Abstract
Key message
Wheat lines with shortened Th. ponticum chromatin carrying Fhb7 and molecular markers linked to Fhb7 will accelerate the transfer of Fhb7 to breeding lines and provide an important resource for future map-based cloning of this gene.
Abstract
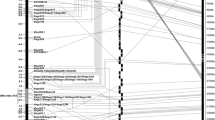
Fusarium head blight is a major wheat disease globally. A major FHB resistance gene, designated as Fhb7, derived from Thinopyrum ponticum, was earlier transferred to common wheat, but was not used in wheat breeding due to linkage drag. The aims of this study were to (1) saturate this FHB resistance gene region; (2) develop and characterize secondary translocation lines with shortened Thinopyrum segments carrying Fhb7 using ph1b; (3) pyramid Fhb7 and Fhb1 by marker-assisted selection. Fhb7 was mapped in a 1.7 cM interval that was flanked by molecular markers XsdauK66 and Xcfa2240 with SSR, diversity arrays technology, EST-derived and conserved markers. KS24-2 carrying Fhb7 was analyzed with molecular markers and genomic in situ hybridization, confirming it was a 7DS.7el2L Robertsonian translocation. To reduce the Thinopyrum chromatin segments carrying Fhb7, a BC1F2 population (Chinese Spring ph1bph1b*2/KS24-2) was developed and genotyped with the markers linked to Fhb7. Two new translocation lines (SDAU1881 and SDAU1886) carrying Fhb7 on shortened alien segments (approximately 16.1 and 17.3 % of the translocation chromosome, respectively) were developed. Furthermore, four wheat lines (SDAU1902, SDAU1903, SDAU1904, and SDAU1906) with the pyramided markers flanking Fhb1 and Fhb7 were developed and the FHB responses indicated lines with mean NDS ranging from 1.3 to 1.6 had successfully combined Fhb7 and Fhb1. Three new molecular markers associated with Fhb7 were identified and validated in 35 common wheat varieties. The translocation lines with shortened alien segments carrying Fhb7 (and Fhb1) and the markers closely linked to Fhb7 will be useful for improving wheat scab resistance.






Similar content being viewed by others
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp P, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Bai G, Shaner G (2004) Management and resistance in wheat and barley to Fusarium head blight 1. Annu Rev Phytopathol 42:135–161
Buerstmayr H, Ban T, Anderson JA (2009) QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed 128:1–26
Cainong JC, Bockus WW, Feng Y, Chen P, Qi L, Sehgal SK, Danilova TV, Koo D-H, Friebe B, Gill BS (2015) Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor Appl Genet 128:1019–1027
Cetin Y, Bullerman LB (2005) Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem Toxicol 43:755–764
Chakraborty S, Newton AC (2011) Climate change, plant diseases and food security: an overview. Plant Pathol 60:2–14
Cuthbert PA, Somers DJ, Thomas J, Cloutier S, Brulé-Babel A (2006) Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor Appl Genet 112:1465–1472
Cuthbert PA, Somers DJ, Brulé-Babel A (2007) Mapping of Fhb2 on chromosome 6BS: a gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor Appl Genet 114:429–437
D’mello J, Placinta C, Macdonald A (1999) Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim Feed Sci Technol 80:183–205
Dewey DR (1983) Historical and current taxonomic perspectives of Agropyron, Elymus, and related genera. Crop Sci 23:637–642
Duveiller E, Singh RP, Nicol JM (2007) The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica 157:417–430
Dvořák J (1980) Homoeology between Agropyron elongatum chromosomes and Triticum aestivum chromosomes. Can J Genet Cytol 22:237–259
Fu S, Lv Z, Qi B, Guo X, Li J, Liu B, Han F (2012) Molecular cytogenetic characterization of wheat—Thinopyrum elongatum addition, substitution and translocation lines with a novel source of resistance to wheat Fusarium head blight. J Genet Genomics 39:103–110
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296:92–100
Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525
Han F, Gao Z, Birchler JA (2009) Reactivation of an inactive centromere reveals epigenetic and structural components for centromere specification in maize. Plant Cell 21:1929–1939
He X, Singh PK, Schlang N, Duveiller E, Dreisigacker S, Payne T, He Z (2014) Characterization of Chinese wheat germplasm for resistance to Fusarium head blight at CIMMYT, Mexico. Euphytica 195:383–395
Jauhar PP (2014) Durum wheat genetic stocks involving chromosome 1E of diploid wheatgrass: resistance to Fusarium head blight. Nucleus 57:19–23
Jia J, Zhao S, Kong X, Li Y, Zhao G, He W, Appels R, Pfeifer M, Tao Y, Zhang X, Jing R, Zhang C, Ma Y, Gao L, Gao C, Spannagl M, Mayer K, Li D, Pan S, Zheng F, Hu Q, Xia X, Li J, Liang Q, Chen J, Wicker T, Gou C, Kuang H, He G, Luo Y, Keller B, Xia Q, Lu P, Wang J, Zou H, Zhang R, Xu J, Gao J, Middleton C, Quan Z, Liu G, Wang J, International Wheat Genome Sequencing Consortium, Yang H, Liu X, He Z, Mao L, Wang J (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Kim NS, Armstrong K, Knott D (1993) Molecular detection of Lophopyrum chromatin in wheat-Lophopyrum recombinants and their use in the physical mapping of chromosome 7D. Theor Appl Genet 85:561–567
Kong L, Cambron SE, Ohm HW (2008) Hessian fly resistance genes H16 and H17 are mapped to a resistance gene cluster in the distal region of chromosome 1AS in wheat. Mol Breed 21:183–194
Kosambi D (1943) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Ling HQ, Zhao S, Liu D, Wang J, Sun H, Zhang C, Fan H, Li D, Dong L, Tao Y (2013) Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 496:87–90
Liu S, Anderson JA (2003) Targeted molecular mapping of a major wheat QTL for Fusarium head blight resistance using wheat ESTs and synteny with rice. Genome 46:817–823
Liu S, Zhang X, Pumphrey MO, Stack RW, Gill BS, Anderson JA (2006) Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct Integr Genomics 6:83–89
Marais G, Kotze L, Eksteen A (2010) Allosyndetic recombinants of the Aegilops peregrina-derived Lr59 translocation in common wheat. Plant Breed. doi:10.1111/j.1439-0523.2009.01713.x
Mayer KF, Rogers J, Doležel J, Pozniak C, Eversole K, Feuillet C, Gill B, Friebe B, Lukaszewski AJ, Sourdille P (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788
Niu Z, Klindworth DL, Friesen TL, Chao S, Jin Y, Cai X, Xu SS (2011) Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics 187:1011–1021
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Qi L, Friebe B, Zhang P, Gill BS (2007) Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res 15:3–19
Qi L, Pumphrey M, Friebe B, Chen P, Gill B (2008) Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor Appl Genet 117:1155–1166
Roberts MA, Reader SM, Dalgliesh C, Miller TE, Foote TN, Fish LJ, Snape JW, Moore G (1999) Induction and characterization of Ph1 wheat mutants. Genetics 153:1909–1918
Semagn K, Bjørnstad Å, Skinnes H, Marøy AG, Tarkegne Y, William M (2006) Distribution of DArT, AFLP, and SSR markers in a genetic linkage map of a doubled-haploid hexaploid wheat population. Genome 49:545–555
Shen X, Ohm H (2007) Molecular mapping of Thinopyrum-derived Fusarium head blight resistance in common wheat. Mol Breed 20:131–140
Shen X, Kong L, Ohm H (2004) Fusarium head blight resistance in hexaploid wheat (Triticum aestivum)-Lophopyrum genetic lines and tagging of the alien chromatin by PCR markers. Theor Appl Genet 108:808–813
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Van Ooijen JW (2006) JoinMap® 4.0: software for the calculation of genetic linkage maps in experimental population. Kyazma BV, Wageningen, p. 33
Vogel JP, Garvin DF, Mockler TC, Schmutz J, Rokhsar D, Bevan MW, Barry K, Lucas S, Harmon-Smith M, Lail K (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463:763–768
Wang RR (2011) Agropyron and Psathyrostachys. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, cereals, 1st edn. Springer, Berlin, pp 77–108
Wang RR, Lu B (2014) Biosystematics and evolutionary relationships of perennial Triticeae species revealed by genomic analyses. J Syst Evol 52:697–705
Wang J, Li H, Zhang L, Meng L (2014a) Users’ Manual of QTL IciMapping. The Quantitative Genetics Group, Institute of Crop Science, Chinese Academy of Agricultural Sciences (CAAS), Beijing 100081, China, and Genetic Resources Program, International Maize and Wheat Improvement Center (CIMMYT), Apdo. Postal 6-641, 06600 Mexico, D.F., Mexico
Wang Z, Cui Y, Chen Y, Zhang D, Liang Y, Zhang D, Wu Q, Xie J, Ouyang S, Li D (2014b) Comparative genetic mapping and genomic region collinearity analysis of the powdery mildew resistance gene Pm41. Theor Appl Genet 127:1741–1751
Wenzl P, Carling J, Kudrna D, Jaccoud D, Huttner E, Kleinhofs A, Kilian A (2004) Diversity arrays technology (DArT) for whole-genome profiling of barley. Proc Natl Acad Sci USA 101:9915–9920
West JS, Holdgate S, Townsend JA, Edwards SG, Jennings P, Fitt BD (2012) Impacts of changing climate and agronomic factors on Fusarium ear blight of wheat in the UK. Fungal Ecol 5:53–61
Xue S, Li G, Jia H, Xu F, Lin F, Tang M, Wang Y, An X, Xu H, Zhang L (2010) Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor Appl Genet 121:147–156
Xue S, Xu F, Tang M, Zhou Y, Li G, An X, Lin F, Xu H, Jia H, Zhang L (2011) Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor Appl Genet 123:1055–1063
You FM, Huo N, Gu YQ, Lazo GR, Dvorak J, Anderson OD (2009) ConservedPrimers 2.0: a high-throughput pipeline for comparative genome referenced intron-flanking PCR primer design and its application in wheat SNP discovery. BMC Bioinformatics 10:331
Yu J, Bai G, Cai S, Dong Y, Ban T (2008) New Fusarium head blight-resistant sources from Asian wheat germplasm. Crop Sci 48:1090–1097
Zhang X, Shen X, Hao Y, Cai J, Ohm HW, Kong L (2011) A genetic map of Lophopyrum ponticum chromosome 7E, harboring resistance genes to Fusarium head blight and leaf rust. Theor Appl Genet 122:263–270
Acknowledgments
The authors thank Robert McIntosh, University of Sydney Plant Breeding Institute–Cobbitty, PB4011, Narellan, NSW 2567, Australia, for review of the manuscript before submission. We thank Dr. B. S. Gill, Kansas state university for providing seeds of wheat nulli-tetra and deletion stocks of group 7. We acknowledge financial supports by the National High-Tech R&D Program of China (2011AA100102 and 2012AA101105), the NSF of China (Grant nos. 31171553 and 31471488), Shandong Seed Engineering Project (2015-2019), and the International Collaboration Program (948 Project, 2013-S19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by H. Buerstmayr.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, J., Zhang, X., Hou, Y. et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor Appl Genet 128, 2301–2316 (2015). https://doi.org/10.1007/s00122-015-2586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2586-x