Abstract
Aims/hypothesis
Being born small for gestational age (SGA) is associated with an increased risk of type 2 diabetes in an affluent society, but could confer an improved chance of survival during sparse living conditions. We studied whether insulin action and other metabolic responses to prolonged fasting differed between 21 young adults born SGA and 18 matched controls born appropriate for gestational age (AGA).
Methods
A frequently sampled IVGTT and indirect calorimetry measurements were performed after a 36 h fast. Endogenous glucose production, insulin sensitivity (SI), first-phase insulin secretion and glucose effectiveness were estimated by stable isotope tracer techniques and minimal modelling. Muscle and fat biopsies were obtained after 35 h of fasting.
Results
During fasting, SGA individuals experienced a more pronounced decrease in serum insulin and lower plasma triacylglycerol levels compared with AGA individuals. In addition, energy expenditure decreased in SGA but increased in AGA individuals. After fasting, SGA individuals displayed lower fat oxidation than AGA individuals. SG was reduced in SGA compared with AGA individuals, whereas hepatic or whole body insulin action (SI) did not differ between groups. SGA individuals had increased muscle PPARGC1A DNA methylation. We found no differences in adipose tissue PPARGC1A DNA methylation, muscle and adipose tissue PPARGC1A mRNA expression, or muscle glycogen levels between the groups.
Conclusion
Compared with AGA individuals, SGA individuals displayed a more energy-conserving and energy-conserving cardiometabolic response to 36 h fasting. The role of increased muscle PPARGC1A DNA methylation in mediating this response requires further study.
Similar content being viewed by others
Introduction
Individuals born small for gestational age (SGA) have an increased risk of type 2 diabetes and cardiovascular disease later in life [1–5]. We and others have previously documented several metabolic defects in young adult SGA individuals, including altered fat tissue distribution [6], muscle and hepatic insulin resistance [7], reduced beta cell function [8], increased lipolysis [9], reduced expression of key insulin signalling proteins in fat and muscle tissue [10, 11], abnormal epigenetic regulation of the peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PPARGC1A) transcription factor in muscle and fat [12, 13] and immature muscle fibre composition [14]. The development of peripheral insulin resistance may represent a beneficial response to fasting by facilitating sufficient energy delivery to vital organs, including the brain and heart [9]. We hypothesised that the observed adverse phenotype of SGA individuals in adulthood may be primarily driven by a programmed enforced tolerance of fasting. Thus, SGA individuals may cope with fasting in a different and more energy-conserving manner compared with those born appropriate for gestational age (AGA), potentially including increased insulin resistance and/or lower total energy expenditure.
We studied young men born SGA and age- and BMI-matched AGA controls during 36 h of fasting. A frequently sampled IVGTT was then performed to test the hypothesis that SGA individuals may respond to energy deprivation in a different and potentially more energy-conserving manner than AGA individuals. Furthermore, we hypothesised that improved tolerance to fasting might unmask or influence adverse metabolic and epigenetic changes previously shown to occur in the muscle and adipose tissue of SGA individuals, including disproportionately increased DNA methylation and correspondingly reduced PPARGC1A gene expression and muscle glycogen content [12, 13, 15]. The PPARGC1A gene influences mitochondrial function and is thought to be involved in the control of both insulin action and energy metabolism [16]. These properties, together with its known aberrant regulation in type 2 diabetes [17, 18] and in SGA individuals [12, 13], suggest that this gene could contribute to a putative altered energy-conserving response to fasting in SGA vs AGA individuals.
Methods
Study population
Thirty-nine young healthy white men born at term (week 39–41) were recruited from the Danish National Birth Register. SGA was defined as a birthweight lower than the 10th percentile, and AGA as the 50th to 75th percentile. Exclusion criteria were diabetes in two generations of the family, a BMI >30 kg/m2, exercise for >10 h/week or the use of medication known to affect metabolism. Written informed consent was obtained from all participants. The study protocol was in accordance with the Helsinki II declaration and approved by the local ethics committee.
Thirty-six hour fasting protocol
Participants received standardised, precooked meals and abstained from exercise, alcohol and soft drinks for 3 days prior to the study (electronic supplementary material (ESM) Fig. 1). During this period, the calorie intake was fixed at 10 MJ/day, and comprised 35% fat, 15% protein and 50% carbohydrate. Participants arrived at the laboratory at 19:30, received a standardised meal and fasted thereafter. Water was allowed ad libitum. Smokers were allowed to use a 10 mg Nicorette inhaler (McNeil, Birkerød, Denmark).
Day 1
Anthropometry was determined and an intravenous cannula was placed in an antecubital vein. Blood samples were drawn at 08:00 and 14:00. All participants spent two 10-min sessions on an ergometer bicycle. A dual energy x-ray absorption scan was performed at 14:30 (Hologic Discovery QDR Series, Bedford, MA, USA). At 20:00, a second intravenous antecubital cannula was placed in the other arm. Participants were instructed to go to sleep at 22:00.
Overnight
Blood samples were drawn every 15 min from 20:00 throughout the night until 07:59 the next morning to test for growth hormone (not shown), and hourly to test for glucose, insulin and NEFAs. At 05:00 (33 h fast), a primed infusion of [6,6-2H2]glucose (4 mg/kg followed by 0.04 mg/kg/min) and [1,1,2,3,3-2H5]glycerol (0.15 mg/kg followed by 0.01 mg/kg/min) was administered. Tracers had been tested for pyrogenicity and prepared in saline in ampoules at the Department of Pharmacy and Medicines Management, the Ipswich Hospital NHS Trust, Ipswich, UK. Infusions were given for 180 min to allow equilibration with endogenous glucose and glycerol pools, and then blood samples were taken at 5 min intervals from 07:30 to 07:59 hours to determine basal endogenous glucose production and lipolysis. At 06:45, each participant was transferred to the laboratory by wheelchair. Biopsies were obtained between 07:05 and 07:20. Biopsies were obtained from the vastus lateralis muscle and abdominal subcutaneous adipose tissue (SAT) under local anaesthesia using a Bergström needle, snap frozen in liquid nitrogen and stored at −80°C.
Day 2
At 08:00 hours (36 h fast), a bolus of 270 mg/kg naturally abundant and 30 mg/kg [6,6-2H2]glucose as a 20% solution was administered intravenously over a period of 2 min. Blood samples for measuring plasma glucose, serum insulin and serum C-peptide were collected after 0, 2, 3, 4, 5, 6, 8, 10, 15, 19, 22, 23, 25, 27, 30, 35, 40, 60, 90, 120, 150 and 180 min. Additional samples for plasma NEFA determination were collected at 0, 10, 19, 30 and 180 min. A bolus of 0.02 IU insulin/kg (Actrapid; Novo Nordisk, Bagsværd, Denmark) was administered intravenously at 08:20 hours to improve estimates of insulin sensitivity. For each participant, a hand and forearm was placed in a heated Plexiglas box to arterialise the blood. Indirect calorimetry was performed for 30 min at baseline (12 h fast) and after 31 h and 34 h of fasting to assess energy expenditure (EE) and substrate oxidation rates (Deltatrac monitor; Datex instruments, Baldwin Park, CA, USA). Urine samples were collected after 12 and 36 h of fasting. All samples were stored at −80°C except for those for cholesterol testing, which were analysed the same day.
Biochemical analysis
Blood ketone levels were measured using one-touch, insulin and C-peptide levels were analysed by fluoroimmunoassay and lipids, glucose and carbamide were analysed on a Vitros 5600. HbA1c was measured using high performance liquid chromatography. LDL-cholesterol was calculated as described in the ESM. NEFA levels were measured using a human NEFA kit. Isotopic analysis of glucose and glycerol were performed using GC/MS, see ESM for further details.
Muscle glycogen content was determined by a fluorometric system, PPARGC1A DNA methylation levels were measured using PyroSequencing and gene expression was determined by quantitative real-time PCR as described in further details in the ESM.
Calculations
Total AUC for glucose, insulin and C-peptide was determined using the trapezoid rule. The RQ was calculated as expired CO2 divided by consumed O2. Net lipid and glucose oxidation rates were calculated using the non-protein RQ [19]. The first-phase insulin response (FPIR) was calculated as the baseline-adjusted AUC of insulin from 0 to 10 min of the IVGTT, and the Phi1 index as FPIR divided by the baseline-adjusted glucose response from 0 to 10 min of the IVGTT. Hepatic insulin clearance was calculated by dividing serum C-peptide levels by serum insulin levels from 12 to 36 h of fasting [20]. Basal endogenous glucose production (EGP) was estimated from the quotient of the labelled glucose infusion rate and the tracer:trace ratio. Hepatic insulin resistance was calculated as the product of EGP multiplied by the serum insulin level at 06:00 [21].
The insulin sensitivity index (SI) and glucose effectiveness (SG) were calculated from the minimal model using MinMod Millenium v 6.02 (Richard N. Bergman, Los Angeles, CA, USA), an implementation of the minimal model of Bergman [22]. Glucose tolerance (KG) was determined as the rate constant for glucose clearance after the insulin bolus, expressed as an exponential function.
Statistical analysis
Paired and unpaired Student’s t tests were performed to compare single measurements. An unpaired t test with Welch’s correction and a paired Wilcoxon signed rank test were used to analyse groups with unequal variance.
Development of peripheral insulin resistance was defined a priori as the primary endpoint. We had a power of >90% to detect a 50% difference in SI between groups with a significance level of 0.05 including at least 14 individuals in each group.
Correlation analyses were performed using the Spearman’s rank correlation test. A mixed effects model was used to analyse the longitudinal measurements of plasma and serum, with an appropriate choice of correlation model depending on the data structure, variable numbers and residual variances. In the statistical analysis, all insulin and triacylglycerol data from the fasting period as well as C-peptide and glucose data from the IVGTT period were logarithmically transformed. The reported p values indicate the overall differences in effect over time between groups. Since waking up led to large fluctuations in all values measured after 06:00, mixed effects models were only conducted until this time point except for blood ketones and lipids. A p value of ≤0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA) and GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA).
Results
Characteristics of SGA and AGA groups
SGA individuals were lighter at birth (p < 0.0001), shorter (p = 0.01) and had reduced lean body mass (p = 0.03) than AGA individuals. The proportion of fat mass was similar in the two groups. Baseline lipid and glucose profiles did not differ between the groups (Table 1).
Response to 12–36 h of fasting
Indirect calorimetry
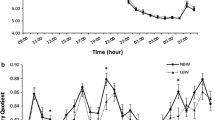
There were no differences in EE between groups at baseline (Fig. 1a–f). The RQ reached a stable level after 31 h of fasting in the SGA group but decreased continuously in the AGA group, resulting in a tendency towards a lower RQ (p = 0.055) after 34 h of fasting in the AGA group. This difference resulted from a significantly higher fat oxidation rate (Fig. 1d) in AGA individuals after a 34 h fast (p = 0.02). There was no difference in glucose oxidation at any time point between the two groups (Fig. 1c).
From 12 to 31 h of fasting, the EE decreased slightly in SGA individuals; in contrast, it increased significantly (p = 0.02) in AGA individuals (Fig. 1b). Therefore, SGA individuals had a lower EE after 31 h (p = 0.02) and 34-h fast (p = 0.03) compared with AGA individuals. This was related to a reduced amount of exhaled CO2 in the SGA group (p = 0.0004; Fig. 1e) and increased O2 consumption in the AGA group (p = 0.005; Fig. 1f).
Plasma and serum samples
Plasma glucose and serum C-peptide levels decreased equally during fasting in both groups (p = 0.72 and p = 0.21 for the effect over time; Fig. 2a, c). There was a significant difference in the change in serum insulin concentrations over time (12–34 h fasting) between the two groups (p = 0.05), with a 42% reduction in the AGA group compared with a 60% reduction in the SGA group (Fig. 2b). A borderline significant difference in hepatic insulin clearance over time between the two groups was observed (p = 0.07; Fig. 2d). Baseline-adjusted AUC of hepatic insulin clearance from 12 to 34 h of fasting was significantly higher in the SGA group (116 ± 85) than in the AGA group (−5.96 ± 230; p = 0.045).
(a) Glucose, (b) insulin, (c) C-peptide and (d) hepatic insulin clearance after 12–36 h of fasting. Changes during the fasting period within groups were analysed using paired t tests; differences between time points were analysed using unpaired t tests and differences in changes over time were determined using a mixed effects model. Solid line, AGA group; dotted line, SGA group. Data are presented as means ± SEM
During the fasting period, plasma NEFA concentrations increased significantly (p < 0.001) and to the same extent (effect over time p = 0.53) in both groups (Fig. 3a). Levels of total cholesterol (Fig. 3b) increased from 12 to 24 h of fasting in the AGA group only (p < 0.05), but no difference in effect over time was found between the groups from 12 to 36 h of fasting (p = 0.47). Plasma HDL-cholesterol and LDL-cholesterol (Fig. 3c, d) levels increased slightly but significantly from 12 to 24 h of fasting in both groups (p < 0.04 and p = 0.001), and decreased again during sleep, but with no differences in effect over time between the groups (HDL-cholesterol p = 0.46, LDL-cholesterol p = 0.42). Plasma triacylglycerol levels (Fig. 3e) decreased steeply from 12 to 24 h of fasting in both groups (AGA p = 0.0003, SGA p < 0.0001), but increased again in the morning. The SGA group did not return to the baseline level, resulting in a significantly lower triacylglycerol level after 36 h of fasting compared with the AGA group (p = 0.02); in fact, the triacylglycerol level was significantly lower after 36 h than after 12 h (p = 0.008). There was no difference in the effect of time between groups (p = 0.11). Blood ketone levels (Fig. 3f) increased (p < 0.0001) from 12 to 36 h of fasting in both groups, to a similar extent (p = 0.19).
Lipid and ketone levels after 12 to 36 h of fasting. (a) NEFA, (b) cholesterol, (c) HDL-cholesterol, (d) LDL-cholesterol, (e) triacylglycerol and (f) ketones. Changes during the fasting period within groups were analysed using paired t tests, differences between time points were analysed using unpaired t tests, and differences in changes over time were determined using a mixed effects model. Solid line, AGA group; dotted line, SGA group; data are presented as means ± SEM
Stable isotopes
The absolute rate of EGP, expressed as the rate of appearance (Ra) of glucose was lower in SGA individuals than in AGA individuals after 36 h of fasting (p = 0.046; Table 2). However, no difference in EGP was found between groups when expressed in relation to total or lean body mass (Table 2). There was no difference between the groups in lipolysis expressed as Ra of glycerol (p = 0.95).
Frequently sampled IVGTT
We observed no differences in plasma glucose, serum insulin or serum C-peptide levels in response to injected insulin during the IVGTT between groups (Fig. 4a–c).
Plasma NEFA levels were suppressed by 56% in the AGA group and by 63% in the SGA group (p = 0.15) during the first 30 min of the IVGTT; however, 3 h after the glucose bolus plasma NEFA levels had returned to pre-IVGTT levels in both groups (Fig. 4d). Blood ketone levels were approximately halved in both groups after the IVGTT (Fig. 4e). There was no difference between the two groups in effect over time for any of the above-mentioned variables, and the calculated FPIR and Phi1 values did not differ (Table 2).
Stable isotopes
SG estimated using the glucose tracer (hot SG) was lower in the SGA group than the AGA group after 36 h of fasting (p = 0.05). In contrast, SG estimated from glucose and insulin levels during the IVGTT (cold SG) did not differ (p = 0.83; Table 2). There was no difference in SI or KG values between groups (Table 2).
Muscle glycogen content
No difference in muscle glycogen content was found after fasting between SGA (535 ± 142 nmol glucose/mg) and AGA individuals (488 ± 98 nmol glucose/mg; p = 0.27; ESM Fig. 2).
PPARGC1A DNA methylation and gene expression
The degree of muscle PPARGC1A DNA methylation was higher (4.5%) in SGA individuals than in AGA individuals after fasting at two out of the three CpG sites investigated (CpG-816, p = 0.03; CpG-783, p = 0.02; Fig. 5a). The average extent of DNA methylation across the three sites was also significantly higher in SGA individuals (p = 0.05; Fig. 5a). The degree of PPARGC1A DNA methylation in SAT did not differ between groups at any of the CpG sites studied (Fig. 5b). PPARGC1A expression levels were not different in muscle (p = 0.82) or SAT (p = 0.99; Fig. 5C + D), and we did not observe any significant associations between PPARGC1A expression levels and average degree of DNA methylation across the three CpG sites in either muscle (p = 0.16) or SAT (p = 0.58; ESM Fig. 3).
Effect of birthweight on PPARGC1A DNA methylation in (a) skeletal muscle tissue and (b) SAT, and on PPARGC1A gene expression levels in (c) skeletal muscle (n = 18 SGA individuals, 16 AGA individuals) and (d) SAT (n = 20 SGA individuals, 13 AGA individuals). *p < 0.05. Grey bars, AGA group; white bars, SGA group; data are presented as means ± SEM. Av., average; meth., methylation
Discussion
In the current study, SGA and AGA individuals displayed a similar degree of insulin resistance, as determined by minimal modelling of IVGTT data after fasting. However, SGA individuals showed a more pronounced decline in serum insulin and plasma triacylglycerol levels during 36 h of fasting. Interestingly, SGA individuals showed a significantly greater decrease in the total rate of EE during fasting compared with AGA controls.
A number of studies have confirmed an association between SGA and an increased risk of type 2 diabetes [23–26], as well as the presence of multiple metabolic abnormalities that can precede diabetes, including hepatic and peripheral insulin resistance in healthy SGA individuals [1]. However, we are unaware of any human experimental studies that have provided evidence to support the thrifty phenotype hypothesis, which claims that SGA is detrimental during conditions of affluent living conditions and potentially beneficial during sparse living conditions. Indeed, our finding that energy deprivation is associated with differential energy saving metabolic responses including lower EE, as well as a tendency towards lower glucose effectiveness in SGA individuals is in accordance with the thrifty phenotype hypothesis.
In contrast to our a priori hypothesis, we did not find any differential response in SGA individuals regarding whole body SI. That is, both study groups responded to 36 h of fasting by developing severe insulin resistance approaching the detection limit for whole body SI. The finding of a steeper decline in serum insulin and lower plasma triacylglycerol levels during fasting in SGA individuals than in AGA individuals, with no difference in SI, should be seen in the context of previous consistent reports that SGA is associated with increased fasting insulin levels [2], insulin resistance [3, 7, 9] and elevated plasma triacylglycerol levels after an 8–10 h overnight fast [4]. Whether the observed alterations during prolonged fasting in our study are associated with beneficial changes in other cardiometabolic risk factors is currently unknown.
In line with the lower serum insulin and triacylglycerol levels in SGA individuals during fasting, the calculated hepatic insulin resistance value tended to be lower after 36 h fasting in the SGA group. This contrasts with the otherwise consistent finding of increased hepatic insulin resistance in SGA individuals after an 8 h overnight fast [7]. However, the exact role of insulin vs other metabolic and hormonal substances in controlling hepatic glucose production during prolonged fasting is unclear, and the importance of estimated hepatic insulin action may be questioned in this metabolic condition. Studies have shown that SGA individuals, despite commonly having slightly elevated fasting insulin levels, display a disproportionate reduction in insulin secretion in relation to their ambient degree of peripheral insulin action [8]. Furthermore, animal studies have consistently shown that fetal energy deprivation is associated with impaired pancreatic development and reduced postnatal pancreatic insulin secretion [27]. Accordingly, our finding of lower serum insulin levels in SGA individuals during fasting conditions could be a reflection of their primary reduction in basal pancreatic insulin secretion. However, the reduced insulin levels in SGA individuals could also be caused by an increased ability to extract insulin in the liver during fasting, when assessed as the ratio between serum C-peptide and insulin levels. However, this needs to be documented using more direct assessments of in vivo insulin clearance rates. A similar FPIR was found during the IVGTT, suggesting that the capability of pancreatic beta cells to sense and respond to glucose is conserved in SGA individuals.
Several lines of evidence suggest that the plasma triacylglycerol level is an independent risk factor for cardiovascular disease in people with and without diabetes [28]. Hepatic VLDL production represents an important source of fasting plasma triacylglycerols, and hepatic VLDL production is elevated in people with hepatic insulin resistance [29]. Accordingly, it is likely that lower fasting plasma triacylglycerol levels in SGA individuals may be caused by lower hepatic VLDL production associated with a trend towards improved hepatic insulin action.
EE has previously been shown to increase during acute fasting [30], and this has been suggested to result from increased plasma noradrenaline levels [31]. The lower nocturnal EE in fasting SGA individuals was associated with a lower lipid oxidation rate. We previously found a disproportionately elevated rate of lipid oxidation in SGA individuals during the night after ingestion of an isocaloric diet including an evening meal [32], as well as after 5 days of high fat overfeeding [7]. Thus, our current finding of a disproportionately lower fat oxidation rate in SGA individuals after 36 h of fasting is another example of a metabolic alteration in SGA individuals that is reversed in response to fasting.
We previously found an elevated rate of whole body and SAT lipolysis in SGA individuals after overnight fasting [9, 32]. Recent studies suggest that individuals born SGA display significant alterations to their SAT functions, of which impaired expandability and capacity to store excess fat represent core metabolic changes [33, 34]. It is likely that the finding of increased lipolysis in SGA individuals after an 8 h overnight fast may be a metabolic manifestation of their impaired capability to accumulate fat, and that “excess fat” is therefore released at an increased rate after an overnight fast. In contrast, upon examination after 36 h of fasting, we did not find an increased rate of whole body lipolysis among SGA compared with AGA individuals. These data support our overall conclusion that SGA individuals may have a preferential metabolic response to long-term fasting.
Interestingly, the lower lipid oxidation rate was not reflected in an increased glucose oxidation rate, as otherwise predicted from the glucose-fatty acid cycle [35]. This may represent another manifestation of a preferential energy-conserving phenotype in SGA individuals with respect to glucose turnover rates. However, it should be mentioned that the RQ is also to some extent influenced by ketogenesis, and that the increased rate of oxygen consumption with no change in carbon dioxide production seen in SGA individuals during fasting could theoretically be explained by an increased rate of ketogenesis, which may not be reflected in any changes in plasma ketone body levels.
The notion of an energy-conserving metabolic state in fasted SGA individuals was further supported by our finding of reduced SG reflecting glucose-mediated glucose uptake and suppression of hepatic glucose production during the IVGTT (Table 2).
We previously showed that SGA individuals tend to exhibit lower muscle glycogen levels after 3 months of exercise training compared with healthy AGA individuals [15]. The finding in the present study of similar muscle glycogen levels in SGA and AGA individuals provides some support for the idea that SGA individuals at least do not exhibit a reduced capability to conserve energy during fasting.
Increased PPARGC1A DNA methylation has been reported in skeletal muscle, SAT and isolated pancreatic islets from type 2 diabetic patients [13, 36, 37]. We previously reported increased muscle PPARGC1A DNA methylation in SGA compared with AGA individuals after overnight fasting, which subsequently disappeared after 5 days of high fat overfeeding because of an increased PPARGC1A DNA methylation response to overfeeding in healthy AGA controls [12]. Interestingly, in the present study fasting did not normalise the increased PPARGC1A DNA methylation in skeletal muscle biopsies from SGA individuals despite reversal of the dysmetabolic traits. PPARGC1A is thought to play a key role in the control of mitochondrion content and function, as well as in the control of energy metabolism and insulin action [18, 38]. To the best of our knowledge, increased PPARGC1A methylation represents the most consistent epigenetic alteration in the skeletal muscle of SGA individuals [39]. Transcriptional repression of PPARGC1A among SGA individuals during fasting could therefore theoretically explain the decreased mitochondrial EE in SGA participants during fasting. However, in accordance with previous studies of our group [12, 13], no correlation was found between PPARGC1A DNA methylation and gene expression during fasting, and PPARGC1A mRNA expression was not significantly reduced in SGA individuals. Therefore, alternative mechanisms and/or more data are needed to explain the extent to which differential changes in response to fasting in SGA individuals may be influenced by their altered skeletal muscle PPARGC1A methylation levels. The differential response to fasting was not associated with any differences in adipose tissue PPARGC1A DNA methylation or mRNA expression between SGA and AGA individuals (Fig. 5).
Taken together, our data provide further support for the thrifty phenotype hypothesis, including a preferential metabolic and energy-conserving phenotype as well as unchanged muscle glycogen content in young adults born SGA compared with AGA individuals when exposed to 36 h of fasting. The extent to which the consistent finding of increased muscle PPARGC1A DNA methylation during fasting may contribute mechanistically to the more energy-conserving phenotype of SGA individuals needs further exploration.
Abbreviations
- AGA:
-
Appropriate for gestational age
- EE:
-
Energy expenditure
- EGP:
-
Endogenous glucose production
- FPIR:
-
First-phase insulin response
- KG :
-
Glucose tolerance
- Ra :
-
Rate of appearance
- SAT:
-
Subcutaneous adipose tissue
- SG :
-
Glucose effectiveness
- SGA:
-
Small for gestational age
- SI :
-
Insulin sensitivity
References
Vaag AA, Grunnet LG, Arora GP, Brøns C (2012) The thrifty phenotype hypothesis revisited. Diabetologia 55:2085–2088
Brøns C, Jensen CB, Storgaard H et al (2008) Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. J Clin Endocrinol Metab 93:3885–3892
Hermann TS, Rask-Madsen C, Ihlemann N et al (2003) Normal insulin-stimulated endothelial function and impaired insulin-stimulated muscle glucose uptake in young adults with low birth weight. J Clin Endocrinol Metab 88:1252–1257
Vielwerth SE, Jensen RB, Larsen T et al (2008) The effect of birthweight upon insulin resistance and associated cardiovascular risk factors in adolescence is not explained by fetal growth velocity in the third trimester as measured by repeated ultrasound fetometry. Diabetologia 51:1483–1492
Garg M, Thamotharan M, Dai Y et al (2012) Early postnatal caloric restriction protects adult male intrauterine growth-restricted offspring from obesity. Diabetes 61:1391–1398
Rasmussen EL, Malis C, Jensen CB et al (2005) Altered fat tissue distribution in young adult men who had low birth weight. Diabetes Care 28:151–153
Brøns C, Jacobsen S, Hiscock N et al (2012) Effects of high-fat overfeeding on mitochondrial function, glucose and fat metabolism, and adipokine levels in low-birth-weight subjects. Am J Physiol Endocrinol Metab 302:E43–E51
Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA (2002) Early differential defects of insulin secretion and action in 19-year-old Caucasian men who had low birth weight. Diabetes 51:1271–1280
Alibegovic AC, Højbjerre L, Sonne MP et al (2010) Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab 298:E555–E564
Ozanne S, Jensen C, Tingey K et al (2006) Decreased protein levels of key insulin signalling molecules in adipose tissue from young men with a low birthweight: potential link to increased risk of diabetes? Diabetologia 49:2993–2999
Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA (2005) Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48:547–552
Brøns C, Jacobsen S, Nilsson E et al (2010) Deoxyribonucleic acid methylation and gene expression of PPARGC1A in human muscle is influenced by high-fat overfeeding in a birth-weight-dependent manner. J Clin Endocrinol Metab 95:3048–3056
Gillberg L, Jacobsen SC, Ronn T, Brons C, Vaag A (2014) PPARGC1A DNA methylation in subcutaneous adipose tissue in low birth weight subjects—impact of 5 days of high-fat overfeeding. Metabolism 63:263–271
Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA (2007) Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab 92:1530–1534
Mortensen B, Hingst JR, Frederiksen N et al (2013) Effect of birth weight and 12 weeks of exercise training on exercise-induced AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab 304:E1379–E1390
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622
Patti ME, Butte AJ, Crunkhorn S et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100:8466–8471
Mootha VK, Lindgren CM, Eriksson KF et al (2003) PGC-1á-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Polonsky KS, Rubenstein AH (1984) C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 33:486–494
Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA (2007) Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30:89–94
Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467
Hales CN, Barker DJ, Clark PM et al (1991) Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022
Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A (2007) Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 165:849–857
Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP (1994) Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 37:624–631
Poulsen P, Vaag AA, Kyvik KO, Moller JD, Beck-Nielsen H (1997) Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia 40:439–446
Portha B, Chavey A, Movassat J (2011) Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res 2011:105076
Kolovou GD, Mikhailidis DP, Kovar J et al (2011) Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol 9:258–270
Sparks JD, Sparks CE, Adeli K (2012) Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol 32:2104–2112
Norrelund H, Nair KS, Jorgensen JO, Christiansen JS, Moller N (2001) The protein-retaining effects of growth hormone during fasting involve inhibition of muscle-protein breakdown. Diabetes 50:96–104
Zauner C, Schneeweiss B, Kranz A et al (2000) Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr 71:1511–1515
Højbjerre L, Alibegovic AC, Sonne MP et al (2011) Increased lipolysis but diminished gene expression of lipases in subcutaneous adipose tissue of healthy young males with intrauterine growth retardation. J Appl Physiol 111:1863–1870
Ferland-McCollough D, Fernandez-Twinn DS, Cannell IG et al (2012) Programming of adipose tissue miR-483–3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ 19:1003–1012
de Zegher F, Diaz M, Sebastiani G et al (2012) Abundance of circulating preadipocyte factor 1 in early life. Diabetes Care 35:848–849
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Barres R, Osler ME, Yan J et al (2009) Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10:189–198
Ling C, del Guerra S, Lupi R et al (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51:615–622
Wu Z (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Zeng Y, Gu P, Liu K, Huang P (2013) Maternal protein restriction in rats leads to reduced PGC-1alpha expression via altered DNA methylation in skeletal muscle. Mol Med Rep 7:306–312
Acknowledgements
This paper is dedicated to the memory of our colleague, Dr. Les Bluck, who passed away while the manuscript was under revision. We thank the Core Biochemical Assay Laboratory, NIHR Cambridge Biomedical Research Centre, for their assistance, as well as M. Modest and L. S. Koch of the Steno Diabetes Center, Gentofte, for technical support and laboratory assistance. S. Wassell assisted with the isotopes at the Medical Research Council (MRC) Human Nutrition Research Unit, Cambridge, UK, where the work was carried out under UK MRC programme number MC_UP_A090_1005. We are especially grateful to all the young men who participated in this study.
Funding
The study was funded by the European Foundation for the Study of Diabetes (EFSD), the Danish Council for Strategic Research, the Augustinus Foundation and the EU 6th Framework EXGENESIS grant. L. Bluck’s salary was paid for by the UK Medical Research Council (MRC).
Duality of interest
CB, KF, MF and AV are shareholders in Novo Nordisk. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
SWJ designed and performed the study, researched data, wrote the first draft of the manuscript, and reviewed and edited the manuscript. LB analysed the isotopes and performed the mathematical modeling of the IVGTT data, and contributed to, reviewed and edited the manuscript. CB, DBD and AAV designed the study, contributed to discussions, and reviewed and edited the manuscript. LH collected data, conducted the mixed effects model analysis and analysed the biopsies. LG analysed the biopsies. KF, AT, LH, LG and MF contributed to data interpretation and discussions, and reviewed and edited the manuscript. SWJ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors, except for LB, who died on 14 May 2014, approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr Les Bluck died on 14 May 2014 before publication of this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 168 kb)
ESM Fig. 1
(PDF 213 kb)
ESM Fig. 2
(PDF 81 kb)
ESM Fig. 3
(PDF 11 kb)
Rights and permissions
About this article
Cite this article
Jørgensen, S.W., Brøns, C., Bluck, L. et al. Metabolic response to 36 hours of fasting in young men born small vs appropriate for gestational age. Diabetologia 58, 178–187 (2015). https://doi.org/10.1007/s00125-014-3406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3406-6