Abstract
Key message
We mapped Ryd4Hb in a 66.5 kbp interval in barley and dissociated it from a sublethality factor. These results will enable a targeted selection of the resistance in barley breeding.
Abstract
Virus diseases are causing high yield losses in crops worldwide. The Barley yellow dwarf virus (BYDV) complex is responsible for one of the most widespread and economically important viral diseases of cereals. While no gene conferring complete resistance (immunity) has been uncovered in the primary gene pool of barley, sources of resistance were searched and identified in the wild relative Hordeum bulbosum, representing the secondary gene pool of barley. One such locus, Ryd4Hb, has been previously introgressed into barley, and was allocated to chromosome 3H, but is tightly linked to a sublethality factor that prevents the incorporation and utilization of Ryd4Hb in barley varieties. To solve this problem, we fine-mapped Ryd4Hb and separated it from this negative factor. We narrowed the Ryd4Hb locus to a corresponding 66.5 kbp physical interval in the barley ‘Morex’ reference genome. The region comprises a gene from the nucleotide-binding and leucine-rich repeat immune receptor family, typical of dominant virus resistance genes. The closest homolog to this Ryd4Hb candidate gene is the wheat Sr35 stem rust resistance gene. In addition to the fine mapping, we reduced the interval bearing the sublethality factor to 600 kbp in barley. Aphid feeding experiments demonstrated that Ryd4Hb provides a resistance to BYDV rather than to its vector. The presented results, including the high-throughput molecular markers, will permit a more targeted selection of the resistance in breeding, enabling the use of Ryd4Hb in barley varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Virus diseases cause significant yield losses and represent an increasing threat to agricultural crop production worldwide (Oerke 2006). Among them, the Barley yellow dwarf virus (BYDV) complex is responsible for one of the most widespread and economically important viral diseases of cereals. Transmitted in a persistent and circulative manner by several species of aphids, BYDV causes dwarfing and leaf discoloration, leading to significant yield loss in major cereal crops, in particular barley, wheat, maize, and oats (Ali et al. 2018). In recent years, it has become increasingly important in winter barley with an incidence that could reach 70% and yield loss of up to 80% (Beoni et al. 2016; Dedryver et al. 2010; Ordon et al. 2009). As climate change scenarios predict longer and warmer autumns, which favor aphid infestations of winter crop fields, BYDV could become one of the most threatening diseases of cereal crops (Roos et al. 2011; Trebicki 2020). Reduction of yield losses by insecticide-based vector control is possible in principle, but undesirable for ecological reasons. To ensure sustainable barley cultivation in the expanding infestation areas and thus secure yields and quality in the long term, the cultivation of virus resistant varieties would provide the best solution.
So far, three genes and some QTLs have been described as providing partial resistance or tolerance to BYDV in barley. The gene ryd1, providing recessive intermediate tolerance, was identified by Suneson (1955) but is still not cloned (Niks et al. 2004). Its effectiveness is low and it is rarely used in breeding. Ryd2 was identified from an Ethiopian barley landrace (Schaller et al. 1964). It provides field tolerance to the virus serotypes BYDV-PAV, BYDV-MAV, and BYDV-SGV (Baltenberger et al. 1987). Mapped close to the centromere of chromosome 3H (Collins et al. 1996), Ryd2 is used in several breeding lines (Kosova et al. 2008) where it can reduce significantly the yield loss caused by BYDV (Beoni et al. 2016). The third gene, Ryd3 was also identified from an Ethiopian barley landrace (Niks et al. 2004). The gene was mapped in the centromeric region of chromosome 6H but, despite fine mapping on more than 3,000 F2 plants (Lüpken et al. 2014), the mapping interval is still large. Ryd3 has been transferred to commercial varieties where it provides a quantitative resistance, improved when in combination with Ryd2 (Riedel et al. 2011). QTLs on chromosomes 1H, 2H, 4H, 5H, and 7H have been reported, however, providing only a limited level of tolerance (Toojinda et al. 2000; Riedel et al. 2011; Hu et al. 2019). No complete resistance to BYDV or its aphid vectors is known in barley, and broadening the genetic basis of resistance is therefore needed to ensure a durable and stable production of winter barley fields.
The secondary gene pool of barley, consisting of the species Hordeum bulbosum, has not yet been used to improve resistance to the BYDV complex. Michel (1996) identified resistance to BYDV in the tetraploid (2n = 4x = 28) Hordeum bulbosum accession A17 (Bu10/2) from the Botanical Garden of Montevideo, Uruguay. Plants of this accession remained ELISA-negative for BYDV after several inoculations with aphids charged with the virus isolates BYDV-PAV1 Aschersleben, BYDV-MAV1 Aschersleben, and CYDV (Cereal yellow dwarf virus)-RPV Dittersbach (Habekuß et al. 2004). A17 was used as a parent in interspecific crosses and backcrosses with H. vulgare cv. Igri to generate an H. bulbosum introgression to barley. Its resistance was described as complete, dominant, and monogenic, and the locus, assigned to chromosome 3H, was named Ryd4Hb (Scholz et al. 2009). Adversely, a recessive sublethality factor was cosegregating with Ryd4Hb in the respective introgression. A study revealed low aphid feeding on the H. bulbosum A17 accession, suggesting that resistance may not be acting on the virus but rather against the aphid vector (Schliephake et al. 2013).
The present study reports the fine mapping of the Ryd4Hb locus, the identification of candidate genes, and the description of aphid feeding behavior on susceptible and resistant introgression lines.
Material and methods
Plant material
BC2F5 and BC2F6 families derived from BC2F4 plants from the Scholz et al. (2009) population were used for the low-resolution linkage mapping and development of an introgression line lacking the sublethality factor. This population is named LM_Pop.
Two additional populations were generated to map Ryd4Hb at a higher resolution. FM_Pop1 was derived from a BC2F7 plant from LM_Pop crossed successively with three different barley elite varieties. The pedigree of the 15 lineages that constitute FM_Pop1 is presented in supplementary Table 1. The donor of resistance in the FM_Pop2 was a BC2F8 plant derived from the BC2F6 line JKI-5215 homozygous for the H. vulgare (Hv)-allele in the sublethality factor locus. As for FM_Pop1, the resistance donor was crossed successively with two different barley elite varieties. The pedigree of the lines of five lineages that constitute FM_Pop2 is presented in Supplementary Table 2. The F1 plants from the successive crosses were checked with markers to ensure the presence of the H. bulbosum (Hb)-allele at the Ryd4Hb locus and were further selfed to obtain the F2 lineages forming FM_Pop1 and FM_Pop2.
Test of resistance to BYDV
Five to ten Rhopalosiphum padi aphids of the biotype R07, that were reared on BYDV-PAV1 Aschersleben (PAV1-ASL) infected plants as described by Kern et al. (2022), were placed on each one-week-old seedlings to be phenotyped in an air-conditioned greenhouse (20 °C, 16 h photoperiod, 10 klx). The aphids were killed after two days using the insecticide Confidor®WG 70 (Bayer CropScience AG, Germany). Further cultivation of the plants was carried out. Five to six weeks after inoculation, leaf samples of 50 mg from two leaves were taken and tested by a double-antibody sandwich-enzyme-linked immunosorbent assay (DAS-ELISA) according to Clark and Adams (1977) using custom-made antibodies. The viral content was evaluated by measuring extinction at 405 nm on a microtiter plate reader (Opsys MR, ThermoLabsystems or Tecan Sunrise, Tecan) one hour after the addition of the enzyme substrate. Based on negative controls, a extinction threshold was set in each experiment, usually at 0.1, under which a plant was classified as resistant. Phenotyping tests for gene mapping were performed on ten to 15 seeds of progenies of each genotype.
DNA extraction
Genomic DNA from the LM-Pop was either isolated following a slightly modified protocol after Stein et al. (2001) or using the BioSprint 96 DNA Plant Kit (Qiagen) and the BioSprint 96 working station (Qiagen) following the manufacturer’s instructions. DNA was dissolved in TE buffer, quantified via photometric approaches (NanoQuant, Tecan, Austria) and diluted to a working concentration of 10 ng/µl. DNA extractions of plants from FM_Pop1 and FM_Pop2 were carried out according to the protocol described in Milner et al. (2019).
Marker development for low-resolution linkage mapping
The EST-derived simple sequence repeat (SSR) anchor markers GBM1050 and GBM1059 (Scholz et al. 2009; Stein et al. 2007; Thiel et al. 2003; Varshney et al. 2007) were kindly provided by Prof. Andreas Graner (Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben). The sequence-tagged sites (STS) markers GLMWG883 and GLABC161 were derived from the sequence of RFLP probe MWG883 (Rostoks et al. 2005; Szűcs et al. 2009) and barley anchor marker ABC161 (Close et al. 2009), respectively. Orthology of the interval on the rice chromosome 1 using the mapping of the anchor marker ABC161 at 40.43 Mbp on said chromosome allowed for the development of 18 tentative consensus (TC) markers polymorphic between Hv and Hb on chromosome 3HL.
Additional polymorphisms between the H. bulbosum and H. vulgare genomes for marker development were identified by RNA-seq and Massive analysis of cDNA-ends (MACE). To this end, 1,000 plants from BC2F5 of LM_Pop were screened with the TC marker TC173485. Among them, 200 plants homozygous for the Hb-allele, considered resistant, and 200 plants homozygous for the Hv-allele, considered susceptible, were selected. In total, 100 plants of each category were inoculated by aphids carrying the isolate BYDV-PAV1, and an equal number of plants were infested with control aphids without virus in a separate chamber. 1h, 4h, 8h, and 24h after inoculation, plant material of 25 genotypes per category and treatment was harvested and sent to GenXPro (Frankfurt am Main, Germany) for RNA isolation and sequencing. RNA-seq and MACE were performed as described in Santos et al. (2018) and Braun et al. (2019), respectively. In short, the raw data were cleaned of adapter sequences using the software TagDust (Lassmann et al. 2009). All RNA-seq datasets were combined to create a reference library. Assembly was performed using the software Trinity (Grabherr et al. 2011). The reads of the individual libraries were hereafter mapped to the reference library and single-nucleotide polymorphisms (SNPs) were identified using the software SNVMix (Goya et al. 2010). SNPs between the H. bulbosum and H. vulgare genomes were identified, and sequences 100 bps up- and downstream of each SNP were determined. Annotation of the SNP-containing sequences was done by using the database Swiss-Prot (Boeckmann et al. 2003).
Additional polymorphisms were retrieved from exome capture sequencing, performed according to Wendler et al. (2014) on the H. bulbosum parent A17 (Wendler et al. 2015) and the BC2F4 plant 5194/5, homozygous for the 3HL-H. bulbosum introgression and mapped on the first barley genome assembly (International Barley Genome Sequencing Consortium 2012). Single-nucleotide variants between H. vulgare and H. bulbosum were called with samtools (SNP call score < 200). Variants located within 200 bp of the end of a reference sequence contig or supported by less than fivefold sequence read coverage were excluded from further evaluation. The flanking sequences (50–60 bp) of variant positions were used for marker assay development.
The primer design for PCR markers was carried out using Primer 3 (Untergasser et al. 2012). Conversion of SNPs to cleaved amplified polymorphic sequences (CAPS) markers was done by using SNP2CAPS (Thiel et al. 2004). All markers used for the low-resolution linkage mapping are described in Supplementary Table 3.
Marker development for high-resolution linkage mapping
Exome capture data of the H. bulbosum A17 parent and of the BC2F4 plant 5194/5 were remapped onto the barley reference genome MorexV3 (Mascher et al. 2021) together with the exome capture data of 13 barley varieties from (Russell et al. 2016) (cultivars ‘Barke’, ‘Bonus’, ‘Borwina’, ‘Bowman’, ‘Foma’, ‘Gull’, ‘Harrington’, ‘Haruna Nijo’, ‘Igri’, ‘Kindred’, ‘Morex’, ‘Steptoe’, and ‘Vogelsanger Gold’). Reads mapping and variant calling were performed as described in Milner et al. (2019). The SNP matrix was filtered on the following criteria: Heterozygous and homozygous calls had to have a minimum mapping quality score of three and five, respectively, and be supported by a minimum of ten reads. SNP sites were retained if they had less than 20% missing data and less than 20% heterozygous calls. SNPs that were within the Ryd4Hb 20 Mbp interval defined by the low-resolution linkage mapping, homozygous for one allele in all barley varieties and for the other allele in A17 and the BC2F4 introgression line, were retained.
For six SNPs, a 100 bp sequence containing the SNP in its center was provided to LGC genomics (Berlin, Germany) for KASP marker production (Supplementary Table 4). Within the sublethality factor interval, ten more SNPs were retrieved and sequences of 100 bp around each one were sent to 3CR Bioscience (Welwyn Garden City, UK) for PACE assay design (Supplementary Table 5). Primers were ordered from Metabion (Germany) and mixed according to 3CR Bioscience (Welwyn Garden City, UK) recommendations.
Thirteen CAPS markers (Supplementary Table 6) were developed using NEBcutter (Vincze et al. 2003) to identify the cutting enzyme and Primer 3 (Untergasser et al. 2012) to design the PCR primers. Ryd4_CAPS1, Ryd4_CAPS2, Ryd4_CAPS3, and Ryd4_CAPS4 were designed based on the same or a very close SNP to the one genotyped by Ryd4_KASP1, Ryd4_KASP2, Ryd4_KASP5, and Ryd4_KASP3, respectively.
Genotyping assays
For PCRs of SSR, STS, and CAPS markers were carried out in a volume of 10µL containing 50-100ng of DNA, 1X PCR buffer (Qiagen), 0.5 µM of each primer, 0.5 U of Taq DNA polymerase (Qiagen), and 0.2 mM of dNTPs. PCR amplification was carried out with an initial 10 min step at 95°C, followed by a touchdown profile of ten cycles at 95 °C for 30 s, 60 °C for 30 s with a 0.5 °C reduction per cycle, and 72 °C for 1 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s then 72 °C for 1 min, and a last step of 7 min at 72 °C. For CAPS markers, a 5 µl aliquot of the PCR product was digested in 10 µL with 1 U of restriction enzyme and 1 × of the appropriate digestion buffer at the temperature recommended by the manufacturer. Pre- and post-digestion PCR products were separated on 2.5% agarose gels followed by ethidium bromide staining or in 10% polyacrylamide gels followed by silver nitrate staining according to Budowle et al. (1991).
Detection of SNPs as genetic markers was performed by high-resolution melt analysis (HRM) by using the Rotor Gene Technology (Qiagen). PCR was carried out in 20 µl volume containing 20 ng template DNA, 1 × buffer (Promega), 2.5 mM MgCl2, 0.8 mM dNTP mix, 0.5 µM of each primer, 1 × EvaGreen Dye (Biotium, Inc.), and 0.3 U Taq DNA polymerase (Promega). A touchdown PCR protocol was conducted with a temperature gradient from 60 to 50 °C. The melt curve analysis was conducted by ramping from 65 to 95 °C with a 0.1 °C decrease per capture.
Genotyping assays with KASP and PACE markers were carried out as described in Pidon et al. (2020).
Genetic linkage analysis
LM_Pop was genotyped with 4 EST-derived SSR and STS markers, 18 TC markers (16 STS and 2 CAPS), 19 markers derived from MACE (6 SNP markers scored using HRM, and 13 STS markers denoted as MACE_b), 9 markers derived from RNA-seq experiment (6 SNP and 3 CAPS markers) denoted as RNASeq_b and comp, and 3 markers derived from exome capture (one CAPS, STS and SNP markers). Linkage analysis was performed using the JoinMap® 4.1 software (Van Ooijen 2006). Genetic maps were displayed and edited in MapChart2.2 (Voorrips 2002).
Pan-genome comparison
The flanking markers and the Ryd4Hb interval in the MorexV3 genome were searched on the 19 other assemblies of the barley pan-genome (Jayakodi et al. 2020) using BLAST + (Camacho et al. 2009). The resulting intervals were extracted and reannotated through a combination of alignments of the Morex candidate genes to them, the search for the presence of conserved domains using NCBI conserved domains (Lu et al. 2019), and nucleotide-binding and leucine-rich repeat immune receptors (NLR) annotation with NLR-Annotator (Steuernagel et al. 2020). The interval structures were compared using Easyfig with blastn (Sullivan et al. 2011).
Aphid feeding experiment
In order to test if Ryd4Hb provides resistance to aphids, a resistant and a susceptible progeny of the heterozygous lines at Ryd4Hb locus FM2_C05_3_206 and FM2_C01_5_228 were selected. The susceptible progenies FM2_C05_3_206_2 and FM2_C01_5_228_2 were carrying Hv-alleles at both Ryd4_CAPS19 and Ryd4_CAPS24, while the resistant lines FM2_C05_3_206_4 and FM2_C01_5_228_6 displayed a recombination event in the interval and carrying a Hb-allele at Ryd4_CAPS24 or Ryd4_CAPS19, respectively. The feeding behavior on the respective genotypes of adult apterous non-viruliferous R. padi (clone R07) of random age was observed using the electrical penetration graph (EPG) technique (Tjallingii 1978). For each genotype, the feeding behavior of 12 to 16 aphids, each on an individual healthy plant, was measured. Plants for EPG experiments were reared in a greenhouse and were used at a 3–4 leaf stage where aphids were placed on the lower side of the second leaf. Aphids were reared as described before (Kern et al. 2022). Aphids were starved for 1 h before they were placed on the leaf. The observation period was set to 8 h, and recording was started after all aphids were placed. For data acquisition, the GIGA-8 EPG amplifier and EPG stylet software (EPG Systems, Wageningen, the Netherlands) were used and data were analyzed with the EPG stylet analysis module. Waveforms were annotated according to Tjallingii (1978) and Tjallingii & Esch (1993). Subsequently, selected parameters were analyzed by using an Excel workbook (Alvarez et al., 2021). Recordings of aphids that fell from the leaf or escaped during the experiment were not used.
Results
Low-resolution linkage mapping of Ryd4 Hb
From LM_Pop, 1,125 BC2F5 and BC2F6 were used for the low-resolution linkage mapping of Ryd4Hb. Phenotyping of progenies revealed 276 susceptible and 849 resistant plants, 279 of which died at early stages. This high mortality rate was expected due to the recessively inherited sublethality factor closely linked to Ryd4Hb and therefore segregating with the resistance. This locus prevents normal plant development, resulting in severely repressed growth and premature plant death (Fig. 1). Because of the close linkage between the two loci, we considered that all plants that died in the BC2F5 and BC2F6 families could be defined as homozygous resistant, while the resistant plants that survived would be heterozygous at Ryd4Hb. The phenotype distribution would indeed fit the expected 1:2:1 ratio of homozygous resistant, heterozygous resistant, and homozygous susceptible genotypes for a dominant monogenic inheritance of Ryd4Hb resistance (Chi-square goodness of fit test: χ2 = 0.216, p = 0.90), confirming the previous observation for that locus on the BC2F4 generation (Scholz et al. 2009).
Non-infected five-month-old homozygous resistant plants either homozygous Hb (left) or Hv (right) for the sublethality locus. The growth of plants homozygous for the sublethality factor is greatly reduced compared to plants carrying the resistance locus but lacking the sublethality locus. Almost all such plants will have died before reaching heading stage
The population was genotyped with 53 codominant markers, and the linkage mapping was performed on the 1,014 individuals with a low amount of missing data. The introgression, defined by the largest interval between polymorphic markers, was estimated to be 18.7 cM-long, delimited distally by the marker EXCAP_16 and proximally by the marker MACE_b_79 (Fig. 2). On the MorexV3 reference, the linkage map spans 22.5 Mbp in the terminal region of chromosome 3HL, between the coordinates 573.9 and 596.4 Mbp. The resistance locus Ryd4Hb was flanked by the MACE marker Mace_b_53 and the TC marker TC262452 with a genetic distance of 0.3 cM proximally and 0.5 cM distally, respectively.
Development of a recombinant introgression line lacking the sublethality factor
The exploitation of Ryd4Hb for breeding programs requires the selection of homozygous resistant and vital (not sublethal) plants. To select recombinants lacking the sublethality factor, 12,133 BC2F6 plants of LM_Pop were screened with markers TC262452 and Mace_b_53. Among those, 3,103 plants were homozygous for Hb-alleles, 6,020 heterozygous (Hb/Hv), and 3,010 homozygous for Hv-alleles at both markers. The 3,103 plants homozygous for Hb-alleles were propagated and the progenies were checked for resistance. One progeny named JKI-5215 was both resistant and vital, hence homozygous Hb at the two markers Ryd4Hb flanking markers, and heterozygous or homozygous Hv at the sublethality factor.
The JKI-5215 population, made of 43 BC2F7 progeny of plant JKI-5215, was genotyped with all 16 markers that mapped distally from Ryd4Hb. Markers TC262452 to RNASeq_b_1 were homozygous for Hb-alleles, whereas Mace_b_30 and all the remaining markers distally located were segregating in a 1:2:1 fashion (Fig. 3). The JKI-5215 plant was therefore homozygous for Hb-alleles from Mace_b_53 to RNASeq_b_1 and heterozygous from Mace_b_30 to EXCAP_16. The eight BC2F7 plants homozygous for the Hb-alleles in the distal fragment were sublethal, while the others were non-lethal. The recombination occurred within the initial 3HL introgression and resulted in a reduced Hb segment of 3.4 cM. A BC2F8 from the family JKI-5215 homozygous for the Hv segment in the sublethality factor interval was selfed and used as the resistant donor for the FM_Pop2.
Characterization of 43 plants from the family JKI-5215. Each vertical bar represents one offspring from JKI-5215. White, black, and gray fragments represent Hb fragments, Hv fragments, and heterozygous genotypes at the markers indicated on the right side, respectively. The light gray fragments at the dominant marker Mace_b_69 are either Hv or heterozygous genotypes. The phenotype is indicated below the figure as ‘nL’ for vital plants and ‘L’ for sublethal ones. For better readability, marker positions are not to scale
High-resolution mapping of Ryd4 Hb in two F2 populations
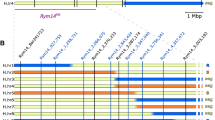
To precisely map Ryd4Hb, 5,589 F2 plants from FM_Pop1 and 10,155 F2 plants from FM_Pop2 were genotyped with two KASP markers to identify recombination at the locus. The four KASP markers designed to this end were close to the flanking markers identified by low-resolution mapping for Ryd4_KASP2 and Ryd4_KASP5, and around 10 kbp upward and 5 kbp downward for Ryd4_KASP1 and Ryd4_KASP4 on the MorexV3 genome assembly, respectively. Plants from FM_Pop1 were screened with Ryd4_KASP1 and Ryd4_KASP4, and plants from FM_Pop2 with Ryd4_KASP1 and Ryd4_KASP5. Indeed, Ryd4_KASP4 is located in the sublethality factor interval and was segregating in FM_Pop1, but not in FM_Pop2 where it was fixed Hv in its resistant JKI-5215-derived donor. We identified 46 and 68 recombinant plants in FM_Pop1 and FM_Pop2, corresponding to 0.41% and 0.33% of recombination per meiosis, respectively. The positions of the SNPs of the markers Ryd4_KASP1, Ryd4_KASP4, and Ryd4_KASP5 were identified in the GBS mapping data from 92 recombinant inbred lines of the cross ‘Barke’ x ‘Morex’ (Mascher et al. 2013). A distance of 15 cM was observed between the markers Ryd4_KASP1 and Ryd4_KASP4, and of 12.6 cM between Ryd4_KASP1 and Ryd4_KASP5, indicating by definition a 15 and 12.6% probability of recombination in pure H. vulgare background in these intervals, respectively. The observed rate of recombination at the Ryd4Hb locus is therefore about 35–40 times lower than expected for the same genetic interval in a pure intraspecific barley cross.
Recombinants were phenotyped on up to 15 offsprings and genotyped with the 13 CAPS markers. The resulting interval for Ryd4Hb comprised 66.5 kbp in the MorexV3 genome assembly between the coordinates 592,685,940 and 592,752,329 flanked by CAPS19_2 and CAPS24, describing an interval harboring six recombination events (Fig. 4, Supplementary Table 7). Four genes are annotated with high confidence on the MorexV3 genome in that interval: HORVU.MOREX.r3.3HG0318400, HORVU.MOREX.r3.3HG0318420, HORVU.MOREX.r3.3HG0318450, and HORVU.MOREX.r3.3HG0318470, respectively, annotated as encoding for an S-formylglutathione hydrolase, a partial NLR with a coiled-coil domain (CNL) lacking LRR domain which is likely a pseudogene, a complete CNL, and an ankyrin-repeat-containing protein. Those genes are referred to SFGH, pCNL1, CNL2, and ANK, respectively. The genes’ homology revealed that locus Ryd4Hb is syntenic to the Triticum monococcum locus Sr35, conferring resistance to wheat stem rust (Saintenac et al. 2013). The candidate genes present a high similarity to one of the Sr35 candidate genes. In particular, the translation of the complete CNL2 gene sequence from the MorexV3 reference genome shows 83% identity with the SR35 protein while the respective coding sequence shows 88.7% nucleotide identity. The Ryd4Hb interval also overlaps almost completely the ones of the Rph13 leaf rust resistance gene from the H. vulgare ssp. spontaneum accession Hs2986 (Jost et al. 2020) and of the Jmv2 resistance gene to the Japanese soil-borne wheat mosaic virus from the barley cultivar ‘Sukai Golden’ (Okada et al. 2022). Rph13 is located on chr3H between the coordinates 592,658,337 and 592,786,929 on MovexV3 (128.6 kbp). Comparing the number of recombinants, the size of the interval in which they occurred, and the size of the mapping population for Rph13 and Ryd4Hb (four recombinants in 128.6 kbp out of 719 plants and six recombinant plants in 66.5 kbp out of 15,774, respectively), the recombination rate observed in the Ryd4hb populations is 7.5 times lower than observed in the intraspecific cross used to map Rph13.
High-resolution mapping of the Ryd4Hb locus. (a) High-resolution mapping in the 5,589 F2 plants from FM_Pop1 and 10,155 plants of FM_Pop2. The numbers in brackets below markers show the sum of individual recombinants between the resistance locus and the corresponding marker in FM_Pop1 and FM_Pop2, respectively. The white numbers in the gray bar indicate the total number of recombinants between adjacent markers in the two populations. The discrepancy between the two types of recombinant numbers in the Ryd4_CAPS18-Ryd4_KASP5 interval is due to four sublethal recombinants whose position relative to Ryd4Hb could not be ascertained due to their lack of progeny to phenotype. (b) Marker saturation using 18 recombinant plants at the Ryd4Hb locus. Recombinants from FM_Pop1 that died are excluded. (c) The 66.5 kbp final Ryd4Hb interval in H. vulgare ‘Morex’ with annotation of the candidate genes compared the T. monococcum DV92 orthologous genes in the Sr35 interval (only fragments with more than 80% nucleotide identity are shown)
Ryd4 Hb locus diversity in the barley pan-genome
The orthologous intervals of the MorexV3 Ryd4Hb region were retrieved from 19 diverse genome assemblies of a published barley pan-genome (Jayakodi et al. 2020) (Supplementary Table 8). We annotated the intervals by using a combination of methods: mapping the ‘Morex’ genes, searching for NLR genes with NLR-Annotator (Steuernagel et al. 2020), and confirming the absence of additional conserved domains with NCBI conserved domains (Lu et al. 2019). The analysis revealed a very large divergence of the Ryd4Hb interval in the different genotypes (Fig. 5a, Supplementary Fig. 1). The shortest orthologous interval is the one of MorexV3. The largest is the one of the accession ‘HOR 21599’, 406 kbp-long and containing 10 NLRs, of which five are complete. The interval in the cultivars ‘Akashinriki’ and ‘Du-Li Huang’ is affected by a large inversion relative to MorexV3 of around 500 kbp (Fig. 5b). The observed diversity between haplotypes is mainly explained by the presence of different repetitive elements and duplications. The degree of divergence in the H. vulgare gene pool in this interval suggests that an even greater diversity and divergence may be anticipated for the corresponding region in the H. bulbosum genome.
Graphical representations of the Ryd4Hb interval in barley diversity. (a) Graphical representation of the haplotype size and gene composition in the barley pan-genome. Gray horizontal bars represent the size of the Ryd4Hb corresponding intervals in sequenced barley genotypes. Forward slashes represent the breakpoint due to the large inversion in Akashinriki and Du-Li Huang comprising 440 kbp homologous a region outside of the Ryd4Hb interval in the other genome assemblies. Genes are displayed as arrows: S-formylglutathione hydrolase genes (yellow), partial NLRs (orange), complete NLRs (green), and ankyrin-repeats-containing genes (blue). (b) Schematic representation of the inversion between Morex and Akashinriki at the locus. Inverted and non-inverted regions are shown in blue and red, respectively. Lighter colors represent regions outside of the Ryd4Hb interval in the MorexV3 assembly (colour figure online)
Mapping of the sublethality factor
To better understand why the Ryd4Hb carrying H. bulbosum chromatin is causing sublethality when introgressed into H. vulgare, we used recombinants identified in the frame of the Ryd4Hb mapping to pinpoint the responsible factor more precisely. The low-resolution linkage mapping located it distally from the marker RNASeq_b_1, which corresponds to position 594,019,595 on chromosome 3H of the MorexV3 genome. To precise its interval, ten PACE markers were designed between Ryd4_KASP18 and Ryd4_KASP22 (Supplementary Table 5), and used to genotype plants recombining in the interval. Six recombination events were available: the one of JKI-5215 that we mapped using 24 non-recombinant F2 plants from lineage FM2_C01 of FM_Pop2, and the ones of five F2 plants from FM_Pop1 recombining between Ryd4_KASP5 and Ryd4_CAPS22, of which four were vital plants and one was sublethal (plant FM1_C08_340_48, which died before heading, Fig. 6). The genotyping of 24 non-recombinant FM_Pop2 F2 plants confirmed that the JKI-5215 recombination event occurred between Ryd4_KASP5 and Ryd4_CAPS22 (Fig. 5), and more precisely between markers Ryd4_leth3 and Ryd4_leth4 (594,290,776 to 594,700,400 bp). The genotyping and phenotyping of thirty-two F3 plants from each of the four vital plants recombining between Ryd4_KASP5 and Ryd4_CAPS22 placed the sublethality factor proximally of marker Ryd4_leth7. This was confirmed by the genotyping of the sublethal plant FM1_C08_340_48 from FM_Pop1 which was identified as recombinant between Ryd4_leth6 and Ryd4_leth7 (Fig. 6). The sublethality factor could therefore be assigned to a 483 kbp interval between markers Ryd4_leth3 and Ryd4_leth7 (594,290,776–594,773,972 bp on chromosome 3H of MorexV3). This interval in MorexV3 is annotated with 15 high-confidence genes described in Table 1. Among those genes, one or several could be essential genes for plant development with no orthologs in the corresponding region of the H. bulbosum genome.
Graphical representation of the genotype of lines recombining in the sublethality interval. Genotypes of FM1_C09_457_13, FM1_C01_42_106, FM1_C12_559_101, and FM1_C08_344_20 are inferred from those of 32 of their offsprings. The genotype of JKI-5215 is reconstructed from those of 24 F2 plants of FM_Pop2 from the lineage FM2_C01. Markers are depicted as vertical black lines and genotypes as horizontal bars. White, black, and gray segments represent Hv, Hb, and heterozygotes genotypes, respectively. Phenotypes are described on the right as sublethal (L), sublethality segregating in the progeny (HL), and vital (nL). For better readability, marker positions are not to scale
Ryd4 Hb does not prevent aphid feeding
Resistance to insect-transmitted viruses can either act at the level of resistance to the virus or to the vector. To test if Ryd4Hb provides resistance to the BYDV aphid vector, we monitored the feeding of 12 to 16 aphids by EPG on five lines: two susceptible F4 lines (FM2_C05_3_206_2 and FM2_C01_5_228_2), their two resistant sister lines (FM2_C05_3_206_4 and FM2_C01_5_228_6), and the susceptible barley cultivar ‘Igri’ which was the susceptible parent of LM-Pop and JKI-5215 (Fig. 7a). As none of the selected EPG parameters showed a normal distribution according to a Shapiro–Wilk test with a p-value threshold of 0.05, we selected the Kruskal–Wallis test for multiple comparisons. No significant differences between the lines were observed for the selected parameters s_Np (χ2 = 6.78, df = 4, p = 0.148), s_C (χ2 = 2.35, df = 4, p = 0.671), s_F (χ2 = 4.64, df = 4, p = 0.327), s_G (χ2 = 2.96, df = 4, p = 0.565), s_E1 (χ2 = 1.35, df = 4, p = 0.854), s_E2 (χ2 = 3.16, df = 4, p = 0.534), and s_sE2 (χ2 = 3.52, df = 4, p = 0.474) (Fig. 7b, Supplementary Table 9). The most divergent line was Igri, with an increased median duration for s_Np and decreased median durations for s_E2 and s_sE2, probably due to differences in the genetic background with the other lines.
Aphid feeding behavior on resistant and susceptible lines (a) Graphical representation of the lines used to test the aphid feeding behavior on resistant and susceptible lines. Loci are depicted as vertical black lines and genotypes as horizontal bars. White and black, segments represent Hv and Hb genotypes, respectively. (b) Duration of the different feeding events, indicated by different EPG waveforms, on the five lines summed up by type. s_Np: total duration of all non-probing events. s_C: total duration of pathway phase. s_F: total duration of penetration problems. s_G: total duration of xylem drinking. s_E1: total duration of secretion of watery saliva. s_E2: total duration of phloem sap ingestion. s_sE2: total duration of sustained phloem sap ingestion (E2 > 10 min). (c) Duration of selected EPG parameters indicating different layers of resistance regarding aphid–plant interaction. t > 1Pr: Time from the start of recording to 1st probing—epidermal resistance factors. t > 1E: Time to 1st sieve element contact indicated by E1 behavior—including epidermal ad mesophyll located resistance. t > 1E2: Time to 1st ingestion indicated by waveform E2—including epidermal, mesophyll, and sieve element located resistance. t > 1sE2: Time to 1st sustained ingestion—including epidermal, mesophyll, and sieve element located resistance interfering with establishment of long-term feeding sites. Lines in the box plots indicate the median, and whiskers show the upper and lower 1.5xIQR (interquartile range) with dots indicating outliers
In addition to these general parameters, parameters associated with epidermis (t > 1Pr) and mesophyll located (t > 1E) but also sieve element located (t > 1E2, t > 1sE2) defense responses were inspected. Igri showed the longest median durations for reaching the sieve elements (t > 1E) and to reach ingestion (t > 1E2 and t > 1sE2). However, none of the parameters t > 1Pr (χ2 = 3.83, df = 4, p = 0.43), t > 1E (χ2 = 6.32, df = 4, p = 0.176), t > 1E2 (χ2 = 3.43, df = 4, p = 0.489), and t > sE2 (χ2 = 5.42, df = 4, p = 0.247) differed significantly between the tested lines (Fig. 7c, Supplementary Table 9).
Discussion
BYDV is a major threat to barley cultivation that is expected to increase in the following years, as autumns become longer and warmer in Northern Europe (Roos et al. 2011; Trebicki 2020), thus breeding for BYDV resistance increases in priority. So far only partial resistance has been discovered in the H. vulgare primary gene pool (Baltenberger et al. 1987; Collins et al. 1996; Lüpken et al. 2014; Niks et al. 2004; Schaller et al. 1964; Suneson 1955). Here, we report the high-resolution mapping of Ryd4Hb, the first resistance gene to BYDV in barley, originating from the wild relative and secondary gene pool species H. bulbosum. We mapped the gene in an interval corresponding to a physical segment of 66.5 kbp in the MorexV3 barley reference genome. Mapping was achieved despite linkage of the resistance locus to a sublethality factor and despite the heavily reduced recombination frequency between the orthologous H. vulgare and H. bulbosum genomes. Although we identified six recombination events in this interval, the high sequence repetitiveness and complexity at that locus in barley prevented us from designing additional markers and further reducing it in the absence of genomic information from H. bulbosum.
At the Ryd4Hb locus, four genes are annotated with high confidence on the MorexV3 genome, including two genes from the CNL family, one pseudogene, and one likely to be functional. CNL genes are part of the larger NLR family which are the most common class of resistance genes to biotic stress. They code for intracellular proteins that form complexes (Wang et al. 2019) recognizing, directly or indirectly, pathogen effector molecules and typically induce local cell death responses. More than 30 NLR genes conferring resistance to viruses have been cloned so far (Boualem et al. 2016; Sett et al. 2022) and more are candidates. An H. bulbosum homolog of pCNL1 or CNL2 is therefore a very promising candidate for Ryd4Hb. The Ryd4Hb locus is orthologous to the Sr35 resistance locus from the wheat wild relative T. monococcum (Saintenac et al. 2013). Sr35 codes for a CNL protein that shares 83% identity with the translated H. vulgare sequence of CNL2 and provides resistance to the fungal pathogen Puccinia graminis f. sp. tritici causing wheat stem rust. Ryd4Hb interval is also overlapping the one of the Rph13 resistance to leaf rust in the H. vulgare spp. spontaneum accession ‘PI 531849’ (Jost et al. 2020), and the large Jmv2 interval providing resistance to the Japanese soil-borne wheat mosaic virus from the barley cultivar ‘Sukai Golden’ (Okada et al. 2022). Interestingly, the best homolog of Sr35 in rice is LOC_Os11g43700, which was identified as a resistance gene to the Rice yellow mottle virus in the African rice species Oryza glaberrima (Pidon et al. 2017; Bonnamy et al. 2023). None of those two viruses is part of the Tombusviridae to which BYDV belongs. It is not rare that closely related NLRs provide resistance to different classes of pathogens. A good example is the potato NLRs genes GPA2 and RX1 which provide resistance against the nematode Globodera pallida and potato virus X, respectively, and share 88% of their amino acid sequence (Van Der Vossen et al. 2000). The comparison of the Ryd4Hb interval in the barley pan-genome (Jayakodi et al. 2020) demonstrated a very large diversity at this locus, including NLR duplications. NLRs genes are indeed frequently under diversifying selection and tend to evolve and duplicate by interallelic recombination between orthologs and by unequal crossing-over between paralogs (Baggs et al. 2017; Chen et al. 2010; Ding et al. 2007; Guo et al. 2011; Li et al. 2010; Michelmore & Meyers 1998; Zhou et al. 2004). The Sr35/Ryd4Hb locus is one of those very diverse and dynamic loci that could be described as R gene factories. Together with the homology with other resistance loci, this locus’ NLR diversity strongly suggests Ryd4Hb to be a CNL. However, in addition to the CNL genes, a H. bulbosum ortholog of the ANK could also be a good candidate. Indeed, the structure of the encoded protein is close to the one of Arabidopsis ACCELERATED CELL DEATH 6 (ACD6) protein. ACD6 confers enhanced resistance to bacterial pathogens, including Pseudomonas syringae, by increasing the level of salicylic acid and inducing spontaneous cell death (Rate et al. 1999; Dong 2004; Lu et al. 2003, 2005). We also cannot exclude that Ryd4Hb resistance is due to presence/absence variation of a gene in the primary and the secondary gene pool of barley; thus, the resistance gene from H. bulbosum may have no ortholog in the H. vulgare interval. Cloning of Ryd4Hb would therefore most likely require a de novo genome assembly of the Ryd4Hb interval in a resistant genotype (introgression line of H. vulgare or resistance donor genotype of H.bulbosum).
Resistance to insect-transmitted viruses, like the one provided by Ryd4Hb, can either be a direct resistance to the virus or a resistance to the vector, which would in effect prevent infection and therefore provide indirect virus resistance. The melon NLR Vat resistance gene is the model of this indirect resistance. VAT provides resistance to Aphis gossypii and to all the viruses it transmits tested so far, including the Cucumber mosaic virus (Boissot et al. 2016). It recognizes an effector from A. gossypii and triggers the hypersensitive response, stopping at the same time any viral infection that may have occurred. BYDV cannot be inoculated to barley mechanically, so only resistance to the aphid vector was tested. A previous study showed that R. padi aphids were feeding less and having a shorter salivation time on the Ryd4Hb H. bulbosum resistance donor A17 compared to the BYDV-susceptible H. bulbosum line A21, suggesting that this could be the reason for A17 BYDV resistance (Schliephake et al. 2013). However, our study showed no differences in aphid feeding patterns on closely related resistant and susceptible lines, accompanied by an absence of BYDV infection in the resistant lines, suggesting that the preliminary observation on the H. bulbosum donor was probably due to A17 genetic background rather than Ryd4Hb. We therefore conclude that Ryd4Hb provides direct resistance to BYDV.
Ryd4Hb is a prime example of the importance of crop wild relatives serving as genetic resources and gene donors in breeding schemes to achieve efficient and durable disease resistance. The advantage of using a crop wild relative in prebreeding schemes as a unique source of resistance, however, comes at a cost. First of all, the lack of genetic collinearity could lead to the definition of an incorrect interval. In the case of the H. vulgare x H. bulbosum cross, a high genetic collinearity between the two genomes was previously reported (Wendler et al. 2017). We could confirm that this is also the case at this locus, as no discrepancy between the recombination pattern and the physical map could be detected in this study. The risk also arises as genetic distances typically translate into large physical distances due to the reduced frequency of recombination between the two genomes. In the case of the Ryd4Hb locus, recombination is reduced by a factor of 7.5 compared to an intraspecific barley cross used to map Rph13. To fine map the gene despite this handicap, we screened very large mapping populations with high-throughput genotyping technologies. However, in the absence of a H. bulbosum genome assembly, there is no guarantee that the gene content in the H. vulgare target interval provides full information on candidate gene content in the H. bulbosum resistance donor. That physical collinearity at disease resistance loci may be low between genotypes within one species (Barragan and Weigel 2021; Lee and Chae 2020; Michelmore and Meyers 1998; Van de Weyer et al. 2019), as illustrated by our exploration of the haplotypes at Ryd4Hb locus within barley diversity. Considering the high relatedness between these two Hordeum species, the risk taken is probably not much higher than in an intraspecific cross between divergent H. vulgare accessions.
Interspecific crosses also carry the risk of hybrid incompatibility leading to fertility or lethality problems (Bomblies 2010). At the Ryd4Hb locus, this negative linkage drag was strongly materialized by a sublethality factor characterized by the reduced growth and early death of introgression lines carrying the Hb-allele at homozygous state at this locus. By screening a large number of plants for recombination, we managed to break the linkage, producing a resistance donor lacking the sublethality factor, that could be included in breeding schemes. We mapped the sublethality factor to a 600 kbp interval on the MorexV3 genome. The observed phenotype suggested that sublethal plants are possibly lacking one or a few genes essential for barley development. Those are therefore likely among the genes annotated on the H. vulgare interval but have no ortholog at this locus in the donor H. bulbosum genome. Among the 15 genes annotated with high confidence in the interval, B3 domain-containing proteins are part of a large transcription factor superfamily whose members are playing key roles in various stages of plant development, from embryogenesis to seed maturation (Swaminathan et al. 2008). F-box containing proteins are central parts of the ubiquitin–26S proteasome system and are thus key for different processes like phytohormone signaling, plant development, cell cycle, or self-incompatibility (Stefanowicz et al. 2015). RecA proteins are maintaining DNA integrity during meiosis by initiating double-strand break repair (Emmenecker et al. 2023). Annexins are widely involved in regulating plant processes, from growth and development to responses to stresses (Wu et al. 2022). One of the corresponding genes in the MorexV3 interval could be missing in the A17 haplotype and thus could explain the observed phenotype.
The results of this study are potentially helpful to breed barley varieties with an effective resistance to BYDV. We identified recombinants with a strongly reduced H. bulbosum fragment that can be used in breeding schemes, removing almost completely the negative linkage drag. The markers closely linked to the resistance were designed based on interspecific polymorphisms and should work with the vast majority of barley lines, as confirmed on the 20 accessions of the barley pan-genome. Therefore, they can be used in marker-assisted and genomic selection, postponing the tedious resistance evaluation to the last breeding step. Knowing that Ryd4Hb is a resistance gene to BYDV and not its vector would also make it possible to establish the best strategy to avoid resistance breaking. Such a strategy could be pyramiding it with partial resistance or tolerance sources like Ryd2 and Ryd3. Such resistant varieties would make a major contribution to sustainable barley cultivation.
Data availability
The exome capture sequencing datasets generated and/or analyzed in this study are deposited at EMBL-ENA under the project IDs PRJEB7909 and PRJEB65283.
References
Ali M, Anwar S, Shuja MN, Tripathi RK, Singh J (2018) The genus Luteovirus from infection to disease. Eur J Plant Pathol 151(4):841–860. https://doi.org/10.1007/s10658-018-1425-8
Baggs E, Dagdas G, Krasileva K (2017) NLR diversity, helpers and integrated domains : making sense of the NLR IDentity. Curr Opin Plant Biol 38:59–67. https://doi.org/10.1016/j.pbi.2017.04.012
Baltenberger DE, Ohm HW, Foster JE (1987) Reactions of oat, barley, and wheat to infection with barley yellow Dwarf virus isolates. Crop Sci 27(2):195–198. https://doi.org/10.2135/cropsci1987.0011183X002700020010x
Barragan AC, Weigel D (2021) Plant NLR diversity : the known unknowns of pan-NLRomes. Plant Cell 33(4):814–831. https://doi.org/10.1093/plcell/koaa002
Beoni E, Chrpová J, Jarošová J, Kundu JK (2016) Survey of Barley yellow dwarf virus incidence in winter cereal crops, and assessment of wheat and barley resistance to the virus. Crop Pasture Sci 67(10):1054–1063
Boeckmann B, Bairoch A, Apweiler R, Blatter M-C, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucl Acids Res 31(1):365–370. https://doi.org/10.1093/nar/gkg095
Boissot N, Thomas S, Chovelon V, Lecoq H (2016) NBS-LRR-mediated resistance triggered by aphids: viruses do not adapt; aphids adapt via different mechanisms. BMC Plant Biol 16(1):25. https://doi.org/10.1186/s12870-016-0708-5
Bomblies K (2010) Doomed Lovers : mechanisms of isolation and incompatibility in plants. Annu Rev Plant Biol 61(1):109–124. https://doi.org/10.1146/annurev-arplant-042809-112146
Bonnamy M, Pinel-Galzi A, Gorgues L, Chalvon V, Hébrard E, Chéron S, Nguyen TH, Poulicard N, Sabot F, Pidon H, Champion A, Césari S, Kroj T, Albar L (2023) Rapid evolution of an RNA virus to escape recognition by a rice nucleotide-binding and leucine-rich repeat domain immune receptor. New Phytol 237(3):900–913. https://doi.org/10.1111/nph.18532
Boualem A, Dogimont C, Bendahmane A (2016) The battle for survival between viruses and their host plants. Curr Opin Virol 17:32–38. https://doi.org/10.1016/j.coviro.2015.12.001
Braun E-M, Tsvetkova N, Rotter B, Siekmann D, Schwefel K, Krezdorn N, Plieske J, Winter P, Melz G, Voylokov AV, Hackauf B (2019) Gene expression profiling and fine mapping identifies a gibberellin 2-oxidase gene co-segregating with the dominant dwarfing Gene Ddw1 in Rye (Secale cereale L.). Front Plant Sci. https://doi.org/10.3389/fpls.2019.00857
Budowle B, Chakraborty R, Giusti AM, Eisenberg AJ, Allen RC (1991) Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 48(1):137–144
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinform 10(1):421. https://doi.org/10.1186/1471-2105-10-421
Chen Q, Han Z, Jiang H, Tian D, Yang S (2010) Strong positive selection drives rapid diversification of R-genes in arabidopsis relatives. J Mol Evol 70(2):137–148. https://doi.org/10.1007/s00239-009-9316-4
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34(3):475–483. https://doi.org/10.1099/0022-1317-34-3-475
Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, Ramsay L, Druka A, Stein N, Svensson JT, Wanamaker S, Bozdag S, Roose ML, Moscou MJ, Chao S, Varshney RK, Szűcs P, Sato K, Hayes PM, Matthews DE, Waugh R (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genom 10(1):582. https://doi.org/10.1186/1471-2164-10-582
Collins NC, Paltridge NG, Ford CM, Symons RH (1996) The Yd2 gene for barley yellow dwarf virus resistance maps close to the centromere on the long arm of barley chromosome 3. Theor Appl Genet 92(7):858–864. https://doi.org/10.1007/BF00221898
Dedryver C-A, Le Ralec A, Fabre F (2010) The conflicting relationships between aphids and men: a review of aphid damage and control strategies. CR Biol 333(6):539–553. https://doi.org/10.1016/j.crvi.2010.03.009
Ding J, Cheng H, Jin X, Araki H, Yang Y, Tian D (2007) Contrasting patterns of evolution between allelic groups at a single locus in Arabidopsis. Genetica 129(3):235–242. https://doi.org/10.1007/s10709-006-0002-9
Dong X (2004) The role of membrane-bound ankyrin-repeat protein ACD6 in programmed cell death and plant defense. Sci STKE. https://doi.org/10.1126/stke.2212004pe6
Emmenecker C, Mézard C, Kumar R (2023) Repair of DNA double-strand breaks in plant meiosis: role of eukaryotic RecA recombinases and their modulators. Plant Reprod 36(1):17–41. https://doi.org/10.1007/s00497-022-00443-6
Goya R, Sun MGF, Morin RD, Leung G, Ha G, Wiegand KC, Senz J, Crisan A, Marra MA, Hirst M, Huntsman D, Murphy KP, Aparicio S, Shah SP (2010) SNVMix: predicting single nucleotide variants from next-generation sequencing of tumors. Bioinformatics 26(6):730–736. https://doi.org/10.1093/bioinformatics/btq040
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Regev A (2011) Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nature Biotechnol 29(7):644–652. https://doi.org/10.1038/nbt.1883
Guo Y-L, Fitz J, Schneeberger K, Ossowski S, Cao J, Weigel D (2011) Genome-wide comparison of nucleotide-binding site-leucine-rich repeat-encoding genes in arabidopsis. Plant Physiol 157(2):757–769. https://doi.org/10.1104/pp.111.181990
Habekuß A, Schliephake E, Ehrig F (2004) Hordeum bulbosum—A source for BYDV resistance. In: Proceedings of the 9th international barley genetics symposium, pp 787–791
Hu H, Choudhury S, Shabala S, Gupta S, Zhou M (2019) Genomic regions on chromosome 5H containing a novel QTL conferring barley yellow dwarf virus-PAV (BYDV-PAV) tolerance in barley. Sci Rep. https://doi.org/10.1038/s41598-019-47820-2
International Barley Genome Sequencing Consortium (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491(7426):711–716. https://doi.org/10.1038/nature11543
Jayakodi M, Padmarasu S, Haberer G, Bonthala VS, Gundlach H, Monat C, Lux T, Kamal N, Lang D, Himmelbach A, Ens J, Zhang X-Q, Angessa TT, Zhou G, Tan C, Hill C, Wang P, Schreiber M, Boston LB, Stein N (2020) The barley pan-genome reveals the hidden legacy of mutation breeding. Nature 588(7837):284–289. https://doi.org/10.1038/s41586-020-2947-8
Jost M, Singh D, Lagudah E, Park RF, Dracatos P (2020) Fine mapping of leaf rust resistance gene Rph13 from wild barley. Theor Appl Genet 133(6):1887–1895. https://doi.org/10.1007/s00122-020-03564-6
Kern M, Meiners T, Schliephake E, Habekuss A, Ordon F, Will T (2022) Infection of susceptible/tolerant barley genotypes with Barley yellow dwarf virus alters the host plant preference of Rhopalosiphum padi clones depending upon their ability to transmit BYDV. J Pest Sci 95:215–229. https://doi.org/10.1007/s10340-021-01367-2
Kosova K, Chrpová J, Šíp V (2008) Recent advances in breeding of cereals for resistance to barley yellow dwarf virus. Czech J Genet Plant Breed 44(1):1–10
Lassmann T, Hayashizaki Y, Daub CO (2009) TagDust—A program to eliminate artifacts from next generation sequencing data. Bioinformatics 25(21):2839–2840. https://doi.org/10.1093/bioinformatics/btp527
Lee RRQ, Chae E (2020) Variation patterns of NLR clusters in Arabidopsis thaliana genomes. Plant Commun 1(4):100089. https://doi.org/10.1016/j.xplc.2020.100089
Li J, Ding J, Zhang W, Zhang Y, Tang P, Chen J-Q, Tian D, Yang S (2010) Unique evolutionary pattern of numbers of gramineous NBS–LRR genes. Mol Genet Genom 283(5):427–438. https://doi.org/10.1007/s00438-010-0527-6
Lu H, Liu Y, Greenberg JT (2005) Structure–function analysis of the plasma membrane- localized Arabidopsis defense component ACD6. Plant J 44(5):798–809. https://doi.org/10.1111/j.1365-313X.2005.02567.x
Lu H, Rate DN, Song JT, Greenberg JT (2003) ACD6, a novel Ankyrin protein, is a regulator and an effector of salicylic acid signaling in the arabidopsis defense response. Plant Cell 15(10):2408–2420. https://doi.org/10.1105/tpc.015412
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A (2019) CDD/SPARCLE: the conserved domain database in 2020. Nucl Acids Res 48(D1):D265–D268. https://doi.org/10.1093/nar/gkz991
Lüpken T, Stein N, Perovic D, Habekuß A, Serfling A, Krämer I, Hähnel U, Steuernagel B, Scholz U, Ariyadasa R, Martis M, Mayer K, Niks RE, Collins NC, Friedt W, Ordon F (2014) High-resolution mapping of the barley Ryd3 locus controlling tolerance to BYDV. Mol Breed 33(2):477–488. https://doi.org/10.1007/s11032-013-9966-1
Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, Muñoz-Amatriaín M, Close TJ, Wise RP, Schulman AH, Himmelbach A, Mayer KFX, Scholz U, Poland JA, Stein N, Waugh R (2013) Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ). Plant J 76(4):718–727. https://doi.org/10.1111/tpj.12319
Mascher M, Wicker T, Jenkins J, Plott C, Lux T, Koh CS, Ens J, Gundlach H, Boston LB, Tulpová Z, Holden S, Hernández-Pinzón I, Scholz U, Mayer KFX, Spannagl M, Pozniak CJ, Sharpe AG, Šimková H, Moscou MJ, Stein N (2021) Long-read sequence assembly: a technical evaluation in barley. Plant Cell 33(6):1888–1906. https://doi.org/10.1093/plcell/koab077
Michel M (1996) Untersuchungen zur Übertragung von Resistenzgenen aus der Wildart Hordeam bulbosum L. in die Kulturgerste Hordeum vulgare L. Diss., TU München, Munich, Germany
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8(11):1113–1130. https://doi.org/10.1101/gr.8.11.1113
Milner SG, Jost M, Taketa S, Mazón ER, Himmelbach A, Oppermann M, Weise S, Knüpffer H, Basterrechea M, König P, Schüler D, Sharma R, Pasam RK, Rutten T, Guo G, Xu D, Zhang J, Herren G, Müller T, Stein N (2019) Genebank genomics highlights the diversity of a global barley collection. Nat Genet 51(2):319–326. https://doi.org/10.1038/s41588-018-0266-x
Niks RE, Habekuß A, Bekele B, Ordon F (2004) A novel major gene on chromosome 6H for resistance of barley against the barley yellow dwarf virus. Theor Appl Genet 109(7):1536–1543. https://doi.org/10.1007/s00122-004-1777-7
Oerke E-C (2006) Crop losses to pests. J Agric Sci 144(1):31–43. https://doi.org/10.1017/S0021859605005708
Okada K, Tanaka T, Fukuoka S, Oono Y, Mishina K, Oikawa T, Sato K, Kato T, Komatsuda T, Namai K (2022) Two dominant genes in barley (Hordeum vulgare L.) complementarily encode perfect resistance to Japanese soil-borne wheat mosaic virus. Breed Sci 72(5):372–382. https://doi.org/10.1270/jsbbs.22046
Ordon F, Habekuss A, Kastirr U, Rabenstein F, Kühne T (2009) Virus resistance in cereals: sources of resistance, genetics and breeding. J Phytopathol 157(9):535–545. https://doi.org/10.1111/j.1439-0434.2009.01540.x
Pidon H, Ghesquière A, Chéron S, Issaka S, Hébrard E, Sabot F, Kolade O, Silué D, Albar L (2017) Fine mapping of RYMV3: a new resistance gene to Rice yellow mottle virus from Oryza glaberrima. Theor Appl Genet 130(4):807–818. https://doi.org/10.1007/s00122-017-2853-0
Pidon H, Wendler N, Habekuβ A, Maasberg A, Ruge-Wehling B, Perovic D, Ordon F, Stein N (2020) High-resolution mapping of Rym14Hb, a wild relative resistance gene to barley yellow mosaic disease. Theor Appl Genet. https://doi.org/10.1007/s00122-020-03733-7
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11(9):1695–1708. https://doi.org/10.1105/tpc.11.9.1695
Riedel C, Habekuß A, Schliephake E, Niks R, Broer I, Ordon F (2011) Pyramiding of Ryd2 and Ryd3 conferring tolerance to a German isolate of Barley yellow dwarf virus-PAV (BYDV-PAV-ASL-1) leads to quantitative resistance against this isolate. Theor Appl Genet 123(1):69. https://doi.org/10.1007/s00122-011-1567-y
Roos J, Hopkins R, Kvarnheden A, Dixelius C (2011) The impact of global warming on plant diseases and insect vectors in Sweden. Eur J Plant Pathol 129(1):9–19. https://doi.org/10.1007/s10658-010-9692-z
Rostoks N, Borevitz JO, Hedley PE, Russell J, Mudie S, Morris J, Cardle L, Marshall DF, Waugh R (2005) Single-feature polymorphism discovery in the barley transcriptome. Genome Biol 6:R54. https://doi.org/10.1186/gb-2005-6-6-r54
Russell J, Mascher M, Dawson IK, Kyriakidis S, Calixto C, Freund F, Bayer M, Milne I, Marshall-Griffiths T, Heinen S, Hofstad A, Sharma R, Himmelbach A, Knauft M, van Zonneveld M, Brown JWS, Schmid K, Kilian B, Muehlbauer GJ, Waugh R (2016) Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat Genet 48(9):1024–1030. https://doi.org/10.1038/ng.3612
Saintenac C, Zhang W, Salcedo A, Rouse MN, Trick HN, Akhunov E, Dubcovsky J (2013) Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 341(6147):783–786. https://doi.org/10.1126/science.1239022
Santos E, Benito C, Silva-Navas J, Gallego FJ, Figueiras AM, Pinto-Carnide O, Matos M (2018) Characterization, genetic diversity, phylogenetic relationships, and expression of the aluminum tolerance MATE1 gene in Secale species. Biol Plant 62(1):109–120. https://doi.org/10.1007/s10535-017-0749-0
Schaller CW, Qualset CO, Rutger JN (1964) Inheritance and linkage of the Yd2 gene conditioning resistance to the barley yellow Dwarf virus disease in Barley. Crop Sci 4:544–548. https://doi.org/10.2135/cropsci1964.0011183X000400050034x
Schliephake E, Habekuss A, Scholz M, Ordon F (2013) Barley yellow dwarf virus transmission and feeding behaviour of Rhopalosiphum padi on Hordeum bulbosum clones. Entomol Exp Appl 146(3):347–356. https://doi.org/10.1111/eea.12033
Scholz M, Ruge-Wehling B, Habekuß A, Schrader O, Pendinen G, Fischer K, Wehling P (2009) Ryd4Hb: a novel resistance gene introgressed from Hordeum bulbosum into barley and conferring complete and dominant resistance to the barley yellow dwarf virus. Theor Appl Genet 119(5):837–849. https://doi.org/10.1007/s00122-009-1093-3
Sett S, Prasad A, Prasad M (2022) Resistance genes on the verge of plant–virus interaction. Trends Plant Sci 27(12):1242–1252. https://doi.org/10.1016/j.tplants.2022.07.003
Stefanowicz K, Lannoo N, Van Damme EJM (2015) Plant F-box proteins – judges between life and death. Crit Rev Plant Sci 34(6):523–552. https://doi.org/10.1080/07352689.2015.1024566
Stein N, Herren G, Keller B (2001) A new DNA extraction method for high-throughput marker analysis in a large-genome species such as Triticum aestivum. Plant Breed 120(4):354–356. https://doi.org/10.1046/j.1439-0523.2001.00615.x
Stein N, Prasad M, Scholz U, Thiel T, Zhang H, Wolf M, Kota R, Varshney RK, Perovic D, Grosse I, Graner A (2007) A 1,000-loci transcript map of the barley genome: new anchoring points for integrative grass genomics. Theor Appl Genet 114(5):823–839. https://doi.org/10.1007/s00122-006-0480-2
Steuernagel B, Witek K, Krattinger SG, Ramirez-Gonzalez RH, Schoonbeek H, Yu G, Baggs E, Witek AI, Yadav I, Krasileva KV, Jones JDG, Uauy C, Keller B, Ridout CJ, Wulff BBH (2020) The NLR-annotator tool enables annotation of the intracellular immune receptor repertoire. Plant Physiol 183(2):468–482. https://doi.org/10.1104/pp.19.01273
Sullivan MJ, Petty NK, Beatson SA (2011) Easyfig: a genome comparison visualizer. Bioinformatics (oxford, England) 27(7):1009–1010. https://doi.org/10.1093/bioinformatics/btr039
Suneson CA (1955) Breeding for resistance to yellow Dwarf virus in Barley. Agron J 47(6):283–283. https://doi.org/10.2134/agronj1955.00021962004700060014x
Swaminathan K, Peterson K, Jack T (2008) The plant B3 superfamily. Trends Plant Sci 13(12):647–655. https://doi.org/10.1016/j.tplants.2008.09.006
Szűcs P, Blake VC, Bhat PR, Chao S, Close TJ, Cuesta-Marcos A, Muehlbauer GJ, Ramsay L, Waugh R, Hayes PM (2009) An integrated resource for barley linkage map and malting quality QTL alignment. The Plant Genome. https://doi.org/10.3835/plantgenome2008.01.0005
Thiel T, Kota R, Grosse I, Stein N, Graner A (2004) SNP2CAPS: a SNP and INDEL analysis tool for CAPS marker development. Nucl Acids Res 32(1):e5. https://doi.org/10.1093/nar/gnh006
Thiel T, Michalek W, Varshney R, Graner A (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet 106(3):411–422. https://doi.org/10.1007/s00122-002-1031-0
Tjallingii WF (1978) Electronic recording of penetration behaviour by aphids. Entomol Exp Appl 24(3):721–730. https://doi.org/10.1111/j.1570-7458.1978.tb02836.x
Tjallingii WF, Esch ThH (1993) Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol Entomol 18(3):317–328. https://doi.org/10.1111/j.1365-3032.1993.tb00604.x
Toojinda T, Broers LH, Chen XM, Hayes PM, Kleinhofs A, Korte J, Kudrna D, Leung H, Line RF, Powell W, Ramsay L, Vivar H, Waugh R (2000) Mapping quantitative and qualitative disease resistance genes in a doubled haploid population of barley (Hordeum vulgare). Theor Appl Genet 101(4):580–589. https://doi.org/10.1007/s001220051519
Trebicki P (2020) Climate change and plant virus epidemiology. Virus Res 286:198059. https://doi.org/10.1016/j.virusres.2020.198059
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—New capabilities and interfaces. Nucl Acids Res 40(15):e115. https://doi.org/10.1093/nar/gks596
Van de Weyer A-L, Monteiro F, Furzer OJ, Nishimura MT, Cevik V, Witek K, Jones JDG, Dangl JL, Weigel D, Bemm F (2019) A species-wide inventory of NLR genes and alleles in Arabidopsis thaliana. Cell 178(5):1260-1272.e14. https://doi.org/10.1016/j.cell.2019.07.038
Van Der Vossen EAG, Van Der Voort JNAMR, Kanyuka K, Bendahmane A, Sandbrink H, Baulcombe DC, Bakker J, Stiekema WJ, Klein-Lankhorst RM (2000) Homologues of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J 23(5):567–576. https://doi.org/10.1046/j.1365-313x.2000.00814.x
Van Ooijen JW (2006) JoinMap 4: software for the calculation of genetic linkage maps in experimental populations of diploid species. Wageningen, Netherlands: Plant Research International BV and Kayazma BV
Varshney RK, Marcel TC, Ramsay L, Russell J, Röder MS, Stein N, Waugh R, Langridge P, Niks RE, Graner A (2007) A high density barley microsatellite consensus map with 775 SSR loci. Theor Appl Genet 114(6):1091–1103. https://doi.org/10.1007/s00122-007-0503-7
Vincze T, Posfai J, Roberts RJ (2003) NEBcutter : a program to cleave DNA with restriction enzymes. Nucl Acids Res 31(13):3688–3691. https://doi.org/10.1093/nar/gkg526
Voorrips RE (2002) MapChart : software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78. https://doi.org/10.1093/jhered/93.1.77
Wang J, Hu M, Wang J, Qi J, Han Z, Wang G, Qi Y, Wang H-W, Zhou J-M, Chai J (2019) Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364(6435):eaav5870. https://doi.org/10.1126/science.aav5870
Wendler N, Mascher M, Himmelbach A, Bini F, Kumlehn J, Stein N (2017) A high-density, sequence-enriched genetic map of Hordeum bulbosum and its collinearity to H. vulgare. The Plant Genome. https://doi.org/10.3835/plantgenome2017.06.0049
Wendler N, Mascher M, Himmelbach A, Johnston P, Pickering R, Stein N (2015) Bulbosum to go: a toolbox to Utilize Hordeum vulgare/bulbosum introgressions for breeding and beyond. Mol Plant 8(10):1507–1519. https://doi.org/10.1016/j.molp.2015.05.004
Wendler N, Mascher M, Nöh C, Himmelbach A, Scholz U, Ruge-Wehling B, Stein N (2014) Unlocking the secondary gene-pool of barley with next-generation sequencing. Plant Biotechnol J 12(8):1122–1131. https://doi.org/10.1111/pbi.12219
Wu X, Wang Y, Bian Y, Ren Y, Xu X, Zhou F, Ding H (2022) A critical review on plant annexin: structure, function, and mechanism. Plant Physiol Biochem 190:81–89. https://doi.org/10.1016/j.plaphy.2022.08.019
Zhou T, Wang Y, Chen J-Q, Araki H, Jing Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Genet Genom 271(4):402–415. https://doi.org/10.1007/s00438-004-0990-z
Acknowledgements
We gratefully acknowledge the excellent technical support by Manuela Kretschmann in DNA extraction and KASP genotyping, Dörte Grau in BaMMV resistance phenotyping, and Evelyn Betke, Katharina Stein, and Kerstin Welzel in aphid rearing. We thank Anne Fiebig for raw data submission and Kristin Kutter for marker analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported as part of the collaborative projects ‘Dwarfbulb’ (grant 2814501910 from the German Federal Ministry of Food and Agriculture (BMEL)) and ‘BulbOmics’ (grant 2818201615 from the German Federal Ministry of Food and Agriculture (BMEL)). The exome capture data production was part of the collaborative project ‘TRANSBULB’ (grant 0315966 from the German Federal Ministry of Education and Research (BMBF)).
Author information
Authors and Affiliations
Contributions
BR-W, NS, NW, and VK conceived the project and acquired the funding. BR-W, NW, KO, AM-P, and VK designed and built the mapping populations. BR-W and KF performed the initial linkage mapping and broke the linkage between the resistance and the sublethality loci. BR-W and AH performed the phenotyping experiments. NW did the exome capture experiment. TW performed and analyzed the aphid feeding experiments. HP performed the high-resolution mapping and the pan-genome analysis and drafted the manuscript. NS supervised the project. All authors provided critical feedback and helped shape the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Neele Wedler, Viktor Korzun, Klaus Oldach, and Anja Maasberg-Prelle are employed at KWS SAAT SE & Co and KWS LOCHOW. The other authors declare no conflict of interest.
Additional information
Communicated by Takao Komatsuda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pidon, H., Ruge-Wehling, B., Will, T. et al. High-resolution mapping of Ryd4Hb, a major resistance gene to Barley yellow dwarf virus from Hordeum bulbosum. Theor Appl Genet 137, 60 (2024). https://doi.org/10.1007/s00122-024-04542-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04542-y