Abstract
Key message
A point mutation in the AHAS1 gene leading to resistance to imidazolinone in chickpea was identified. The resistance is inherited as a single gene. A KASP marker targeting the mutation was developed.
Abstract
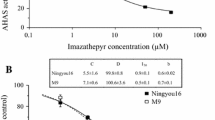
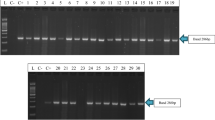
Weed control in chickpea (Cicer arietinum L.) is challenging due to poor crop competition ability and limited herbicide options. A chickpea genotype with resistance to imidazolinone (IMI) herbicides has been identified, but the genetic inheritance and the mechanism were unknown. In many plant species, resistance to IMI is caused by point mutation(s) in the acetohydroxyacid synthase (AHAS) gene resulting in an amino acid substitution preventing herbicide attachment to the molecule. The main objective of this research was to characterize the resistance to IMI herbicides in chickpea. Two homologous AHAS genes namely AHAS1 and AHAS2 sharing 80 % amino acid sequence similarity were identified in the chickpea genome. Cluster analysis indicated independent grouping of AHAS1 and AHAS2 across legume species. A point mutation in the AHAS1 gene at C675 to T675 resulting in an amino acid substitution from Ala205 to Val205 confers the resistance to IMI in chickpea. A KASP marker targeting the point mutation was developed and effectively predicted the response to IMI herbicides in a recombinant inbred (RI) population of chickpea. The RI population was used in molecular mapping where the major locus for the reaction to IMI herbicide was mapped to chromosome 5. Segregation analysis across an F2 population and RI population demonstrated that the resistance is inherited as a single gene in a semi-dominant fashion. The simple genetic inheritance and the availability of KASP marker generated in this study would speed up development of chickpea varieties with resistance to IMI herbicides.




Similar content being viewed by others
References
Anbessa Y, Taran B, Warkentin TD, Tullu A, Vandenberg A (2009) Genetic analyses and conservation of QTL for ascochyta blight resistance in chickpea (Cicer arietinum L.). Theor Appl Genet 119:757–765
Ashigh J, Tardif FJ (2007) An Ala205val substitution in acetohydroxyacid synthase of Eastern Black Nightshade (Solanum Ptychanthum) reduces sensitivity to herbicides and feedback inhibition. Weed Sci 55:558–565
Baker CJ, Saxton KE, Ritchie WR, Chamen WCT, Reicosky DC, Ribeiro MFS, Justice SE, Hobbs PR (1996) No-tillage seeding in conservation agriculture. CAB International, Wallingford
Beckie HJ, Tardif FJ (2012) Herbicide cross resistance in weeds. Crop Prot 35:15–28
de Givry S, Bouchez M, Chabrier P, Milan D, Schiex T (2005) CARHTA GENE: multipopulation integrated genetic and radiation hybrid mapping. Bioinformatics 21:1703–1704
Doyle JJ, Doyle JL (1987) CTAB DNA extraction in plants. Phytochem Bull 19:11–15
Duggleby RG, Pang SS (2000) Acetohydroxyacid synthase. J Biochem Mol Biol 33:1–36
Duggleby SG, Pang SS, Yu H, Guddat LW (2003) Systematic characterization of mutations in yeast acetohydroxyacid synthase: interpretation of herbicide-resistance data. Eur J Biochem 270:2895–2904
Ganal MW, Altmann T, Roder MS (2009) SNP identification in crop plants. Curr Opin Plant Biol 12:211–217
Garg R, Patel RK, Jhanwar S, Priya P, Bhattacharjee A, Yadav G, Bhatia S, Chattopadhyay D, Tyagi AK, Jain M (2011) Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant Physiol 156:1661–1678
Ghio C, Ramos ML, Altieri E, Bulos M, Sala CA (2013) Molecular characterization of Als1, an acetohydroxyacid synthase mutation conferring resistance to sulfonylurea herbicides in soybean. Theor Appl Genet 126:2957–2968
Han H, Yu Q, Purba E, Li M, Walsh M, Friesen S, Powles SB (2012) A novel amino acid substitution Ala-122-Tyr in ALS confers high-level and broad resistance across ALS-inhibiting herbicides. Pest Manag Sci 68:1164–1170
Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204:430–434
Haughn GW, Smith J, Mazur B, Somerville C (1988) Transformation with a mutant Arabidopsis acetolactate synthase gene renders tobacco resistant to sulfonylurea herbicides. Mol Gen Genet 211:266–271
Heap I (2013) The International Survey of Herbicide Resistant Weeds. http://www.weedscience.org. Accessed 2 May 2013
Hiremath PJ, Kumar A, Penmetsa RV, Farmer A, Schlueter JA, Chamarthi SK, Whaley AM, Carrasquilla-Garcia N, Gaur PM, Upadhyaya HD, Kavi Kishor PB, Shah TM, Cook DR, Varshney RK (2012) Large-scale development of cost-effective SNP marker assays for diversity assessment and genetic mapping in chickpea and comparative mapping in legumes. Plant Biotechnol J 10:716–732
Kantar F, Elkoca E, Zengin H (1999) Chemical and agronomical weed control in Chickpea (Cicer arietinum L. cv. Aziziye-94). Tr J Agric For 23:631–635
Kolkman JM, Slabaugh MB, Bruniard JM, Berry S, Bushman BS, Olungu C, Maes N, Abratti G, Zambelli A, Miller JF, Leon A, Knapp SJ (2004) Acetohydroxyacid synthase mutations conferring resistance to imidazolinone or sulfonylurea herbicides in sunflower. Theor Appl Genet 109:1147–1159
Kosambi DD (1943) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lee H, Rustgi S, Kumar N, Burke I, Yenish JP, Gill KS, von Wettstein D, Ullrich SE (2011) Single nucleotide mutation in the barley acetohydroxy acid synthase (AHAS) gene confers resistance to imidazolinone herbicides. Proc Natl Acad Sci USA 108:8909–8913
Li D, Barclay I, Jose K, Stefanova K, Appels R (2008) A mutation at the Ala122 position of acetohydroxyacid synthase (AHAS) located on chromosome 6D of wheat: improved resistance to imidazolinone and a faster assay for marker assisted selection. Mol Breed 22:217–225
Manabe Y, Tinker N, Colville A, Miki B (2007) CSR1, the sole target of imidazolinone herbicide in Arabidopsis thaliana. Plant Cell Physiol 48:1340–1358
McCourt JA, Pang SS, King-Scott J, Guddat LW, Duggleby RG (2006) Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc Natl Acad Sci USA 103:569–573
McNaughton KE, Letarte J, Lee EA, Tardif FJ (2005) Mutations in ALS confer herbicide resistance in Redroot Pigweed (Amaranthus retroflexus) and Powell Amaranth (Amaranthus powellii). Weed Sci 53:17–22
Miflin BJ (1974) The location of nitrite reductase and other enzymes related to amino acid biosynthesis in the plastids of root and leaves. Plant Physiol 54:550–555
Mourad G, King J (1992) Effect of four classes of herbicides on growth and acetolactate-synthase activity in several variants of Arabidopsis thaliana. Planta 188:491–497
Muhitch MJ, Shaner DL, Stidham MA (1987) Imidazolinones and Acetohydroxyacid synthase from higher plants. Plant Physiol 83:451–456
Oldach KH, Peck DM, Cheong J, Williams KJ, Nair RM (2008) Identification of a chemically induced point mutation mediating herbicide tolerance in annual medics (Medicago spp.). Ann Bot 101:997–1005
Ouellet R, Rutledge RG, Miki BL (1992) Members of the acetohydroxyacid synthase multigene family of Brassica napus have divergent patterns of expression. Plant J 2:321–330
Padbury G, Waltman S, Caprio J, Coen G, McGinn S, Mortensen D, Sinclair R (2002) Agroecosystems and land resources of the northern Great Plains. Agron J 94:251–261
Pozniak CJ, Hucl PJ (2004) Genetic analysis of imidazolinone resistance in mutation-derived lines of common wheat. Crop Sci 44:23–30
Rafalski A (2002) Applications of single nucleotide polymorphisms in crop genetics. Curr Opin Plant Biol 5:94–100
Rutledge RG, Quellet T, Hattori J, Miki BL (1991) Molecular characterization and genetic origin of the Brassica napus acetohydroxyacid synthase multigene family. Mol Gen Genet 229:31–40
Saskatchewan Ministry of Agriculture (2013) Guide to Crop Protection. 3085 Albert Street Regina, Saskatchewan, Canada
Smith K, Schloss JV, Mazur BJ (1989) Functional expression of plant acetolactate synthase genes in Escherichia coli. Proc Natl Acad Sci 86:4179–4183
Süzer S, Büyük H (2010) Residual effects of spraying imidazolinone-family herbicides on Clearfield®* sunflower production from the point of view of crop rotation. Helia 33:25–35
Syvanen AC (2005) Toward genome-wide SNP genotyping. Nat Genet 37(Suppl):S5–S10
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan S, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
Taran B, Warkentin TD, Vandenberg A, Holm FA (2010) Variation in chickpea germplasm for tolerance to imazethapyr and imazamox herbicides. Can J Plant Sci 90:139–142
Taran B, Holm F, Banniza S (2013) Response of chickpea cultivars to pre- and post-emergence herbicide applications. Can J Plant Sci 93:279–286
Tar’an B, Warkentin TD, Tullu A, Vandenberg A (2007) Genetic mapping of ascochyta blight resistance in chickpea (Cicer arietinum L.) using a simple sequence repeat linkage map. Genome 50:26–34
Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar’an B, Millan T, Zhang X, Ramsay LD, Iwata A, Wang Y, Nelson W, Farmer AD, Gaur PM, Soderlund C, Penmetsa RV, Xu C, Bharti AK, He W, Winter P, Zhao S, Hane JK, Carrasquilla-Garcia N, Condie JA, Upadhyaya HD, Luo MC, Thudi M, Gowda CL, Singh NP, Lichtenzveig J, Gali KK, Rubio J, Nadarajan N, Dolezel J, Bansal KC, Xu X, Edwards D, Zhang G, Kahl G, Gil J, Singh KB, Datta SK, Jackson SA, Wang J, Cook DR (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat Biotechnol 31:240–246
Weed Science Society of America (2007) Herbicide handbook, 9th edn. Weed Science Society of America, Lawrence
White AD, Graham MA, Owen MDK (2003) Isolation of acetolactate synthase homologs in common sunflower. Weed Sci 51:845–853
Wright TR, Penner D (1998) Cell selection and inheritance of imidazolinone resistance in sugarbeet. Theor Appl Genet 96:612–620
Xu Y, Crouch JH (2008) Marker-assisted selection in plant breeding: from publications to practice. Crop Sci 48:391
Yadav G (2007) Weed management in Chickpea. In: Yadav SS, Reden RJ, Chen W, Sharma B (eds) Chickpea breeding and management. CABI, Oxfordshire, UK, pp 233–245
Acknowledgments
The authors thank Parvaneh Hashemi, Carmen Breitkreutz, Robert Stonehouse, Mauricio Parada, Ambuj Jha and Amit Deokar for their technical support. Funding for this research was provided by the Saskatchewan Pulse Growers (BRE#0906 and BRE#1010).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments conducted in this research comply with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Diers.
Rights and permissions
About this article
Cite this article
Thompson, C., Tar’an, B. Genetic characterization of the acetohydroxyacid synthase (AHAS) gene responsible for resistance to imidazolinone in chickpea (Cicer arietinum L.). Theor Appl Genet 127, 1583–1591 (2014). https://doi.org/10.1007/s00122-014-2320-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2320-0