Abstract
Key message
Using associative transcriptomics, our study identifies genes conferring resistance to four diverse fungal pathogens in crops, emphasizing key genetic determinants of multi-pathogen resistance.
Abstract
Crops are affected by several pathogens, but these are rarely studied in parallel to identify common and unique genetic factors controlling diseases. Broad-spectrum quantitative disease resistance (QDR) is desirable for crop breeding as it confers resistance to several pathogen species. Here, we use associative transcriptomics (AT) to identify candidate gene loci associated with Brassica napus constitutive QDR to four contrasting fungal pathogens: Alternaria brassicicola, Botrytis cinerea, Pyrenopeziza brassicae, and Verticillium longisporum. We did not identify any shared loci associated with broad-spectrum QDR to fungal pathogens with contrasting lifestyles. Instead, we observed QDR dependent on the lifestyle of the pathogen—hemibiotrophic and necrotrophic pathogens had distinct QDR responses and associated loci, including some loci associated with early immunity. Furthermore, we identify a genomic deletion associated with resistance to V. longisporum and potentially broad-spectrum QDR. This is the first time AT has been used for several pathosystems simultaneously to identify host genetic loci involved in broad-spectrum QDR. We highlight constitutive expressed candidate loci for broad-spectrum QDR with no antagonistic effects on susceptibility to the other pathogens studies as candidates for crop breeding. In conclusion, this study represents an advancement in our understanding of broad-spectrum QDR in B. napus and is a significant resource for the scientific community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop-infecting fungi are responsible for approximately 20% of global crop losses annually (Fisher et al. 2018). Developing broad-spectrum resistance is vital for effective crop disease management, as it protects against several pathogen species or most races or isolates of the same pathogen (Kou and Wang 2010). Despite this, plant interactions with different pathogens are rarely studied together in collaborative programs to identify common and unique genetic factors controlling the diseases.
Most research and breeding focus on “R” (resistance) gene-mediated qualitative resistance, often providing complete resistance to certain races of a pathogen (McDowell and Woffenden 2003). R gene-mediated resistance can be effective at controlling disease but often breaks down when pathogen effector genes mutate. In contrast, quantitative disease resistance (QDR)—also known as partial resistance—presents a continuous distribution of phenotypes from susceptible to resistant.
QDR has great potential for crop improvement due to its broad-spectrum and durable nature; however, it is not frequently targeted in breeding efforts due to a lack of insights into its underlying molecular determinants (Roux et al. 2014; Nelson et al. 2018). QDR is generally conferred by multiple genes, limiting the ability of pathogens to evolve resistance. QDR is likely broad-spectrum as it is often associated with constitutive features that affect the growth of diverse pathogens such as host plant morphology, basal defense, and signal transduction (Raman et al. 2020; Amas et al. 2022). Constitutive QDR may also function in combination with pathogen-specific resistance to prolong the effectiveness of R genes (Pilet-Nayel et al. 2017; Brun et al. 2010). Although few QDR genes have been cloned and functionally validated, reported mechanisms include enhanced cell wall synthesis (Wisser et al. 2005; Corwin et al. 2016), enhanced secondary metabolism (Benson et al. 2015), modified transport processes (Moore et al. 2015; Deppe et al. 2018), hormone synthesis, and transcriptional regulation (Qasim et al. 2020).
Identifying loci involved in broad-spectrum QDR to multiple pathogens is challenging. Different pathogens employ distinct infection and feeding strategies, including feeding on living host cells (biotrophy); killing plant cells to feed on their contents (necrotrophy); and keeping cells alive while establishing infection before switching to a necrotrophic mode (hemibiotrophy) (Kemen and Jones 2012). These diverse strategies necessitate differing plant defense mechanisms for effective resistance. For example, reactive oxygen species (ROS) production and cell death can provide resistance to biotrophic and hemibiotrophic pathogens (Jia et al. 2000; Vleeshouwers et al. 2000; Vetter et al. 2012). However, ROS accumulation and cell death can promote pathogenicity for some necrotrophic pathogens that use necrosis to facilitate their colonization (Tiedemann 1997; Govrin and Levine 2000; Lorang et al. 2007). Additionally, certain plant hormones such as salicylic acid (SA) and jasmonic acid (JA) exhibit opposing effects on resistance dependent on pathogen lifestyle (Navarro et al. 2008): The activation of the JA signaling provides resistance against necrotrophic pathogens, but the activation of SA signaling protects against biotrophic and hemibiotrophic pathogens (Glazebrook 2005).
In addition, some broad-spectrum QDR mechanisms could relate to innate immunity based on the detection of shared pathogenic features irrespective of their lifestyle. For example, some quantitative trait loci (QTLs) implicated in QDR resemble cell surface pattern recognition receptors (PRRs) or receptor-like kinases (RLKs), akin to those in pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) (Nelson et al 2018; Wisser et al. 2005; Lacombe et al. 2010; Schweizer and Stein 2011; Hurni et al. 2015; Schoonbeek et al. 2015). PRRs like CHITIN ELICITOR RECEPTOR-LIKE KINASE 1 (CERK1), FLAGELLIN SENSING 2 (FLS2), and EF-TU RECEPTOR (EFR) detect PAMPs such as chitin, flg22, and elf18, respectively (Miya et al. 2007; Gómez-Gómez and Boller 2000; Zipfel et al. 2006). Upon PAMP recognition, plants rapidly produce ROS like H2O2, followed by mitogen-activated protein kinase (MAPK) activation and defense gene induction. Measurement of ROS production by purified PAMPs has been used extensively in various laboratories to provide consistent and reliable PTI assessment and to study the relationship between PTI response and QDR (Vetter et al. 2012; Samira et al. 2020).
We aimed to identify broad-spectrum QDR loci in oilseed rape (Brassica napus) using a common panel of genotypes. Brassica napus is a major crop worldwide, producing edible oil, biodiesel, and animal feed protein. Disease susceptibility significantly impacts B. napus yields, so enhanced resistance is a major breeding objective. Employing associative transcriptomics (AT) (Harper et al. 2012; Havlickova et al. 2018), we previously identified single nucleotide polymorphism (SNP) and gene expression marker (GEM) candidate loci linked to B. napus QDR against Pyrenopeziza brassicae (Fell et al. 2023). The GEM analysis utilizes transcriptomic data representing a baseline pre-infection expression level—a foundation for investigating constitutive QDR. The AT pipeline has been successfully applied to diverse phenotypes including pathogen resistance, flowering time, and seed glucosinolate content (Harper et al. 2012; Woodhouse et al. 2021; Dakouri et al. 2021; Roy et al. 2021). However, to our knowledge, AT has never been used to research the similarities and differences between the loci underlying resistance to multiple pathogens.
Three hypotheses underlie our study. Firstly, using a diverse B. napus panel— comprising a range of crop types including winter, semi-winter, and spring oilseed rape, swede, and kale, resulting in 219k SNP variants across the genome—we hypothesized there would be variable pathogen resistance within the population due to the high level of genetic and morphological variation. This variation might correlate with constitutive defenses or early innate immunity since PAMP recognition is conserved across pathogens. Therefore, our second hypothesis is that shared QDR loci may be associated with resistance to multiple pathogens, including those with contrasting lifestyles. Finally, given pathogens of different lifestyles utilize different mechanisms for their infection, our third hypothesis is that there would be some QDR loci dependent on the lifestyle of the pathogen. To test these hypotheses, we examined resistance to two necrotrophs, Alternaria brassicicola and Botrytis cinerea, and two hemibiotrophs, P. brassicae and Verticillium longisporum, within the diversity panel. We used AT to identify the associated loci that define the “host genetic signature” of QDR in B. napus. Additionally, we explored the presence of loci associated with both QDR and PTI (measured by the production of PAMP-induced ROS).
For the first time, we demonstrate that AT analyses from several pathogens can be combined to identify candidate loci for broad-spectrum QDR. We identify GEMs associated with either resistance or susceptibility dependent on the pathogen lifestyle. Additionally, we identify a potential deletion associated with resistance to V. longisporum and potentially broad-spectrum QDR. This study provides new insight into the commonalities underlying broad-spectrum QDR and provides a resource for further mechanistic exploration and improving broad-spectrum resistance to pathogens in B. napus.
Materials and methods
Pathogen infection assays
We selected a subset of the 193 B. napus genotypes for phenotyping, ensuring feasible pathology assays with ample replication across all participating laboratories. To mitigate environmental effects during seed production, all genotypes were cultivated under consistent environmental conditions before distribution to laboratories. Nevertheless, in some pathosystems, certain lines yielded unreliable data, leading to variable numbers of genotypes used for analyses.
Alternaria brassicicola
One-hundred-fifty-four B. napus accessions were grown in peat soil (pH 5.6–6.8) with 1/30 perlite, 16-h day/8-h night photoperiod under fluorescent light (Super TLD Philips 865, 100 μmol m−2 s−1), 19 °C ± 2 °C, and approximately 70% relative humidity. The wild-type A. brassicicola strain (ATCC 96836) was cultured and previously described (Macioszek et al. 2018). A conidial suspension diluted to 3.5 × 105 conidia ml−1 in distilled water was applied in two 10-μl drops to the third leaf of five-week-old plants. Necrotic areas were measured in four complete block experimental replicates, with one plant per genotype, at five days post-inoculation using WinDIAS_3 Imaging Analysis System (Delta-T Devices, UK).
Botrytis cinerea
We divided 190 B. napus accessions into four blocks for screening. Plants were grown in Levington F2 compost with 15% 4-mm grit in growth chambers for 6–7 weeks under TL-tubes with a near-sunlight spectrum of 100 µmol m−2 s−1 with a photoperiod of 10 h. Temperatures were 20–22/18–20 °C day/night. Botrytis cinerea strain B05.10 (Schoonbeek et al. 2001) was grown as described previously (Stefanato et al. 2009) and used for inoculations as described (Lloyd et al. 2014). Spore suspensions (200 µl at 2.5 × 106 spores ml−1) were spread on 1/10 PDA (2.5 g l−1 potato dextrose broth, 12 g l−1 agar), and after 24 h at 21 °C, 4-mm agar plugs were used for B. napus inoculations of 22-mm leaf disks on 0.6% water agar. The lesion diameter was measured after 48 h at 21 °C, 85–100% relative humidity, and low light (10–20 µmol m−2 s−1). Reference genotypes, Tapidor and Ningyou 7, were included on each plate, and data were normalized to the mean of these reference lines before subsequent statistical analyses. Three full experimental replicates (divided into four blocks) within a randomized alpha block design were performed. From this, the two most reproducible experimental replicates, as determined by Pearson's coefficient, were selected for subsequent analyses. Sixteen replicates per genotype were performed per experimental replicate.
Pyrenopeziza brassicae
The P. brassicae methods and phenotype dataset used were the same as in our previous publication (Fell et al. 2022). The experiment comprised three complete experimental replicates, each divided into 10 blocks, following a randomized alpha block design. Within each experiment, five replicates were conducted for each genotype. Disease severity was scored on a scale of 1–6—with a score of 1 for no sporulation and 6 for the most sporulation—and was calculated for the 129 genotypes.
Verticillium longisporum
We divided 191 B. napus accessions into four blocks for screening. Four reference lines (Falcon, SEM, Zhongyou, Loras) were used for normalization. Each line had 20 plants for both mock and V. longisporum inoculation. The preparation of fungal inoculum and disease assessment were done as described previously (Zheng 2018; Zheng et al. 2019). Ten-day-old conidial suspension of V. longisporum isolate VL43 obtained from a diseased B. napus plant (Zeise et al. 2001) was used for inoculation. Ten-day-old plants with unfolded cotyledons were inoculated with water or 1 × 106 CFU ml−1 spore suspension for 50 min. Plants were kept in a climate chamber with a 16-h photoperiod and 22 ± 2 °C temperature. Stem disease severity ranging from 1 (healthy) to 9 (dead) was assessed at 7, 14, 21, and 28 days post-inoculation. The area under the disease progress curve was calculated and normalized against reference lines.
ROS assays
One-hundred-ninety B. napus accessions were split into four groups and cultivated in Levington F2 compost with 15% 4-mm grit in growth chambers. A near-sunlight spectrum at around 100 µmol m-2 s-1 and a 10-h photoperiod were maintained, with temperatures of 20–22 °C/18–20 °C day/night. ROS measurements employed a luminol/peroxidase-based assay. For each accession, two 4-mm-diameter leaf disks were taken from the second most recently expanded leaf of four individual plants. Two leaf disks per plant were taken, and the values obtained were averaged, resulting in four replicates per genotype. Leaf disks were incubated in 200-µl sterile water in a 96-well plate for 16–24 h in darkness. Subsequently, water was removed, and a solution was added containing 34-mg l-1 luminol, 20-mg l-1 horseradish peroxidase (HRP), and PAMP: either chitin (100 g l-1, NA-COS-Y, Yaizu Suisankagaku Industry CO., YSK, Yaizu, Japan); flg22 (QRLSTGSRINSAKDDAAGLQIA); or elf18 (SKEKFERTKPHVNVGTIG, both 10 mM, Peptron, http://www.peptron.co.kr). Luminescence was recorded over 40 min, and the total ROS response was quantified using area under the curve calculations. Reference genotypes, Tapidor and Ningyou 7, were included in each plate, and data were normalized to the mean of these reference lines before subsequent statistical analyses. Three full experimental replicates (divided into four blocks) within a randomized alpha block design were performed. From this, the two most reproducible experimental replicates, as determined by Pearson's coefficient, were selected for subsequent analyses.
Phenotypic data processing for association analysis
Each dataset (infection phenotypes for A. brassicicola, B. cinerea, P. brassicae, or V. longisporum, ROS measurements for chitin, flg22, or elf18) is available on Zenodo (https://zenodo.org/records/10499917). Statistical analysis was performed in R using the linear mixed model (lmer (trait ~ genotype_id) + (1|rep/block)) with genotype as a fixed effect and experimental replicate and block as nested random effects (with block being nested within replicate). Estimated marginal means were calculated for each B. napus genotype using emmeans Version 1.8.0 (R Core Team 2023). These mean values contributed by each collaborating laboratory for each pathosystem were utilized as input for the AT pipeline. These data and standard errors are provided in Table S1. For combined data visualization, estimated marginal means were normalized to a 0–1 range. In pathogen datasets, the disease score is indicative of “susceptibility” to the pathogen. To represent “resistance” to the pathogen, the reciprocal of these values was calculated.
Associative transcriptomics
We used genotype (SNP) and expression level datasets (Havlickova et al. 2018) from York Knowledgebase (http://yorknowledgebase.info) and refined to include only the lines used within this study. Functional genotypes were determined via 100-base mRNAseq for the third true leaf using the Illumina HiSeq 2000 platform. Sequence reads were mapped to the CDS gene model-based Brassica AC pan-transcriptome reference (He et al. 2015), which comprised 116,098 gene models for SNP scoring and read quantification; 219,454 SNPs with maternal allele frequencies greater than 5% were used for downstream analysis. Genome-wide Association (GWA) and GEM mapping were done using the R-based GWA and GEM Automation (GAGA) pipeline (Nichols 2022), which utilizes GAPIT Version 3 (Lipka et al. 2012; Wang 2021). GAGA was run using our recently updated population structure (Fell et al. 2023) and B. napus pan-transcriptome Version 11 (Havlickova et al 2018). For GWA analyses, generalized linear model (GLM), Bayesian-information and Linkage-disequilibrium Iteratively Nested Keyway (BLINK) (Huang et al. 2019), and Fixed and random model Circulating Probability Unification (FarmCPU) (Liu et al. 2016) models were compared using QQ plots to select the best-fitting model.
GEM association analyses were performed within the GAGA pipeline based on methodology by Harper et al. (2012). Associations were determined by linear regression using Reads per Kilobase of the transcript per Million mapped reads (RPKM) to predict a quantitative outcome of the trait value. Markers with an average expression below 0.5 RPKM were excluded prior to analysis resulting in 53,883 expression values for association analysis. The Pearson’s coefficient was utilized to assess the correlation between expression and resistance phenotype for each GEM.
To determine the statistical significance threshold in GWA analysis, various methods that account for multiple testing have been proposed, including the Bonferroni correction and False Discovery Rate (FDR). Due to the prevalence of linked markers in modern GWA, the Bonferroni correction threshold can often have an inflated significance level, detecting only strong, major gene effects as significant. To address this, we utilized the FDR to manage the expected proportion of false positives among significant associations. FDR values for both GEM and GWA were determined using the q value R package (Storey 2011). Bonferroni thresholds for GWA and GEM are 6.64 and 6.03-Log10P, respectively, (P < 0.05) and 6.34 and 5.73 −Log10P (P < 0.1).
Linkage disequilibrium (LD) varies based on chromosome position and selection level. To ascertain the LD at the A09 locus associated with V. longisporum resistance, we calculated the mean pairwise R2 between this marker and all others on the chromosome using the TASSEL Version 5.0 site by all analysis options (Bradbury et al. 2007). Markers were deemed in LD when R2 > 0.2.
Interpretation of data post-AT
To determine query coverage (proportion of query sequence aligning with reference sequence) for the genomic region in linkage disequilibrium with the most significant SNP marker associated with V. longisporum resistance, we conducted a command line BLAST analysis against the B. napus pan-transcriptome. The analysis was performed for genotypes included in our study with published reference genomes: Quinta, Tapidor, Westar, and Zhongshuang 11 (Song et al. 2020).
We identified potential Arabidopsis thaliana orthologs of B. napus genes using BLASTN analysis against the A. thaliana transcriptome (TAIR Version 10), selecting the best hit based on e value. Hypergeometric probability was used for to determine whether the number of overlapping GEMs observed was greater than that expected by chance as detailed by Kim et al. (2001).
We conducted weighted gene co-expression analysis (WGCNA) using the R-based WGCNA library (v 1.72) (Langfilder and Horvath 2008). Lowly expressed genes with 0 RPKM value in half of the samples were removed, and the rest of the data were used to perform a signed WGCNA analysis to detect modules of genes using the blockwiseModules() function. A soft threshold power of 6 was selected based on minimum mean connectivity using the pickSoftThreshold() function within the WGCNA package. Correlation and P values with phenotypic trait data were calculated using the “Cor()” and “CorPvalueStudent()” functions. Gene Ontology (GO) enrichment analysis (FDR < 0.05) was conducted using a Fisher´s exact test for GEMs within WCGNA modules.
Results
QDR to A. brassicicola, B. cinerea, P. brassica e, and V. longisporum is present in the B. napus diversity panel
Given the extensive genetic diversity in our B. napus panel, we hypothesized that QDR to A. brassicicola, B. cinerea, P. brassicae, and V. longisporum would be present. We did pathogenicity assays and found that the B. napus genotypes demonstrated varying levels of resistance to the pathogens, confirming the presence of QDR in the panel (Fig. 1a, Table S1).
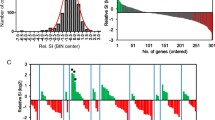
Brassica napus genotypes have common GEMs associated with resistance pathogens of the same lifestyle. a Brassica napus disease resistance to fungal pathogens is quantitative. Resistance phenotype (arbitrary units (a.u.)) of different B. napus genotypes to Alternaria brassicicola (A.b), Botrytis cinerea (B.c), Pyrenopeziza brassicae (P.b), or Verticillium longisporum (V.l). Arbitrary units signify resistance values obtained through the reciprocal of disease susceptibility scores, derived from estimated marginal means and normalized to a 0–1 range for comparative data visualization. The total number of genotypes used for each pathogen assay and the position of reference genotypes Quinta (Q), Tapidor (T), Westar (W), and Zhongshuang 11 (Z) are indicated. b Correlation between the resistance phenotype of B. napus lines to the fungal pathogens. Positive correlations (green), negative correlations (red), and no correlation (n.s.); strengths of correlation (sizes of circles) are indicated between pairwise comparisons of resistance responses. c Manhattan plot of B. napus genome showing marker-trait association of statistically significant GEMs for resistance to each fungal pathogen. The x-axis indicates GEM location along the chromosome; the y-axis indicates the − log10(p) (P value). d The numbers of resistance and susceptibility gene expression markers (GEMs) shared between pairwise comparisons of pathogens. e The numbers of GEMs associated with resistance to one pathogen and susceptibility to another pathogen. f Venn diagrams showing the overlap between A.b and B.c susceptibility GEMs and P.b and V.l resistance GEMs (right) and the overlap between A.b and B.c resistance GEMs and P.b and V.l susceptibility GEMs (left). g Linear regression analysis of gene expression (RPKM) of Cab032851.1 relative to resistance to fungal pathogens (arbitrary units (a.u.), normalized values between zero and one)
We hypothesized that if broad-spectrum QDR was present, there would be a positive correlation between phenotypic responses to pathogens. We initially examined disease resistance in four B. napus genotypes with published reference genomes: Quinta, Tapidor, Westar, and Zhongshuang 11. Quinta showed low resistance to both hemibiotrophic pathogens, while Zhongshuang 11 displayed high resistance to both necrotrophic pathogens (Fig. 1a). However, the ranking of these reference genotypes within the diversity panel varied. To explore phenotypic correlations between responses to pathogens across the entire B. napus panel, we generated a correlation matrix for pairwise fungal pathogen comparisons using the respective phenotypic resistance scores for each genotype (Fig. 1b). There was a positive correlation between resistance to necrotrophic pathogens A. brassicicola and B. cinerea. Conversely, negative correlations emerged between resistance to different pathogen lifestyles; for example, hemibiotrophic V. longisporum showed negative correlations with necrotrophic pathogens A. brassicicola and B. cinerea.
GEMs are associated with resistance to pathogens of the same lifestyle
To identify QDR loci, we performed GWA analysis on our datasets from the four fungal pathogens. Minor GWA association peaks could be observed (-Log10P > 5); however, we did not identify any significant marker associations with resistance to any of the pathogens, using either a Bonferroni threshold of P = 0.1 or a False Discovery Rate (FDR) of 0.05. All association data provided are in Table S2. We performed GEM analyses using the transcriptomes of the B. napus genotypes to associate resistance with the expression of all gene models (Table S3). Numerous GEMs showed significant associations with fungal pathogen resistance (FDR < 0.05), and these were distributed evenly across the B. napus genome (Fig. 1c): 2129 GEMs for A. brassicicola, 370 for B. cinerea, 659 for P. brassicae, and 3222 for V. longisporum. We classified GEMs based on their positive or negative correlation with resistance or susceptibility, naming them “resistance GEMs” or “susceptibility GEMs,” respectively (Table 1).
We hypothesized that there might be shared QDR GEMs associated with all four fungal pathogens given that some early innate defense mechanisms such as PAMP recognition are conserved across most pathogens. To investigate this, we compared the lists of resistance and susceptibility GEMs across the fungal pathogens (Fig. 1d). While necrotrophic pathogens (A. brassicicola and B. cinerea) had shared GEMs, as did hemibiotrophic pathogens (P. brassicae and V. longisporum), there were no common GEMs between necrotrophic and hemibiotrophic pathogens. Consequently, there were no shared GEMs associated with broad-spectrum resistance spanning pathogens with differing lifestyles.
Given the existence of shared QDR GEMs associated with pathogens of the same lifestyle and that pathogens with similar lifestyles have similarities related to their infection, feeding strategies, and plant processes required for defense, we conducted overrepresentation analyses to compare the number of shared QDR GEMs between pathogens of the same lifestyle against what would be expected by chance (Plaisier et al. 2010). There was significant overrepresentation in the number of shared GEMs between necrotrophic pathogens A. brassicicola and B. cinerea (73 resistance GEMs and 56 susceptibility GEMs), as well as between hemibiotrophic pathogens P. brassicae and V. longisporum (89 resistance GEMs and 42 susceptibility GEMs) (Fig. 1d, Tables S4, S5).
GEMs are associated antagonistically with resistance to pathogens of different lifestyles
Hemibiotrophic and necrotrophic pathogens employ distinct plant mechanisms for their infection, with certain genes that confer resistance to hemibiotrophic pathogens leading to susceptibility against necrotrophic pathogens (Lorang et al. 2007). Thus, we hypothesized the presence of QDR GEMs with antagonistic associations based on pathogen lifestyle. To explore this, we compared resistance GEMs with susceptibility GEMs for each combination of fungal pathogens (Fig. 1e). As anticipated, there were no GEMs with antagonistic effects for pairs of either necrotrophic or hemibiotrophic pathogens. However, intriguingly, we observed an overrepresentation of GEMs with antagonistic resistance associations between necrotrophic and hemibiotrophic pathogens. For instance, 1100 (769 + 331) GEMs with opposite resistance associations were linked to A. brassicicola and V. longisporum (Tables S4, S5).
Broad-spectrum resistance GEMs with no antagonistic effects on susceptibility to other pathogens hold promise for crop breeding. To identify such candidates, we compared necrotrophic pathogen susceptibility GEMs to hemibiotrophic pathogen resistance GEMs. Similarly, we compared necrotrophic pathogen resistance GEMs to hemibiotrophic pathogen susceptibility GEMs (Fig. 1f, Table S4). Thirteen GEMs were associated with resistance to both A. brassicicola and B. cinerea with no associations with susceptibility to P. brassicae and V. longisporum (Table 2). Forty-eight GEMs were associated with resistance to both P. brassicae and V. longisporum with no associations with susceptibility to A. brassicicola and B. cinerea (Table 3). Notably, four GEMs homologous to A. thaliana ACIP1 were associated with resistance to both hemibiotrophic pathogens and no antagonistic associations with susceptibility to the necrotrophic pathogens.
Interestingly, there was a significant overrepresentation of GEMs associated with susceptibility or resistance to both necrotrophic pathogens, but with opposing associations to both hemibiotrophic pathogens: 21 GEMs (9 + 12) (Table 4, S6). Notably, the expression of Cab032821.1, the homolog of A. thaliana Fumonisin B1-resistant 12, FBR12, was associated with resistance to both hemibiotrophic pathogens but susceptibility to both necrotrophic pathogens (Fig. 1g), as was the Bo8g081460.1, the homolog of an A. thaliana disease resistance nucleotide-binding leucine-rich repeat (NBS-LRR) protein (Table 4).
We conducted weighted gene co-expression analysis (WGCNA) (Langfilder and Horvath 2008) to explore the co-expression patterns of GEMs in our study. Several GEMs exhibited co-expression patterns across the B. napus population and were organized into co-expression modules. These modules were then assessed for their association with resistance to each pathogen (Table S6). Notably, significantly correlated modules included 22 modules (containing a total of 1075 GEMs) for resistance to A. brassicicola, 18 modules (containing 299 GEMs) for resistance to B. cinerea, 34 modules (containing 575 GEMs) for resistance to P. brassicae, and 26 modules (containing 2075 GEMs) for resistance to V. longisporum. Interestingly, modules correlated with resistance to necrotrophic pathogens displayed susceptibility to hemibiotrophic pathogens, and vice versa, consistent with the findings from our individual GEM results.
To gain further insights, we performed GO enrichment analysis on the putative A. thaliana orthologs of resistance and susceptibility GEMs within each module. Particularly noteworthy was a module linked to susceptibility to necrotrophic pathogens and resistance to hemibiotrophic pathogens, enriched in genes associated with photosynthesis, suggesting that photosynthesis-related processes might exert contrasting effects on resistance based on the pathogen's lifestyle. We examined whether there were any GEMs related to known pathogen-dependent pathways, specifically SA or JA signaling. We first used gene ontology to search for SA- or JA-related GEMs in our whole expression dataset (53,883 markers). No GEMs were linked to SA signaling, and markers associated with JA signaling were not enriched in our GEM analysis or WGCNA modules. This is likely because these genes are usually activated in response to pathogens rather than being constitutively expressed at the represented three-leaf stage in our expression data.
GEMs associated with both PTI and QDR are dependent on pathogen lifestyle
We had originally hypothesized the presence of shared QDR loci associated with resistance to multiple pathogens with contrasting lifestyles possibly linked to early innate immunity due to the conserved nature of PAMP recognition. However, our comparisons of GEMs (Fig. 1d) revealed no shared GEMs between all four pathogens. Nevertheless, previous studies have linked PTI genes to QDR (Nelson et al. 2017; Wisser et al. 2005; Schweiser and Stein 2011; Hurni et al. 2015). Thus, we proceeded to investigate whether any of our pathogen resistance QDR loci were related to PTI.
The B. napus genotypes demonstrated varying levels of PAMP-induced ROS production with all three PAMPs, suggesting quantitative traits (Figs. 2a, S1a). Reference lines (Quinta, Tapidor, Westar, and Zhongshuang 11) had comparable rankings for flg22 and elf18 ROS responses within the panel, and our correlation matrix indicated that the B. napus genotypes exhibited similar rankings of responses across all three PAMPs (Fig. S2b). Minor GWA association peaks could be observed (− Log10P > 5), with a single marker (Cab031873.1:1209:C) significantly associated with flg22-induced ROS production at a Bonferroni threshold of P = 0.1. No significantly associated markers were observed at an FDR of 0.05. All marker associations are detailed in Table S2. We identified 184, 286, and 3251 GEMs associated with chitin-, flg22-, and elf18-induced ROS responses, respectively (Table S3); however, no GEMs corresponded to PRRs. There was a significant overlap between GEMs associated with the different PAMP responses. For example, out of the 184 chitin-induced ROS GEMs, 31% were shared with the flg22 response, 54% with the elf18 response, and 21% with both flg22 and elf18 responses (Table S7, Fig. S1c). These data suggest that response to PAMPs has some general genetic control and some PAMP-dependent distinct elements.
Shared GEMs for QDR and chitin-induced ROS are associated with resistance to hemibiotrophic pathogens but susceptibility to necrotrophic pathogens. a Chitin-induced ROS response (arbitrary units (a.u.)) of the B. napus panel. Arbitrary units signify resistance values obtained through the reciprocal of disease susceptibility scores, derived from estimated marginal means and normalized to a 0–1 range for comparative data visualization. The positions of reference genotypes Quinta (Q), Tapidor (T), Westar (W), and Zhongshuang 11 (Z) are indicated. b Linear regression analysis of chitin-induced ROS response relative to quantitative disease resistance to Alternaria brassicicola (A.b), Botrytis cinerea (B.c), Pyrenopeziza brassicae (P.b), or Verticillium longisporum (V.l) (arbitrary units (a.u.), normalized values between zero and one). c Venn diagrams showing the overlap between GEMs associated with chitin-induced ROS and QDR to the fungal pathogens, Alternaria brassicicola (A.b), Botrytis cinerea (B.c), Pyrenopeziza brassicae (P.b), and Verticillium longisporum (V.l). The percentage of QDR-related GEMs which are also related to chitin-induced ROS is indicated. d The number of gene expression markers (GEMs) whose expression was positively or negatively associated with chitin-induced ROS and the number which was also associated with resistance (R) or susceptibility (S) to the B. napus fungal pathogens. Venn diagram indicating the overlap between GEMs associated with several pathogen interactions. e Linear regression analysis of gene expression (RPKM) of BnaC04g2850D and Cab022449.1 relative to chitin-induced ROS responses and resistance to fungal pathogens
Since chitin is a fungal PAMP and our study centers on fungal pathogens, we concentrated our following investigations on the overlap between chitin-associated and QDR-associated loci. Chitin-induced ROS response did not correlate with QDR to fungal pathogens (Fig. 2b), and only a small fraction (less than 1%) of QDR GEMs for each pathogen were linked to chitin-induced ROS response (Table S7). Notably, there was minimal overlap between GEMs associated with chitin-induced ROS response and QDR to necrotrophic pathogens, and a slight overlap with QDR to hemibiotrophic pathogens (Table S5). This suggests that most QDR loci are independent of early PAMP-induced ROS response (Fig. 2c).
Given that ROS accumulation can influence susceptibility to certain necrotrophic pathogens and our previous observation of QDR GEMs with lifestyle-dependent antagonistic associations, we investigated whether loci linked to chitin-induced ROS production might be associated with resistance against hemibiotrophic pathogens but susceptibility to necrotrophic pathogens. Of the 184 GEMs that were associated with chitin-induced ROS, 142 were positively associated, i.e., increased gene expression was associated with the increased magnitude of chitin-induced ROS response. Remarkably, all positively associated chitin-induced ROS GEMs that coincided with QDR GEMs were exclusively associated with resistance to hemibiotrophic pathogens and susceptibility to necrotrophic pathogens (Fig. 2d, Table 5). These included BnaC04g02850D, a homolog of A. thaliana ACIP1 (also associated with flg22 and elf18 responses), and Cab022449.1, a homolog of A. thaliana Aldehyde dehydrogenase 3H1 (ALDH3H1) (Fig. 2e, Table S4). Likewise, among the 42 GEMs exhibiting negative correlations with chitin-induced ROS response, those overlapping with QDR were associated with resistance against necrotrophic pathogens and susceptibility to hemibiotrophic pathogens. WGCNA analysis revealed that GEMs linked to chitin-induced ROS were grouped into 19 modules (comprising 135 GEMs) (Table S6). In line with our individual GEM results, modules positively associated with chitin-induced ROS production were associated with resistance to hemibiotrophic pathogens and susceptibility to necrotrophic pathogens, including the module enriched in GEMs related to photosynthesis regulation.
A 0.51 MB deletion on chromosome A09 is associated with V. longisporum resistance and potentially broad-spectrum QDR
The genomic distribution of GEMs (Fig. 1C) highlighted a notable peak of five highly significant GEMs associated with V. longisporum resistance on chromosome A09 (Cab013522.1, Cab013524.1, Cab013523.1, Cab013526.1, and Cab013517.1). Notably, Cab013522.1, Cab013524.1, and Cab013526.1 were also linked to P. brassicae resistance, while Cab013522.1, Cab013523.1, Cab013524.1, and Cab013526.1 were associated with susceptibility to A. brassicicola (Table S3). This clustering suggests a potential region of interest for broad-spectrum QDR. Compellingly, this region of chromosome A09 also corresponded to a minor association (P = 5.14e−07, FDR = 0.11) from the V. longisporum resistance GWA analysis (Fig. 3a). Assessment of phenotypic variation segregating with alleles for Cab013526.1.135.A revealed that accessions inheriting a “G” at this locus had resistance values 57% lower than those inheriting an “A” (Fig. 3b). These alleles also segregated with resistance to P. brassicae (with “G” alleles showing 12% lower resistance than “A” alleles) and susceptibility to A. brassicae (with “G” alleles indicating 28% higher resistance than “A” alleles) (Fig. 3b). We calculated the distance in LD with this marker and found a region of 0.51 MB containing 107 genes (Fig. 3c).
Resistance to Verticillium longisporum is associated with a genomic deletion on chromosome A09. a Manhattan plots showing marker-trait association resulting from GEM and GWA analysis of resistance to V. longisporum in 193 Brassica napus genotypes. The x-axis indicates GEM or SNP location along the chromosome; the y-axis indicates the − log10(p) (P value). Gray line indicates the FDR > 0.05 cut-off value, red line highlights the shared region. b Segregation of resistance to V. longisporum (V.l), Pyrenopeziza brassicae (P.b), Alternaria brassicicola (A.b), or Botrytis cinerea (B.c) (arbitrary units (a.u.)) with the highest associating marker, Cab013526.1.651.A, showing the marker effect between A and G (R = A/G). P values were determined by a Student’s t test. Arbitrary units signify resistance values obtained through the reciprocal of disease susceptibility scores, derived from estimated marginal means and normalized to a 0–1 range for comparative data visualization. c Linkage decay plot from marker Cab013526.1.651.A as a function of genetic distance (MB). The gray line indicates an R2 value of 0.2, red lines indicate the area of linkage disequilibrium. d Resistance to V. longisporum (left-hand y-axis) and gene expression (RPKM) of the five highest associating GEMs Cab013522.1, Cab013524.1, Cab013523.1, Cab013526.1, and Cab013517.1 (right-hand y-axis) in reference genotypes Quinta (Q), Tapidor (T), Westar (W), and Zhongshuang 11 (Z). e Heatmap indicating query coverage (compared to the B. napus pan-transcriptome) in Q, T, W, and Z for the 107 genes predicted to be in linkage disequilibrium with Cab013526.1.651.A on chromosome A09
Given the association between the expression of five nearby GEMs and V. longisporum resistance, we speculated that this link could partly be due to a genomic deletion in this region. To explore this, we examined whether any B. napus genotypes with published genomes (Quinta, Tapidor, Westar, and Zhongshuang 11) exhibited low gene expression and high susceptibility (Song et al. 2020). Quinta had little resistance to V. longisporum along with low expression of the five GEMs (expression at such levels could be a result of cross-mapping to the homologous region on the B. napus “C” genome, C08) (Fig. 3d). On the other hand, Tapidor, Westar, and Zhongshuang 11 exhibited moderate resistance and gene expression levels. We hypothesized that Quinta has a deletion on B. napus chromosome A09, whereas Tapidor, Westar, and Zhongshuang 11 have not.
To determine the query coverage, defined as the percentage of the 0.51 MB region (comprising 107 genes in linkage disequilibrium with the most significant SNP marker) that aligned with the B. napus pan-transcriptome, we performed a BLAST analysis against the pan-transcriptome for each reference genotype. Among the 107 genes, a sequence of five consecutive genes displayed nearly 100% query coverage in Tapidor, Westar, and Zhongshuang 11, whereas Quinta exhibited reduced coverage: Cab013522.1 (40%), Cab013523.1 (0%), Cab013524.1 (0%), Cab013525.1 (0%), Cab013526.1 (0%) (Table S8). BLAST analysis of the homologous region on chromosome C08 in Quinta revealed no differences compared to the other genotypes, suggesting a specific deletion on chromosome A09 (Fig. S2a). Linear regression analysis indicated that the expression of these five genes varied across B. napus genotypes and correlated with resistance to V. longisporum (Fig. S2b). This implies that both quantitative and qualitative resistance are associated with this region since both the deletion (qualitative) and gene expression in genotypes without the deletion (quantitative) correlate with resistance.
To identify potential genes within this deletion region that could contribute to V. longisporum resistance, we proposed that their homologs in the B. napus genome might also be relevant GEMs for resistance. Each of the five candidate genes on A09 only had one homolog, on chromosome C08. None of the five homologs were significant GEMs; however, linear regression analysis showed a significant association between gene expression of the Cab013522.1 homolog (Bo8g101810.1) and the Cab013524.1 homolog (Bo8g101830.1) and resistance to V. longisporum (Fig. S2b). Downstream of the five genes were two additional genes with nearly 100% sequence coverage in Quinta but reduced coverage in Tapidor, Westar, and Zhongshuang 11: BnaA09g43290D (0%) and Cab013532.1 (40%). However, neither of these genes was expressed during the third-leaf stage used for GEM analysis. The corresponding C08 homolog of Cab013532.1 (Bo8g101910.1) also was not expressed at this stage, and no C08 homolog for BnaA09g43290D was identified.
To infer the potential function of the five gene candidates, we identified their putative A. thaliana orthologs using BLAST (Table 6). The orthologs included a microtubule-binding motor protein, Kinesin motor family protein, KIN7.2 (Cab013522.1); a carbohydrate kinase, Xylulose kinase 1, XK1 (Cab013523.1); a multidrug and toxin extrusion (MATE) transporter, Enhanced disease susceptibility 5 homolog, EDS5H (Cab013524.1); a plastid isoform aldolase, Fructose-bisphosphate aldolase 1, FBA1 (Cab013525.1); and a B-box zinc finger family transcription factor, B-box domain protein 18, BBX18 (Cab013526.1).
Discussion
QDR is dependent on pathogen lifestyle
In this study, we used an AT pipeline to investigate constitutive QDR to B. napus fungal pathogens: A. brassicicola, B. cinerea, P. brassicae, and V. longisporum and define the “host genetic signature” of common and unique loci associated with resistance or susceptibility.
Throughout our analyses, we observed no significant GWA associations, in contrast to the discovery of numerous strongly significant associations through GEM analysis. While GWA primarily focuses on SNP variation, GEM explores how measurable variation in gene expression levels correlates with phenotypic traits. The limitation of GWA lies in its inability to capture the intricate complexity of gene regulation, interactions, and expression patterns, as it predominantly identifies genomic loci associated with traits without explicitly considering gene expression levels. Notably, SNP variation may or may not affect gene function, whereas gene expression often plays a direct role in determining the functional activity of genes. Moreover, variations such as SNPs and Indels in intergenic and promoter sequences, which have the potential to control gene expression, or variants within genes only induced following pathogen challenge, are not captured in mRNAseq and therefore will not be reflected in our GWA unless gene-based linked markers are present within the region. Additionally, high allelic heterogeneity may be present across the association panel, reducing the ability to detect the effects of causal SNPs on phenotypes (Atwell et al. 2020). GEMs have been shown to be effective in identifying causal genes controlling glucosinolate content (Harper et al. 2012; Lu et al. 2014), seed erucic acid content (Havlickova et al. 2018), seed size, yield parameters, stem strength (Miller et al. 2018, 2019), flowering time (Woodhouse et al. 2021), and freezing stress (Huang et al. 2020). Our analyses further highlight the power of GEM in detecting the overarching molecular mechanisms that influence quantitative resistance traits.
We initially hypothesized broad-spectrum QDR across multiple pathogens with contrasting lifestyles. However, our findings revealed shared QDR loci only within similar pathogen lifestyles, either hemibiotrophic or necrotrophic. This distinction likely stems from differing infection strategies. In fact, phenotypic resistance to necrotrophic pathogens was inversely related to resistance against hemibiotrophic pathogens. This contrast was mirrored by 21 GEMs associated with broad-spectrum resistance or susceptibility to necrotrophic pathogens with an opposite association with hemibiotrophic pathogens.
Immune responses, such as cell death, can effectively counteract the endophytic growth of some pathogens like P. brassicae and V. longisporum, but necrotrophic pathogens like A. brassicicola and B. cinerea may exploit cell death mechanisms to promote their infection (McCombe et al. 2022). Such contrasting effects have been previously observed; for example, an NBS-LRR gene conferring resistance to biotrophic pathogen Puccinia coronata f. sp. avenae acts as a susceptibility factor (LOV1) for necrotrophic pathogen Cochliobolus victoriae (Lorang et al. 2007). Compellingly, in our study, the ortholog of a novel A. thaliana NBS-LRR, Bo8g081460.1, was associated with resistance to both hemibiotrophic pathogens and susceptibility to both necrotrophic pathogens, as was the predicted A. thaliana ortholog of FBR12 which was previously shown to be involved in cell death induced by Pseudomonas syringae (Hopkins et al. 2008).
We explored the overlap between QDR loci and PTI and their varying associations with resistance based on pathogen lifestyle. While most QDR GEMs did not relate to PTI (Corwin et al. 2017), those that were positively associated with chitin-induced ROS response were associated with resistance to hemibiotrophic fungi but susceptibility to necrotrophic fungi. For instance, the B. napus ortholog of A. thaliana ALDH3H1 positively correlated with chitin-induced ROS response and V. longisporum resistance, yet negatively correlated with resistance to B. cinerea. ALDH proteins—for example, a homolog of A. thaliana ALDH3H1, ALDH3I1—can mitigate oxidative stress by scavenging ROS (Kotchoni et al. 2006). Rapid ROS production during PTI can induce programmed cell death, which can confer susceptibility to necrotrophic pathogens like B. cinerea (Levine et al. 1994; Kotchoni and Gachomo 2006). B. cinerea even produces ROS to promote its infection (Govrin and Levine 2000), and ROS scavengers reduce infection severity (Tiedemann 1997). Thus, it follows that genotypes with a potentially greater ability to scavenge ROS would be less susceptible to B. cinerea.
It is intriguing that susceptibility to necrotrophic pathogens, resistance to hemibiotrophic pathogens, and ROS production aligned with a co-expressed module enriched in GEMs linked to photosynthesis regulation. Given that ROS are generated extensively during photosynthesis, it is reasonable to suggest that some genes influencing photosynthesis could impact ROS production levels, consequently influencing resistance to necrotrophic and hemibiotrophic pathogens. Indeed, previous studies have noted distinct regulation of photosynthesis-related genes during A. brassicicola infection (Macioszek et al. 2020).
Identification of a narrow chromosomal region associated with broad-spectrum QDR
By combining SNP and gene expression data through AT, we identified a novel locus associated with resistance to V. longisporum. Further in silico analyses of the region indicated five potential candidate genes, any of which could underlie the resistance phenotype. Alleles of the BBX18 homolog also segregated with resistance to P. brassicae, and KIN7.2, EDS5H, and BBX18 homologs were GEMs for resistance to P. brassicae, suggesting that these could be associated with broad-spectrum QDR. Although we investigated this region using reference genotype with a genomic deletion (qualitative resistance), the expression of the KIN7.2, XK1, EDS5H, FBA1, and BBX18 homologs within other genotypes in the B. napus population varied and was significantly associated with resistance suggesting this locus is related to QDR.
KIN7.2 is involved in microtubule-based movement, and—in the animal field—kinesins have been implicated in protein trafficking for antifungal defense (Kurowska et al. 2012; Ogbomo et al. 2018). EDS5H is a homolog of EDS5, encoding a MATE protein that functions in plant salicylic acid (SA) dependent defense (Nawrath et al. 2002). However, unlike EDS5, in A. thaliana, EDS5H is constitutively expressed in green tissues independent of pathogen infection and does not contribute to pathogen-induced SA accumulation (Parinthawong et al. 2015). Nevertheless, MATE proteins transport a broad range of substrates, such as organic acids, plant hormones, and secondary metabolites, which could influence QDR (Nawrath et al. 2002; Takanashi et al 2014; Su et al. 2022). Conversely, BBX18 and the additional XK1 and FBA1 genes in the potential deletion linked to resistance lack documented defense associations.
With our in silico analyses, we demonstrate the power of the AT pipeline to quickly narrow down on a predicted region of interest for broad-spectrum QDR. Additional functional analyses such as gene editing are needed to validate and identify the causal gene in B. napus and how it affects resistance to V. longisporum and other pathogens during infection.
Broad-spectrum QDR is an opportunity for crop breeding
Achieving broad-spectrum disease resistance without compromising resistance to other pathogens is a crucial goal in crop breeding. For example, GEMs associated with susceptibility and no antagonistic associations with resistance could be interesting targets for gene editing to improve disease control without the introduction of transgenes (Hua et al. 2019). In addition, considering crop breeding for resistance, we identified GEMs associated with resistance to both necrotrophic pathogens and no antagonistic associations with susceptibility to the hemibiotrophic pathogens. We also identified GEMs associated with resistance to both hemibiotrophic pathogens and no antagonistic associations with susceptibility to the necrotrophic pathogens including four GEMs homologous to A. thaliana ACIP1— ACIP1 was previously implicated in flg22-induced ROS response and resistance to Pseudomonas syringae (Cheong et al. 2014). One of these four was positively associated with chitin-induced ROS response, highlighting it as a candidate for further investigation into its role in PTI and broad-spectrum QDR. Future work could extend our findings to evaluate how broad the spectrum of disease control is likely to be using specific fungal strains or mutants, other B. napus pathogens, and different crop species.
Conclusions
Here, we used an AT pipeline to identify novel loci involved in QDR to several B. napus fungal pathogens A. brassicicola, B. cinerea, P. brassicae, and V. longisporum. We observed that broad-spectrum QDR loci were dependent on the lifestyle of the pathogen, as were associations with GEMs related to PAMP-induced ROS production. By combining GWA and GEM analyses, we were able to identify a novel region of interest for V. longisporum resistance and potentially broad-spectrum QDR. To our knowledge, this is the first time this approach with several pathosystems has been used to identify loci involved in broad-spectrum QDR. In summary, this study provides new insight into broad-spectrum QDR and highlights interesting targets for crop breeding.
Data availability
The R-based GAGA pipeline is available at https://github.com/bsnichols/GAGA. Our population structure is available in Fell et al. (2023) [37]. Raw datasets used for the GAGA pipeline are available at Zenodo (https://zenodo.org/records/10499917). Inquiries related to the raw data can be directed to the respective scientific groups responsible for collecting the data and conducting preliminary analyses for each pathogen, as detailed in the author contributions section. Supplemental files are also available at https://zenodo.org/record/8321694.
References
Amas JC, Thomas WJ, Zhang Y, Edwards D, Batley J (2023) Key advances in the new era of genomics-assisted disease resistance improvement of Brassica species. Phytopathology 113(5):771–785. https://doi.org/10.1094/PHYTO-08-22-0289-FI
Atwell S, Huang YS, Vilhjálmsson BJ, Willems G, Horton M, Li Y, Meng D, Platt A, Tarone AM, Hu TT, Jiang R (2020) Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465(7298):627–631. https://doi.org/10.1038/nature08800
Benson JM, Poland JA, Benson BM, Stromberg EL, Nelson RJ (2015) Resistance to gray leaf spot of maize: genetic architecture and mechanisms elucidated through nested association mapping and near-isogenic line analysis. PLoS Genet 11:e1005045. https://doi.org/10.1371/journal.pgen.1005045
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23:2633–2635. https://doi.org/10.1093/bioinformatics/btm308
Brun H, Chèvre AM, Fitt BD, Powers S, Besnard AL, Ermel M, Huteau V, Marquer B, Eber F, Renard M, Andrivon D (2010) Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol 185:285–299. https://doi.org/10.1111/j.1469-8137.2009.03049.x
Cheong MS, Kirik A, Kim JG, Frame K, Kirik V, Mudgett MB (2014) AvrBsT acetylates Arabidopsis ACIP1, a protein that associates with microtubules and is required for immunity. PLoS Pathog 10:e1003952. https://doi.org/10.1371/journal.ppat.1003952
Corwin JA, Kliebenstein DJ (2017) Quantitative resistance: more than just perception of a pathogen. Plant Cell 29:655–665. https://doi.org/10.1105/tpc.16.00915
Corwin JA, Copeland D, Feusier J, Subedy A, Eshbaugh R, Palmer C, Maloof J, Kliebenstein DJ (2016) The quantitative basis of the Arabidopsis innate immune system to endemic pathogens depends on pathogen genetics. PLoS Genet 12:e1005789. https://doi.org/10.1371/journal.pgen.1005789
Dakouri A, Lamara M, Karim M, Wang J, Chen Q, Gossen BD, Strelkov SE, Hwang S-F, Peng G, Yu F (2021) Identification of resistance loci against new pathotypes of Plasmodiophora brassicae in Brassica napus based on genome-wide association mapping. Sci Rep 11:6599. https://doi.org/10.1038/s41598-021-85836-9
Deppe JP, Rabbat R, Hörtensteiner S, Keller B, Martinoia E, Lopéz-Marqués RL (2018) The wheat ABC transporter Lr34 modifies the lipid environment at the plasma membrane. J Biol Chem 293:18667–18679. https://doi.org/10.1074/jbc.RA118.002532
Fell H, Ali AM, Wells R, Mitrousia GK, Woolfenden H, Schoonbeek H-J, Fitt BDL, Ridout CJ, Stotz H (2023) Novel gene loci associated with susceptibility or cryptic quantitative resistance to Pyrenopeziza brassicae in Brassica napus. Theor Appl Genet 136:71. https://doi.org/10.1007/s00122-023-04243-y
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ (2018) Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360:739–742. https://doi.org/10.1126/science.aap7999
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227. https://doi.org/10.1146/annurev.phyto.43.040204.135923
Gómez-Gómez L, Boller T (2000) FLS2: an LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5:1003–1011. https://doi.org/10.1016/S1097-2765(00)80265-8
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757. https://doi.org/10.1016/S0960-9822(00)00560-1
Harper AL, Trick M, Higgins J, Fraser F, Clissold L, Wells R, Hattori C, Werner P, Bancroft I (2012) Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nat Biotechnol 30:798–802. https://doi.org/10.1038/nbt.2302
Havlickova L, He Z, Wang L, Langer S, Harper AL, Kaur H, Broadley MR, Gegas V, Bancroft I (2018) Validation of an updated associative transcriptomics platform for the polyploid crop species Brassica napus by dissection of the genetic architecture of erucic acid and tocopherol isoform variation in seeds. Plant J 93:181–192. https://doi.org/10.1111/tpj.13767
He Z, Cheng F, Li Y, Wang X, Parkin IA, Chalhoub B, Liu S, Bancroft I (2015) Construction of Brassica A and C genome-based ordered pan-transcriptomes for use in rapeseed genomic research. Data Brief 4:357–362. https://doi.org/10.1016/j.dib.2015.06.016
Hopkins MT, Lampi Y, Wang TW, Liu Z, Thompson JE (2008) Eukaryotic translation initiation factor 5A is involved in pathogen-induced cell death and development of disease symptoms in Arabidopsis. Plant Physiol 148:479–489. https://doi.org/10.1104/pp.108.118869
Hua K, Zhang J, Botella JR, Ma C, Kong F, Liu B, Zhu J-K (2019) Perspectives on the application of genome-editing technologies in crop breeding. Mol Plant 12:1047–1059. https://doi.org/10.1016/j.molp.2019.06.009
Huang M, Liu X, Zhou Y, Summers RM, Zhang Z (2019) BLINK: a package for the next level of genome-wide association studies with both individuals and markers in the millions. Gigascience 8:giy154. https://doi.org/10.1093/gigascience/giy154
Huang Y, Hussain MA, Luo D, Xu H, Zeng C, Havlickova L, Bancroft I, Tian Z, Zhang X, Cheng Y, Zou X (2020) A Brassica napus reductase gene dissected by associative transcriptomics enhances plant adaption to freezing stress. Front Plant Sci 26(11):971. https://doi.org/10.3389/fpls.2020.00971
Hurni S, Scheuermann D, Krattinger SG, Kessel B, Wicker T, Herren G, Fitze MN, Breen J, Presterl T, Ouzunova M (2015) The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc Natl Acad Sci 112:8780–8785. https://doi.org/10.1073/pnas.1502522112
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014. https://doi.org/10.1093/emboj/19.15.4004
Kemen E, Jones JDG (2012) Obligate biotroph parasitism: can we link genomes to lifestyles? Trends Plant Sci 17:448–457. https://doi.org/10.1016/j.tplants.2012.04.005
Kim S, Kiraly M, Jiang M, Stuart JM, Eizinger Am Wylie BN, Davidson GS (2001) A gene expression map for Caenorhabditis elegans. Science 5537:2087–2092. https://doi.org/10.1126/science.1061603
Kotchoni SO, Gachomo EW (2006) The reactive oxygen species network pathways: an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci 31:389–404. https://doi.org/10.1007/bf02704112
Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D (2006) Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell Environ 29:1033–1048. https://doi.org/10.1111/j.1365-3040.2005.01458.x
Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13:181–185. https://doi.org/10.1016/j.pbi.2009.12.010
Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint BG, Ménasché G (2012) Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood 119:3879–3889. https://doi.org/10.1182/blood-2011-09-382556
Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, van Esse HP, Smoker M, Rallapalli G, Thomma BP, Staskawicz B et al (2010) Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol 28:365–369. https://doi.org/10.1016/0006-291x(75)90482-9
Langfilder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. https://doi.org/10.1186/1471-2105-9-559
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593. https://doi.org/10.1016/0092-8674(94)90544-4
Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z (2012) GAPIT: genome association and prediction integrated tool. Bioinformatics 28:2397–2399. https://doi.org/10.1093/bioinformatics/bts444
Liu X, Huang M, Fan B, Buckler ES, Zhang Z (2016) Iterative usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet 12:e1005767. https://doi.org/10.1371/journal.pgen.1005767
Lloyd SR, Schoonbeek H-J, Trick M, Zipfel C, Ridout CJ (2014) Methods to study PAMP-triggered immunity in Brassica species. Mol Plant Microb Interact 27:286–295. https://doi.org/10.1094/mpmi-05-13-0154-fi
Lorang JM, Sweat TA, Wolpert TJ (2007) Plant disease susceptibility conferred by a “resistance” gene. Proc Natl Acad Sci 104:14861–14866. https://doi.org/10.1073/pnas.0702572104
Lu G, Harper AL, Trick M, Morgan C, Fraser F, O’Neill C, Bancroft I (2014) Associative transcriptomics study dissects the genetic architecture of seed glucosinolate content in Brassica napus. DNA Res 21(6):613–625. https://doi.org/10.1093/dnares/dsu024
Macioszek VK, Lawrence CB, Kononowicz AK (2018) Infection cycle of Alternaria brassicicola on Brassica oleracea leaves under growth room conditions. Plant Pathol 67:1088–1096. https://doi.org/10.1111/ppa.12828
Macioszek VK, Gapińska M, Zmienko A, Sobczak M, Skoczowski A, Oliwa J, Kononowicz AK (2020) Complexity of Brassica oleracea–Alternaria brassicicola susceptible interaction reveals downregulation of photosynthesis at ultrastructural, transcriptional, and physiological levels. Cells 9:2329. https://doi.org/10.3390/cells9102329
McCombe CL, Greenwood JR, Solomon PS, Williams SJ (2022) Molecular plant immunity against biotrophic, hemibiotrophic, and necrotrophic fungi. Essays Biochem 66:581–593. https://doi.org/10.1042/ebc20210073
McDowell JM, Woffenden BJ (2003) Plant disease resistance genes: recent insights and potential applications. Trends Biotechnol 21:178–183. https://doi.org/10.1016/s0167-7799(03)00053-2
Miller CN, Harper AL, Trick M, Wellner N, Werner P, Waldron KW, Bancroft I (2018) Dissecting the complex regulation of lodging resistance in Brassica napus. Mol Breeding 38:1–18. https://doi.org/10.1007/s11032-018-0781-6
Miller C, Wells R, McKenzie N, Trick M, Ball J, Fatihi A, Dubreucq B, Chardot T, Lepiniec L, Bevan MW (2019) Variation in expression of the HECT E3 ligase UPL3 modulates LEC2 levels, seed size, and crop yields in Brassica napus. Plant Cell 31(10):2370–2385. https://doi.org/10.1105/tpc.18.00577
Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci 104:19613–19618. https://doi.org/10.1073/pnas.0705147104
Moore JW, Herrera-Foessel S, Lan C, Schnippenkoetter W, Ayliffe M, Huerta-Espino J, Lillemo M, Viccars L, Milne R, Periyannan S (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat Genet 47:1494–1498. https://doi.org/10.1038/ng.3439
Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd N, Jones J (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18:650–655. https://doi.org/10.1016/j.cub.2008.03.060
Nawrath C, Heck S, Parinthawong N, Métraux J-P (2002) EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14:275–286. https://doi.org/10.1105/tpc.010376
Nelson R, Wiesner-Hanks T, Wisser R, Balint-Kurti P (2018) Navigating complexity to breed disease-resistant crops. Nat Rev Genet 19:21–33. https://doi.org/10.1038/nrg.2017.82
Nichols B (2022) A pipeline for GEM And GWAS automation. GitHub URL https://github.com/bsnichols/GAGA. https://zenodo.org/badge/latestdoi/512807075
Ogbomo H, Timm-McCann M, Barnes T, Xiang RF, Jamil K, Ganguly A, Stack D, Huston SM, Li SS, Colarusso P (2018) Granule-dependent NK cell killing of cryptococcus requires kinesin to reposition the cytolytic machinery for directed cytotoxicity. Cell Rep 24:3017–3032. https://doi.org/10.1016/j.celrep.2018.08.027
Parinthawong N, Cottier S, Buchala A, Nawrath C, Métraux J-P (2015) Localization and expression of EDS5H a homologue of the SA transporter EDS5. BMC Plant Biol 15:135. https://doi.org/10.1186/s12870-015-0518-1
Pilet-Nayel ML, Moury B, Caffier V, Montarry J, Kerlan MC, Fournet S, Durel CE, Delourme R (2017) Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection. Front Plant Sci 8:1838. https://doi.org/10.3389/fpls.2017.01838
Plaisier SB, Taschereau R, Wong JA, Graeber TG (2010) Rank–rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res 38:e169. https://doi.org/10.1093/nar/gkq636
Qasim MU, Zhao Q, Shahid M, Samad RA, Ahmar S, Wu J, Fan C, Zhou Y (2020) Identification of QTLs containing resistance genes for Sclerotinia stem rot in Brassica napus using comparative transcriptomic studies. Front Plant Sci 11:776. https://doi.org/10.3389/fpls.2020.00776
R Core Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Raman R, Diffey S, Barbulescu DM, Coombes N, Luckett D, Salisbury P, Cowley R, Marcroft S, Raman H (2020) Genetic and physical mapping of loci for resistance to blackleg disease in canola (Brassica napus L.). Sci Rep 10:4416. https://doi.org/10.1038/s41598-020-61211-y
Roux F, Voisin D, Badet T, Balagué C, Barlet X, Huard-Chauveau C, Roby D, Raffaele S (2014) Resistance to phytopathogens e tutti quanti: placing plant quantitative disease resistance on the map. Mol Plant Pathol 15:427–432. https://doi.org/10.1111/mpp.12138
Roy J, Shaikh TM, del Río ML, Hosain S, Chapara V, Rahman M (2021) Genome-wide association mapping and genomic prediction for adult stage sclerotinia stem rot resistance in Brassica napus (L.) under field environments. Sci Rep 11:21773. https://doi.org/10.1038/s41598-021-01272-9
Samira R, Kimball JA, Samayoa LF, Holland JB, Jamann TM, Brown PJ, Stacey G, Balint-Kurti PJ (2020) Genome-wide association analysis of the strength of the MAMP-elicited defense response and resistance to target leaf spot in sorghum. Sci Rep 10:20817. https://doi.org/10.1038/s41598-020-77684-w
Schoonbeek H-J, Del Sorbo G, De Waard MA (2001) The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol Plant Microbe Interact 14:562–571. https://doi.org/10.1094/mpmi.2001.14.4.562
Schoonbeek H-J, Wang HH, Stefanato FL, Craze M, Bowden S, Wallington E, Zipfel C, Ridout CJ (2015) Arabidopsis EF-Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol 206:606–613. https://doi.org/10.1111/nph.13356
Schweizer P, Stein N (2011) Large-scale data integration reveals colocalization of gene functional groups with meta-QTL for multiple disease resistance in barley. Mol Plant Microbe Interact 24:1492–1501. https://doi.org/10.1094/mpmi-05-11-0107
Song J-M, Guan Z, Hu J, Guo C, Yang Z, Wang S, Liu D, Wang B, Lu S, Zhou R (2020) Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nature Plants 6:34–45. https://doi.org/10.1038/s41477-019-0577-7
Stefanato FL, Abou-Mansour E, Buchala A, Kretschmer M, Mosbach A, Hahn M, Bochet CG, Métraux JP, Schoonbeek H-J (2009) The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J 58:499–510. https://doi.org/10.1111/j.1365-313X.2009.03794.x
Storey JD (2011) False discovery rate. In: Lovric M (ed) International encyclopedia of statistical science. Springer, Heidelberg, Berlin, Germany, pp 504–508
Su Q, Rong W, Zhang Z (2022) The pathogen-induced MATE gene TaPIMA1 is required for defense responses to Rhizoctonia cerealis in wheat. Int J Mol Sci 23:3377. https://doi.org/10.3390/ijms23063377
Takanashi K, Shitan N, Yazaki K (2014) The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol 31:417–430. https://doi.org/10.5511/plantbiotechnology.14.0904a
Tiedemann AV (1997) Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol Mol Plant Pathol 50:151–166. https://doi.org/10.1006/pmpp.1996.0076
Vetter MM, Kronholm I, He F, Häweker H, Reymond M, Bergelson J, Robatzek S, de Meaux J (2012) Flagellin perception varies quantitatively in Arabidopsis thaliana and its relatives. Mol Biol Evol 29:1655–1667. https://doi.org/10.1093/molbev/mss011
Vleeshouwers VGAA, van Dooijeweert W, Govers F, Kamoun S, Colon LT (2000) The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta 210:853–864. https://doi.org/10.1007/s004250050690
Wang J, Zhang Z (2021) GAPIT Version 3: boosting power and accuracy for genomic association and prediction. Genom Proteomics Bioinform 19:629–640. https://doi.org/10.1016/j.gpb.2021.08.005
Wisser RJ, Sun Q, Hulbert SH, Kresovich S, Nelson RJ (2005) Identification and characterization of regions of the rice genome associated with broad-spectrum, quantitative disease resistance. Genetics 169:2277–2293. https://doi.org/10.1534/genetics.104.036327
Woodhouse S, He Z, Woolfenden H, Steuernagel B, Haerty W, Bancroft I, Irwin JA, Morris RJ, Wells R (2021) Validation of a novel associative transcriptomics pipeline in Brassica oleracea: identifying candidates for vernalisation response. BMC Genom 22:1–13. https://doi.org/10.1186/s12864-021-07805-w
Zeise K, Tiedemann AV (2001) Morphological and physiological differentiation among vegetative compatibility groups of Verticillium dahliae in relation to V. longisporum. J Phytopathol 149:469–475. https://doi.org/10.1111/j.1439-0434.2001.tb03879.x
Zheng X, Koopmann B, von Tiedemann A (2019) Role of salicylic acid and components of the phenylpropanoid pathway in basal and cultivar-related resistance of oilseed rape (Brassica napus) to Verticillium longisporum. Plants 8:491. https://doi.org/10.3390/plants8110491
Zheng X (2018) Verticillium longisporum on oilseed rape (Brassica napus L.): differential roles of salicylic acid, seed transmission and plant colonization in greenhouse and field conditions. Dissertation, Niedersächsische Staats-und Universitätsbibliothek Göttingen, Germany
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760. https://doi.org/10.1016/j.cell.2006.03.037
Funding
The authors thank Paweł Jedyński and Tomasz Jęcz (University of Lodz, Poland) for help with plant cultivation and inoculation with A. brassicicola and Aiming Qi for advice about P. brassicae data analysis. VKM and AKK were supported by the National Center for Research and Development, Poland (grant no. ERA-CAPS II/1/2015). XZ and AvT were supported by the German Research Foundation (grant no. ERA-CAPS DFG; TI170/13–1). HJS, RW, RB, CJR, HF, GKM, HUS, and BDLF were funded by the Biotechnology and Biological Sciences Research Council (BBSRC) grants BB/N005007/1 and BB/N005112/1 (as part of the ERA-CAPS consortium “MAQBAT”). HJS, RW, CNJ, GSS, RB, and CJR were also funded by the John Innes Institute strategic program grants Plant Health BB/P012574/1 (Response BBS/E/J/000PR9796), Molecules from Nature BB/P012523/1, Genes in the Environment BB/P013511/1, and Brassica Rapeseed and Vegetable Optimization (BRAVO) BB/P003095/1.
Author information
Authors and Affiliations
Contributions
VKM did experiments with A. brassicicola infection and analyzed the data with AKK. XZ did experiments and data curation with V. longisporum infection supervised by AvT. HF and GKM did experiments with P. brassicae and analyzed data with HUS and HF, and HUS and BDLF contributed to the conception and experimental design. RB and HJS did experiments and data curation with B. cinerea and PAMPs, HJS contributed to the conception and experimental design, and HJS and CNJ analyzed the data. CNJ did statistical analysis of all datasets, CNJ and RW did association transcriptomics, GSS did the WGCNA analysis, and BS did the BLAST validation of the genomic deletion. CNJ, RW, and CJR contributed to the conception, data interpretation, and in silico analyses post-AT. The first draft of the manuscript was written by CNJ, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Lixi Jiang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
122_2024_4569_MOESM1_ESM.xlsx
Mean, normalized phenotype data for resistance to fungal pathogens and PAMP-induced ROS response. Pathogens include Alternaria brassicicola, Botrytis cinerea, and Verticillium longisporum and PAMPs include chitin, flg22, and elf18. These data were used for association transcriptomic analysis. Data for Pyrenopeziza brassicae can be found in original publication (Fell et al. 2023). (XLSX 45 KB)
122_2024_4569_MOESM2_ESM.xlsx
Full list of single nucleotide polymorphism (SNP) markers and significance levels from genome-wide association (GWA) analyses for resistance to fungal pathogens and for PAMP-induced ROS response. Pathogens include Alternaria brassicicola, Botrytis cinerea, Pyrenopeziza brassicae, and Verticillium longisporum and PAMPs include chitin, flg22, and elf18. Each excel tab contains the analyses for a single trait. The best-fit model for GWA analysis is indicated in the tab title. Manhattan plots showing marker-trait association are included for data visualization; the x-axis indicates SNP location along the chromosome; the y-axis indicates the -log10(p) (P value). (XLSX 114266 KB)
122_2024_4569_MOESM3_ESM.xlsx
Full list of gene expression markers (GEMs) and significance levels from GEM analyses for resistance to fungal pathogens and for PAMP-induced ROS response. Pathogens include Alternaria brassicicola, Botrytis cinerea, Pyrenopeziza brassicae, and Verticillium longisporum and PAMPs include chitin, flg22, and elf18. Each Excel tab contains the analyses for a single trait. Manhattan plots showing marker-trait association are included for data visualization; the x-axis indicates GEM location along the chromosome; the y-axis indicates the -log10(p) (P value). (XLSX 21442 KB)
122_2024_4569_MOESM4_ESM.xlsx
184 gene expression markers (GEMs) associated with chitin-induced ROS. These GEMs are compared with GEMs associated with resistance to pathogens (Alternaria brassicicola, Botrytis cinerea, Pyrenopeziza brassicae, and Verticillium longisporum) and ROS response induced by flg22, and elf18. Lists correspond to Venn diagrams in Fig. 2. The first tab includes all 184 GEMs associated with chitin-induced ROS. The subsequent tabs include lists of shared GEMs associated with chitin-induced ROS response and each additional trait (quantitative disease resistance (QDR) to each fungal pathogen or additional PAMP-induced ROS responses). The title of each tab indicates the data included in each comparison and the number of shared GEMs. Predicted Arabidopsis thaliana orthologs and corresponding descriptions are shown where possible. (XLSX 1347 KB)
122_2024_4569_MOESM5_ESM.xlsx
Enrichment analyses to determine if the number of gene expression markers (GEMs) shared between different lists is greater than the number of GEMs that would be expected by chance (e.g., lists of quantitative disease resistance (QDR) GEMs for two fungal pathogens). The representation factor is the number of overlapping GEMs divided by the expected number of overlapping GEMs drawn from two independent groups (traits), considering the total number of GEMs sequenced (53884). A representation factor > 1 indicates more overlap than expected of two groups, a representation factor < 1 indicates less overlap than expected, and a representation factor of 1 indicates that the two groups by the number of genes expected for independent groups of genes. (XLSX 12 KB)
122_2024_4569_MOESM6_ESM.xlsx
Results from Weighted Co-expression Gene Network Analysis (WGCNA). The first tab indicates significant modules from WGCNA analysis. Black and magenta modules are associated with antagonistic effects on resistance/susceptibility to all four pathogens. The second tab includes a full list of the GEM markers (Table S3), which are in significant WGCNA modules. The third, fourth, and fifth tabs indicate all significant GEMs in the black module, GO terms associated with GEMs in the black module, and all GO terms associated with the black module, respectively. The sixth, seventh, and eighth tabs indicate all significant GEMs in the magenta module, GO terms associated with GEMs in the magenta module, and all GO terms associated with the magenta module, respectively. (XLSX 5788 KB)
122_2024_4569_MOESM7_ESM.xlsx
Shared gene expression markers (GEMs) associated with resistance to different fungal pathogens (Alternaria brassicicola, Botrytis cinerea, Pyrenopeziza brassicae, and Verticillium longisporum). Lists correspond to matrices and Venn diagrams in Fig 3. The first tab includes all GEMs associated with quantitative disease resistance (QDR) to the fungal pathogens. The subsequent tabs include lists of shared GEMs associated with QDR to two or more fungal pathogens. The title of each tab indicates the data included in each comparison and the number of shared GEMs. Predicted Arabidopsis thaliana orthologs and corresponding descriptions are shown where possible. (XLSX 122 KB)
122_2024_4569_MOESM8_ESM.xlsx
List of genes in linkage disequilibrium with the top marker for Verticillium longisporum resistance. Data from genome-wide association (GWA) analysis on chromosome A09 (107 genes) (Tab 1) and the homoeologous region on C08 (Tab 2). Their percentage identity and query coverage in Brassica napus reference genotypes Quinta, Tapidor, Westar, and Zhongshuang 11 compared to the B. napus pantranscriptome is indicated. Predicted Arabidopsis thaliana orthologs and corresponding descriptions are shown where possible. (XLSX 41 KB)
122_2024_4569_MOESM9_ESM.eps
There is a correlation between phenotypes and GEMs associated with chitin-, flg22, and elf18-induced ROS. (A) Flg22- and elf18-induced ROS response (arbitrary units (a.u.)) of different B. napus genotypes. Arbitrary units signify resistance values obtained through the reciprocal of disease susceptibility scores, derived from estimated marginal means and normalized to a 0-1 range for comparative data visualization. The position of reference genotypes Quinta (Q), Tapidor (T), Westar (W) and Zhongshuang 11 (Z) are indicated. (B) Correlation between the PAMP-induced ROS responses of B. napus lines elicited by chitin, flg22, and elf18. Positive correlations (green) are indicated between pair-wise comparisons of ROS responses. (C) Venn diagrams showing the overlap between GEMs associated with chitin-, flg22- and elf18- induced ROS. (EPS 466 KB)
122_2024_4569_MOESM10_ESM.eps
Chromosome C08 region homologous to the region on A09 associated with resistance to Verticillium longisporum. (A) Heatmap indicating query coverage in Quinta (Q), Tapidor (T), Westar (W), and Zhongshuang 11 (Z) (compared to the Brassica napus pantranscriptome) for a region on chromosome C08, homoeologous to the region on A09 containing 107 genes predicted to be in linkage disequilibrium with Cab013526.1.651.A. The C08 homeolog (Bo8g101810.1), of Cab013526.1 (A09) is indicated. (B) Linear regression analysis of gene expression (RPKM) of the five Gene Expression Markers (GEMs) in the genomic deletion on chromosome A09 and their homeologs on chromosome C08 relative to resistance to V. longisporum (arbitrary units (a.u.) in 193 B. napus genotypes. Arbitrary units signify resistance values obtained through the reciprocal of disease susceptibility scores, derived from estimated marginal means and normalized to a 0-1 range for comparative data visualization. (EPS 817 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacott, C.N., Schoonbeek, Hj., Sidhu, G.S. et al. Pathogen lifestyle determines host genetic signature of quantitative disease resistance loci in oilseed rape (Brassica napus). Theor Appl Genet 137, 65 (2024). https://doi.org/10.1007/s00122-024-04569-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04569-1