiting factor (VIF), a potent endogenous cofactor of angiotensin II Acting on the angiotensin II type 2 receptor. Circulation 131:1426–1434
AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U (2001) The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem 276:39721–39726
Jalowy A, Schulz R, Dorge H, Behrends M, Heusch G (1998) Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J Am Coll Cardiol 32:1787–1796
Jones ES, Black MJ, Widdop RE (2004) Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J Mol Cell Cardiol 37:1023–1030
Barauna VG, Mantuan PR, Magalhaes FC, Campos LC, Krieger JE (2013) AT1 receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr(495) and Ser(1177). Biochem Biophys Res Commun 441:713–719
Zhang GX, Kimura S, Murao K et al (2010) Effects of angiotensin type I receptor blockade on the cardiac Raf/MEK/ERK cascade activated via adrenergic receptors. J Pharmacol Sci 113:224–233
Zuccollo A, Shi C, Mastroianni R et al (2005) The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation 112:3001–3008
Steckelings UM, Fredgart M, Leurgans T et al (2015) Abstract P143: The angiotensin AT2 receptor agonist Compound 21 is a low affinity thromboxane A2 receptor antagonist. Hypertension 66:AP143
Porrello ER, Pfleger KD, Seeber RM et al (2011) Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell Signal 23:1767–1776
Acknowledgements
The authors would like to thank S. Sacca, E. Lastavec and M. Haillay (Baker IDI Heart and Diabetes Institute, Australia) for their technical support. We are grateful to R. Pickering and A. Sharma (Baker IDI Heart and Diabetes Institute, Australia) for providing the monocyte and endothelial cell lines and to A. Ljunggren (Vicore Pharma AB, Göteborg, Sweden) for providing C21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study is supported by a National Health & Medical Research Council of Australia (NHMRC) Project Grant. BSMC is a recipient of a JDRF postdoctoral fellowship. MEC, KAJD and TJA have been supported by NHMRC fellowships.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript. KJD and TJA received Compound 21 from Vicore Pharma AB, Göteborg,, Sweden, as a gift.
Contribution statement
All authors contributed to the study concept and design, and the interpretation of the data. BSMC, CK, UMS, TU, MEC, KAJD and TJA conceived and designed the experiments. BSMC, CK and PK performed the experiments and BSMC, CK, PK, MEC, KAJD and TJA analysed the data. BSMC wrote the paper. The manuscript was edited and reviewed by CK, PK, UMS, TU, MEC, KAJD and TJA. All authors approved the final version of this manuscript. TJA takes full responsibility for the work conducted in this paper.
Additional information
Bryna S. M. Chow and Christine Koulis contributed equally to the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 79 kb)
Rights and permissions
About this article
Cite this article
Chow, B.S.M., Koulis, C., Krishnaswamy, P. et al. The angiotensin II type 2 receptor agonist Compound 21 is protective in experimental diabetes-associated atherosclerosis. Diabetologia 59, 1778–1790 (2016). https://doi.org/10.1007/s00125-016-3977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-016-3977-5