Abstract
Driven by natural and sexual selection, calling behaviours and call parameters can vary within and between individuals. Phenotypic plasticity can be influenced by environmental conditions (e.g., temperature), size, body condition, and age. Crickets have been classic model organisms for studying the evolution of acoustic communication, but previous studies have focused on field crickets, for which males call at a low frequency, while females exhibit phonotaxis. This study holistically investigated the plasticity of calling behaviours and call parameters across a temperature gradient in a species of lebinthine crickets and examined plasticity between and within individuals. These crickets exhibit a unique communication system, including males calling at a near-ultrasonic frequency while actively searching for females. Ten recording assays at different temperatures were done on males of different sizes and body conditions, half of the assays when the males first became adults and another half 1 month later. Size, body condition, and age group of male crickets, as well as the ambient temperature, had different effects on different calling behaviours (e.g., number of songs produced) and call parameters (e.g., call duration, trill syllable period), even when the acoustic traits were correlated. The crickets also exhibited acclimatisation to the experimental conditions in their calling behaviours and acoustic traits to repeated assays. We also found that calling behaviours were less repeatable than temporal call parameters (e.g., call duration, trill duration), which in turn, were less repeatable than the spectral call parameter (dominant frequency).
Similar content being viewed by others
Introduction
Opposing pressures applied by natural and sexual selection can lead to divergence and variation in within- and between-individual behavioural strategies to balance survival and reproductive needs (Zuk and Kolluru 1998; Hedrick and Kortet 2006). For many animals, male individuals produce sounds to attract conspecific females as a form of sexual advertisement (Hill 1998). These signals are used by females as an indication of a conspecific male’s presence and the general location, as well as of a male’s quality and fitness (Ulagaraj and Walker 1973; Brown et al. 2006; Bentsen et al. 2006; Koehler et al. 2017; Drayton et al. 2010). But sound production can also impose considerable costs in terms of heightening predation and parasitism risks and energetic expenditure (e.g., Kotiaho 2001; Callander et al. 2013). How sound-producing behaviours and parameters vary can be complex because they can be driven by the environment as well as variations within and between individuals of the population(s) (Snell-Rood 2013; O'Dea et al. 2020). Hence, studying the processes and variations associated with sound-producing behavioural strategies is intriguing and paramount to enhancing our understanding of the evolution of acoustic communication.
Behavioural variation across environments can occur either through phenotypic plasticity—short-term, reversible, and adaptive response to environmental changes (Snell-Rood 2013; O'Dea et al. 2020)—or microevolutionary responses to selection pressures (Gross et al. 2010). Such environmental-driven responses in acoustic communication have often been observed among crickets, frogs, and birds (e.g., Walker 1962; Morton 1975; Cade and Wyatt 1984; Hill 1998; Martin et al. 2000; Hedrick et al. 2002; Brumm 2004; Witte et al. 2005; Greenfield and Medlock 2007; Singh et al. 2020). These include the effect of temperature on the call parameters of crickets (e.g., Walker 1962; Cade and Wyatt 1984) and the modulation of calls by birds and frogs in response to noises (e.g., Brumm 2004; Witte et al. 2005). Anthropogenic effects on calling behaviours have also recently drawn greater attention (e.g., Gross et al. 2010; Slabbekoorn 2013), as birds and crickets have been observed to change their calling patterns due to changes in human activity during the COVID-19 pandemic lockdowns (Gordo et al. 2021; Tan and Robillard 2021a).
In addition to environmental influences, behaviour can also vary between and within individuals due to age, body condition, and size (Brown et al. 1996; Bertram 2000; Bertram et al. 2011; Jacot et al. 2007). These traits are often indicative of male quality or fitness (e.g., Simmons and Zuk 1992; Ciceran et al. 1994; Bertram et al. 2011; Singh and Jain 2020). For instance, larger male field crickets may call more intensively (e.g., higher call rates, longer call durations, more syllables per call), which in turn are preferred by females (Simmons and Zuk 1992; Singh and Jain 2020; but see Deb et al. 2012). Likewise, females can use the energetic quality of male calls to estimate the age of males and consequently choose younger individuals (Verburgt et al. 2011). Females using these calling behaviours and/or call parameters as an indication of larger and/or younger males suggest that size, age, and body condition correlating to calling behaviours can be important mate choice criteria.
Field crickets (subfamily Gryllinae) have been widely utilised as model organisms for behavioural studies associated with calling and call parameters (e.g., Simmons and Zuk 1992; Ciceran et al. 1994; Tuckerman et al. 1993; Brown et al. 1996; 2006; Bertram et al. 2011; Singh and Jain 2020). Typical field crickets call at low frequencies while remaining at the same location on the ground and rely on female phonotaxis (Simmons 1988; Bennet-Clark 1989). However, there is another subfamily of crickets, i.e., Eneopterinae Saussure, in which the males of many species (those from the tribe Lebinthini Robillard) call at a relatively high frequency and actively search for females, while females remain static and respond to males’ calls with vibrational signals (ter Hofstede et al. 2015; Benavides-Lopez et al. 2020). However, there has not been a concerted effort to examine the phenotypic plasticity of calling behaviours and call parameters of these behaviourally-unique crickets under the influence of the environment, cricket size, age, and body condition. Specifically, we studied the species Lebinthus luae Robillard and Tan (Fig. 1a). This species and other members from the tribe, which can be found mainly in Southeast Asia and in most archipelagos of the Western Pacific region (including New Guinea) (Tan et al., 2021a), have been used as a model organism to study acoustic and exploratory behaviours associated with communication and mate-finding (ter Hofstede et al. 2015; Tan & Robillard 2021a, 2021b; Tan et al., 2021b).
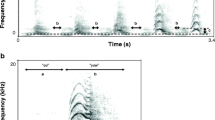
A male adult L. luae in its natural habitat (a) and an example of a male calling song (b) and its spectrogram (c). PCA summarising the calling behaviours and call parameters across 11 individuals of L. luae (d). For the PCA, the Cricket ID refers to the identity of each of the 11 individuals; the arrows indicate how the different traits correlate with one another
Here, we addressed how plastic calling behaviours and/or call parameters are between and within individuals of different sizes and ages and under different temperature treatments. We investigate commonly-studied call parameters (e.g., call duration, syllable period, and dominant frequency) as well as less-explored calling behaviours (e.g., latency or time taken by the cricket to start calling after being introduced to a novel insect cage) and call parameters (e.g., amount of sound produced per call). Latency to start calling can provide indications about the predator-avoidance behaviour of the cricket, such as boldness or tendency to take risks, similar to behavioural/ personality studies which used latency to emerge from a safe refuge in a novel environment (Hedrick 2000; Lewkiewicz and Zuk 2004; Kortet and Hedrick 2007). We also measured the amount of sound produced per call, in addition to call duration, because the latter may not necessarily be sufficient to describe the calling effort by the cricket (Symes et al. 2020). This is because different individuals can produce songs with different call or trill durations with the same number of syllables (Tan and Robillard 2021b). We postulated that different calling behaviours and parameters can be influenced differently by cricket size, age, body condition, and/or temperature, even if they are correlated.
Furthermore, to examine how acoustic traits vary between and within individuals, we also performed repeated recordings for each cricket individual and determined whether the acoustic traits exhibited consistent inter-individual differences after accounting for the effects of the environment and cricket size, age, and body condition. We predicted that some traits are more repeatable than others. For example, some of the call parameters determined primarily by sound-production morphology are likely to be more repeatable than calling behaviours primarily influenced by the environment, cricket size, age, and body condition and neuroendocrine mechanisms.
Materials and methods
Study subjects
Cricket eggs were collected from a few females of a single population in Pulau Ubin of Singapore in 2019 for the experiments. Only a single population was used to minimise confounding effects caused by population differences. Nymphs and male and female adults were held in a community tank in a temperature-light-controlled insect rearing room in the Muséum national d’Histoire naturelle Paris (MNHN). Crickets were kept at 25–27 °C in large plastic tanks (60 cm length × 40 cm height × 40 cm width) with soil as substrate and Hedera helix as vegetation to simulate their natural environment. Colonies were subjected to 13:11 h light:dark cycle. Cotton balls soaked in water and the presence of vegetation helped maintain humidity. The crickets were fed ad libitum with Hedera helix, Affinity ULTIMA Mini Adult dog pellets food, and BJORG Muesli whole grain cereals without added sugar.
Healthy and unscathed males from the second generation of the colony were used in this study as soon as they reached adulthood. We searched for adult males at least once every three days. Each male was subsequently placed in a plastic container (21 cm length × 13 cm width × 7 cm height) in isolation (physically) at least 24 h before the start of the experiments for acclimatisation to the new environment. Each container was cleaned daily, and food (same as in the community tank) and water (a wet cotton ball) were replaced every two days. The fresh weight of each individual was measured using a precise weighing balance (Scaltec SBA 32; Germany) before the first recording session as a proxy of size. Over a period of four months, a total of 11 newly emerged adult males from the same generation were used for this study. Unlike the short life cycle of more prolific typical gryllines (about 30 days to reach adulthood from hatching), L. luae takes about 6 months to reach adulthood. The studied colony only produced a limited number of adult males within each generation.
Recording assays
Each male was recorded in isolation in a sound-attenuating room (2.2 m height, 2.2 m width, 2.9 m length) in MNHN. For each recording assay, one male individual was placed in a sound permeable nylon cage (25 cm in diameter and 33 cm tall) where it was free to roam around and call. To examine the inter- and intra-individual variations, each male was subjected to five recording assays, each lasting for 1 h. Consecutive recording assays were separated by at least 48 h. To account for the effects of circadian rhythm on the calling activity (see Tan and Robillard 2021b), the time of recording assays was randomised for every male.
To test the effect of temperature on calling behaviours and call parameters, each recording assay was done at a randomised temperature range (20–22 °C; 22–24 °C; 24–26 °C; 26–28 °C; 28–30 °C), controlled using a Universal Thermostat UT300 connected to an electric heater (Calor Thermline; 1000 to 2000 W; 67 × 37 × 10 cm). A HOBO 8 K Pendant® Temperature logger (model: UA-001–186 08, Onset, Bourne, MA) was used to track the precise room ambient temperature once every 10 min. A humidifier (Nulaxy Top Fan; Model MH900; Hong Kong) with an incorporated hygrometer, and cotton balls soaked with water were used to maintain the room humidity between 60 and 70%. A lamp with a K10C-A light bulb was lit at a fixed distance from the cage to simulate daylight.
Acoustic recordings were done using a modified condenser microphone (Avisoft Bioacoustics, Berlin; model CM16), with a relatively flat frequency response from 3 to 150 kHz (R. Specht pers. comm.).The program Avisoft Triggering Harddisk Recorder version 2.97 and an 8-Pre MOTU sound card at a sampling frequency of 96 kilo-samples per second (16 bit) were used during trials. The microphone was placed 50 cm horizontally from the nylon cage containing the cricket. The configuration dialogue box in the Avisoft-RECORDER was pre-set to avoid the loss of acoustic information (pre-trigger: 5 s; hold time: 80 s; level of the trigger event: 4%; range: 0–250 kHz; entropy: 35%).
To test the effect of the age of the crickets on calling behaviours and call parameters, the same experiment was repeated for each individual one month later. As we could not identify the exact age of each male, we only differentiated the age of the cricket into two age-group categories (i.e., two experimental phases): young (a few days after moulting) and older (one month later). Between the two phases of the experiments, each cricket was kept in its plastic container (21 cm length × 13 cm width × 7 cm height) in physical isolation. Fresh weight was measured again prior to the new series of recording assays.
After the experiments, the crickets were euthanised in a freezer and pinned for vouchering in the MNHN. The pronotum length was measured. As the fresh weight and pronotum length were correlated (estimate = 0.43 ± 0.18, R2 = 0.32, 95% CI [0.02, 0.84]), we used the fresh weight as a proxy for individual size in the statistical modelling to avoid collinearity problems. We used fresh weight instead of pronotum length because fresh weight can also indicate body condition at both phases of the experiments, when the cricket was younger and older, in addition to an estimate of individual size. We were careful not to harm the crickets during the collection, housing, and experiments.
Acoustic analyses
The calling behaviour was quantified using Raven Pro 1.1.5. (Cornell Lab of Ornithology) by counting the number of calls per assay and the latency to start calling (i.e., the time the cricket takes to start calling after being introduced to a novel insect cage). Although one can expect a cricket that takes a longer time to start calling would naturally call fewer times, we considered both behavioural traits in the subsequent analyses to verify if the assumption is valid and compare how they may differ.
Within-echeme parameters were also quantified. The terminology of the call parameters of L. luae song follows that of Robillard and Tan (2013). The echeme of the calling song of L. luae consists of two parts (Fig. 1b). The first part corresponds to several well-spaced syllables (denoted as ‘clicks’ here), and the second part is a short trill made of several syllables set closer together. Each syllable is made of discrete pulses caused by a discontinuous closing movement of the wings during stridulation. The cricket does not close its wings in a single movement but instead closes its wings slightly to generate a pulse from the first teeth–plectrum interaction and pauses (creating a downtime between the pulses).
Call duration, trill duration, and dominant frequency were obtained using Avisoft-SASLab Pro (version 5.2.15). Call duration refers to the entire duration of the call, whereas trill duration refers to the duration of only the second part of the call. We also quantified the amount of sound produced per call by summing the durations of all the individual syllables for both the clicks and trill part (Symes et al. 2020; Tan and Robillard 2021b). The option ‘Pulse Train Analysis’ in Avisoft-SASLab Pro was used to measure the syllable durations within each call.
Statistical analyses
To examine how the different calling behaviours and call parameters were correlated with one another, the traits were summarised into major gradients of variation by performing a principal component analysis (PCA) using the ‘prcomp’ function (R Core Team 2022). We first obtained the median of the call parameters from each assay. Since PCA cannot handle missing values, we excluded assays in which the cricket did not call.
To investigate how calling behaviours and call parameters are influenced by the size, age group, and/or condition of the cricket, as well as temperature and acclimatisation to experimental conditions, we fitted linear mixed effects models (LMMs) using the ‘lmer’ function from the R package ‘lme4’ (Bates et al. 2014), respectively, for latency to start calling, call duration, amount of sound produced per call, trill duration, trill syllable duration and period, and dominant frequency. We log-transformed all call parameters to improve model fits. We also fitted a generalised mixed-effects model (GLMM) with a negative binomial error using the ‘glmer.nb’ function from the same package for the number of calls to account for the overdispersion of count data. The following fixed effects were fitted in each model: fresh weight as a proxy of size and/or condition, age group (category, young adult, one month older), a quadratic term for temperature, and the number of assays undergone. We log-transformed the number of assays because we expected the cricket to eventually acclimatise to experimental conditions (i.e., the cage in the acoustic room). The size of adult insects was fixed (Chown and Gaston 2010), and we did not find any collinearity between the age group of cricket and fresh weight (estimate = 0.01 ± 0.01, R2 = 0.03, 95% CI [− 0.02, 0.03]). We scaled the fresh weight and temperature about their means. Since more than one measurement was taken from each individual, the individual cricket identity was used as a random intercept. We then performed stepwise simplification to obtain the final model for each acoustic trait.
To estimate the repeatability of the calling behaviours and call parameters, we used the functions ‘rpt’ and ‘rptPoisson’ from the R package ‘rptR’ (Stoffel et al. 2017) to calculate the intraclass correlation coefficient (ICC). The ICC was calculated as the ratio of inter-group variance and the sum of inter- and within-group variance (Nakagawa and Schielzeth 2010), where ‘group’ refers to individual crickets when determining repeatability. We fitted fixed effects from the final model for each calling behaviour and call parameter. We reported the standard errors of the repeatability estimates and performed 500 parametric bootstraps to obtain the 95% confidence intervals for the random effect. Repeatabilities were calculated after controlling for variation due to covariates.
We checked the model and estimation of repeatability of each calling behaviour and call parameters for outliers, heterogeneity of residuals, and variable collinearity to ensure model assumptions were not violated (Zuur et al. 2010; Zuur & Ieno 2016). Although the number of male individuals tested in this study was limited, the models were not overfitted, and the estimation of repeatability was considered reasonable (Bailey et al. 2021).
Results
In total, 11 adult male crickets were studied over four months. All crickets underwent ca. 10 recording assays, except individual 10, which died after five recording assays. A total of 107 recording assays were performed, and 1070 calls were recorded and analysed (n = 10 ± 15 calls recorded per assay). On average, the crickets took about 24 ± 15 min to start singing. The average call parameters were as follows: call duration = 4.3 ± 2.8 s, the amount of sound produced per call = 0.76 ± 0.3 s, trill duration = 1.0 ± 0.6 s, and dominant frequency = 17.8 ± 1.2 kHz, which are not drastically different from the wild population (see Robillard & Tan 2013; Tan & Robillard 2021a).
Correlations
The first two axes of the PCA explained 68.0% of the variance. The calling behaviour and call parameters were summarised into three major gradients of variation (Fig. 1c). Firstly, crickets with a shorter latency to start calling produced a greater number of calls (Fig. 1c). Trill syllable duration, call duration, and amount of sound produced per call were closely correlated (Fig. 1c). Trill syllable period and trill duration were also closely correlated (Fig. 1c).
Calling behaviours
The number of calls produced was influenced by the cricket’s fresh weight and age group and the number of assays undergone (R2m = 0.34, R2c = 0.70) (Fig. 2a). Individuals with a greater fresh weight produced more calls (estimate = 1.04 ± 0.27, 95% CI [0.54, 1.57]) (Fig. 2a). The crickets also produced fewer calls when they were older (estimate = − 1.24 ± 0.38, 95% CI [− 2.00, − 0.49] (Fig. 2a). The crickets showed evidence of acclimatisation to experimental conditions in both age groups by producing more calls during the latter assays within each phase of the experiment (estimate = 2.81 ± 0.70, 95% CI [1.42, 4.18]).
Significant effects of fresh weight and age group on the number of calls produced (a), and significant effects of fresh weight, age group and temperature on time taken to start calling (b). The lines represent predictions based on the final GLMM (a) and LMM (b) after stepwise simplification. The final models only contain the significant explanatory variable(s) as shown in the plots. The effects of fresh weight as a continuous explanatory variable (b) were represented using three lines (0.24 g, 0.27 g, 0.29 g). The data points in circles of different sizes correspond to the individual crickets with different fresh weights in b; otherwise, data points are represented using ‘ + ’ in (a). The data points in green and blue correspond to the ‘young’ and ‘old’ age groups of the cricket
The time taken to start calling was influenced by the fresh weight and age group of the cricket, as well as a quadratic term of temperature and the number of assays undergone (R2m = 0.27, R2c = 0.46). Individuals with greater fresh weight had a shorter latency to start calling than lighter individuals (estimate = − 575.7 ± 158.4, 95% CI [− 951.7, − 176.9]) (Fig. 2b). The crickets also had a longer latency to start calling when they were older (estimate = 1023.8 ± 329.3, 95% CI [377.9, 1655.9] (Fig. 2b).
The crickets had a shorter time to call at higher temperatures until around 25 °C and had a longer time to call at temperatures beyond 25 °C (estimate = 230.4 ± 114.0, 95% CI [7.77, 449.6]) (Fig. 2b). The crickets showed evidence of acclimatisation to experimental conditions in both age groups by taking a shorter latency to start calling the latter assays within each phase of the experiment (estimate = − 1443.8 ± 529.4, 95% CI [− 2476.5, − 420.0]). After accounting for the effects of cricket size, age, body condition, temperature, and acclimatisation, male L. luae also showed consistent inter-individual differences in the number of calls per assay (original-scaled ICC = 0.25 ± 0.09, 95% CI [0.07, 0.40], p-value < 0.001) and time to call (ICC = 0.27 ± 0.12, 95% CI [0.05, 0.52], p-value = 0.010).
Call parameters
Call duration (R2m = 0.08, R2c = 0.44) was influenced by the fresh weight and the number of assays undergone (Fig. 3a). Individuals with greater fresh weight produced calls with longer call duration (log-transformed estimate = 0.09 ± 0.03, 95% CI [0.02, 0.15]) (Fig. 3a). The crickets showed evidence of acclimatisation to experimental conditions in both age groups by producing longer calls during the latter assays within each phase of the experiment (log-transformed estimate = 0.50 ± 0.07, 95% CI [0.37, 0.63]).
Significant effects of fresh weight on call duration (a); significant effects of fresh weight and temperature on the amount of sound produced per call (b); significant effects of age group, fresh weight, and temperature on trill duration (c); significant effects of age group, fresh weight, and temperature on dominant frequency (d). The lines represent predictions based on the final LMMs after stepwise simplification. The final models only contain significant explanatory variable(s), as shown in the plots. The effects of fresh weight as a continuous explanatory variable (b, d) were represented using three lines (0.24 g, 0.28 g, 0.31 g). The data points in circles of different sizes correspond to the individual crickets with different fresh weights in b–d; otherwise, data points are represented using ‘ + ’ in (a). The data points in green and blue correspond to the ‘young’ and ‘old’ age groups of the cricket in c, d; otherwise, data points are represented in black
The amount of sound produced per call (R2m = 0.09, R2c = 0.42) was influenced by the fresh weight, temperature, and the number of assays undergone (Fig. 3b). Individuals with a greater fresh weight produced greater amount of sound per call (log-transformed estimate = 0.05 ± 0.01, 95% CI [0.01, 0.08]) (Fig. 3b). The crickets produced a lower amount of sound per call (log-transformed estimate = − 0.02 ± 0.01, 95% CI [− 0.03, − 0.01]) with increasing temperature (Fig. 3b). The crickets showed evidence of acclimatisation to experimental conditions in both age groups by producing more sound during the latter assays within each phase of the experiment (log-transformed estimate = 0.22 ± 0.03, 95% CI [0.16, 0.28]).
The trill duration (R2m = 0.24, R2c = 0.51) was influenced by the fresh weight, age group of crickets, and temperature (Fig. 3c). Individuals with a greater fresh weight produced marginally longer trills (log-transformed estimate = 0.04 ± 0.01, 95% CI [0.00, 0.06]) (Fig. 3c). Individuals also produced longer trills (log-transformed estimate = 0.23 ± 0.02, 95% CI [0.20, 0.26]) when they were older (Fig. 3c). The crickets generally produced marginally shorter trills (log-transformed estimate = − 0.01 ± 0.01, 95% CI [− 0.03, − 0.00]) with increasing temperature and the decrease in trill duration with increasing temperature appears more prominent after around 25 °C (Fig. 3c).
The dominant frequency (R2m = 0.03, R2c = 0.54) was influenced by the fresh weight and age group of crickets as well as the temperature (Fig. 3d). Individuals with a greater fresh weight produced calls with marginally lower dominant frequency (log-transformed estimate = − 3.1 × 10−3 ± 1.4 × 10−3, 95% CI [− 0.6 × 10−3, − 0.3 × 10−3]) (Fig. 3d). Individuals also produced calls with higher dominant frequency (log-transformed estimate = 6.2 × 10−3 ± 1.7 × 10−3, 95% CI [0.3 × 10−3, 1.0 × 10−3]) when they were older (Fig. 3d). The crickets produced calls with the highest dominant frequency at around 25 °C (log-transformed estimate = − 2.0 × 10−3 ± 0.6 × 10−3, 95% CI [− 3.1 × 10−3, − 0.9 × 10−3]) (Fig. 3d).
The trill syllable duration (R2m = 0.35, R2c = 0.50) was influenced by temperature (Fig. 4a). The crickets generally produced shorter trill syllables at higher temperatures (estimate = − 2.1 × 10−3 ± 0.1 × 10−3, 95% CI [− 2.3 × 10−3, − 2.5 × 10−3]) (Fig. 4a). The trill syllable period (R2m = 0.35, R2c = 0.50) was influenced by the age group of the cricket and temperature (Fig. 4b). The trill syllable period was longer when the crickets were older (estimate = 1.6 × 10−3 ± 0.2 × 10−3, 95% CI [1.1 × 10−3, 2.1 × 10−3]). The syllable period also generally decreased at higher temperatures (estimate = − 2.3 × 10−3 ± 0.2 × 10−3, 95% CI [− 2.4 × 10−3, − 2.1 × 10−3]) (Fig. 4b).
Significant effects of temperature and/or age group on trill syllable duration (a) and period (b). The lines represent predictions based on the final LMMs after stepwise simplification. The final models only contain significant explanatory variable(s), as shown in the plots. The data points in green and blue correspond to the ‘young’ and ‘old’ age groups of the cricket in b; otherwise, data points are represented in black in a
After accounting for the effects of cricket size, age, body condition, temperature, and acclimatisation, male L. luae also showed consistent inter-individual differences in all call parameters (Table 1). Temporal parameters (i.e., call duration, amount of sound produced per call, trill duration, and trill syllable period) had similar ICC estimates, ranging from 0.35 to 0.39. The spectral parameter (i.e., dominant frequency) had a higher ICC estimate than all the temporal parameters (Table 1).
Discussion
Our first objective was to examine how the plasticity of the calling behaviour and call parameters of L. luae is influenced by temperature, cricket size, age, and body condition. In line with our expectations, different calling behaviours and call parameters can vary with the size and age of individuals and/or temperature (Table 2). Even when the calling behaviour and call parameters are correlated, they can interact differently with cricket size, age, body condition, and/or environmental factors (Table 2). For example, even though latency to start calling and the number of calls produced are negatively correlated (Fig. 1c), ambient temperature affected the time taken for the crickets to start calling but not the number of calls. Likewise, ambient temperature also affects the correlated call parameters (i.e., the trill duration vs. trill syllable period and call duration vs. the amount of sound produced per call) differently. We also observed that male crickets had a shorter latency to start calling and produced more and longer calls in the later assays within each experimental phase (i.e., young and one month later), independent of the temperature. This indicates that L. luae crickets gradually acclimatised to the experimental conditions within each of the two experimental periods separated by a month.
How plastic are calling behaviour and call parameters?
In our results, individuals with a greater fresh weight had a shorter latency to start calling and produced more calls and longer calls (in terms of call duration, amount of sound produced per call, and to a smaller extent, trill duration). A plausible explanation, observed in various field crickets (e.g., Bertram et al. 2011; Verburgt et al. 2011), is that larger individuals and/or better body conditions can afford to allocate more energy and resources into calling than smaller males. They may also possess higher muscle performance to sing than smaller males (Verburgt et al. 2011), although this may also not always be the case (e.g., in Acheta domesticus (Linneaus), see Bertram et al. 2011). Previous studies on gryllines have shown that larger-sized field crickets can be attributed to higher protein intake during development and/or higher carbohydrate intake during adulthood (Hunt et al. 2004; Bertram et al. 2011). Higher carbohydrate intake may provide the larger males and/or better body conditions with more energy to increase singing effort (Bertram et al. 2011). Higher nutrient intake during development and adulthood can be particularly crucial for fitness in lebinthines because males need to expend more energy to explore and search for females while calling simultaneously. Hence, larger males and/or better body conditions may be more willing to call and produce longer calls because they can be more confident in exploring further and finding females. However, physiological studies on muscle metabolism should be conducted in lebinthines to confirm our postulation.
We were able to examine the effects of age, independent of size, given that each male was recorded over two time periods of its adulthood (once as a young adult and subsequently a month later as an older adult) and that the size of adult crickets was found to be fixed (Chown and Gaston 2010). Older individuals had a longer latency to start calling and hence produced fewer calls than younger individuals. These results are consistent with those found in gryllines (Brown et al. 1996; Bertram 2000; Jacot et al. 2007; Verburgt et al. 2011). Older individuals may become less rigorous and less able to support the energetic costs of calling than when they were younger. As the males become older, they may experience a decrease in the efficiency of wing muscles owing to senescent physiological changes (Verburgt et al. 2011).
Yet, we also observed males producing more prolonged trills when they were older, which may be attributed to an increase in the syllable period in trills. Our PCA (Fig. 1c), showing a strong correlation between the trill syllable period and trill duration, can support this. As the trill component of the calls is potentially an important indicator of male quality in L. luae, we postulate that older males produce longer trills to attract females’ attention while compensating for less vigorous calling (i.e., the number of songs produced is reduced in older males). Compared to the simpler chirps in typical gryllines, this Lebinthus species has two parts in its calls. The parameters associated with different parts of the calls can be influenced differently by cricket size, age, and body condition because they probably have different communication functions and physiological requirements. Examining the precise physiological and energetic requirements of producing trills and clicks may be needed to test this hypothesis.
Larger males and/or better body conditions also produced calls with a lower dominant frequency, which was more prominent when they were young. A negative correlation between body size and dominant frequency has been consistently observed in crickets (Simmons and Zuk 1992; Simmons 1995; Simmons and Ritchie 1996; Bennet-Clark 1998; Prestwich et al. 2000; Bertram et al. 2011; Deb et al. 2012; Harrison et al. 2013) and sound-producing animals in general (e.g., Hoskin et al. 2009; Sueur et al. 2010). A plausible reason is that larger males produce lower frequency calls because of their larger wing resonators (harp area in crickets; Singh and Jain 2020). Older males producing a higher dominant frequency were also observed in Gryllus rubens Scudder, but only for males of spring-collected (and not fall-collected) parents (Beckers 2020). This can be explained by age-related differences in the stiffness of the resonators of the forewings since stiffer resonant parts of the forewings lead to higher-frequency calls (Koch et al. 1988; Bennet-Clark 2003). It is plausible that older L. luae males may also have stiffer forewings, which can explain why they produce a higher dominant frequency than when they were first recorded.
How does temperature influence calling behaviour and call parameters?
Temperature has far-reaching consequences on the phenotypic plasticity of ectotherms (Moiroux et al. 2016; Brandt et al. 2018), and its effects on calling behaviours and call parameters are also well studied (e.g., Martin et al. 2000; Hedrick et al. 2002; Greenfield and Medlock 2007; Singh et al. 2020). In L. luae, the correlations between temperature and acoustic traits can be more complex than a linear relationship. At higher temperatures, males have a shorter latency to start singing, which is typically expected since ectotherms tend to be more metabolically active at higher temperatures (Martin et al. 2000; Hedrick et al. 2002; Greenfield and Medlock 2007). This effect, however, was reversed when the temperature was too high, and the crickets had a longer latency to start calling. We postulate that the upper limit of the temperature regime in our experiment (i.e., 28–30 °C) may not be physiologically ideal for these crickets, thus deterring them from calling more readily. This is because L. luae are naturally found in the humid and shaded forest floor of tropical rainforests (Robillard and Tan 2013; Baroga-Barbecho et al. 2020). Even though the ambient temperature where they are found can reach beyond 30 °C in tropical Southeast Asia, the microhabitats the crickets occupy (i.e., shaded leaf litters, underneath leaves, on transpiring leaves near the ground) may provide a much cooler and humid microclimate. This may explain why the optimal temperature for calling is around 25 °C rather than closer to the ambient temperature of approximately 30 °C in Singapore. This result hints at the importance of translating similar studies in the field and/or incorporating fine-scale microclimates when studying the effects of temperature on small insects (Pincebourde and Suppo 2016; Pincebourde et al. 2016; Pincebourde and Woods 2020).
The quadratic relationship between temperature and latency to call observed in L. luae was also recently reported in an Indian field cricket, Acanthogryllus asiaticus Gorochov (Singh et al. 2020). For this species, the call duration, syllable duration, and period also increase with temperature to a maximum at 26 °C before decreasing at higher temperatures. However, we observed in L. luae that individuals produced shorter calls based on the amount of sound produced per call and trill duration—both of which may be a direct consequence of the shorter trill syllable duration and period observed with increasing temperature. Furthermore, the decrease in trill part (i.e., trill duration, trill syllable duration and period) was more prominent than the amount of sound at a higher temperature. We inferred that the forewings heat up faster during open-closure at a higher temperature and that producing shorter calls is a way the cricket avoids overheating. These findings led us to believe the effect of temperature on acoustic communication is not necessarily linear and straightforward in crickets (Singh et al. 2020).
Are calling behaviour and call parameters repeatable?
The second objective of the study was to examine the inter-individual differences in calling behaviours and call parameters. After accounting for the effects of cricket size, age, body condition, temperature, and acclimatisation, some individual crickets consistently produce more calls, and all the call parameters in lebinthines were found to be repeatable. These are congruent to the findings by Tan and Robillard (2021a). Our study also takes a step further by demonstrating that the calling behaviours were also repeatable. These findings indicate that some individuals consistently produced more calls and had a shorter latency to start calling than other individuals.
Our results additionally show that calling behaviours are less repeatable than call parameters. Higher lability in the calling behaviours than in the call parameters may be attributed to a stronger influence of the immediate environment, such as eavesdropping by predators (Kolluru 1999; Nandi and Balakrishnan 2013), whereas call parameters may be more stereotyped owing to sexual selection and morphological constraint. Temporal call parameters are less repeatable than the frequency call parameter, which corroborates with previous studies on Lebinthus (Tan and Robillard 2021a) as well as with other orthopterans (Kolluru 1999; Nityananda and Balakrishnan 2008; Deb et al. 2012; Nandi and Balakrishnan 2013; Tan 2020). Call frequency is more constrained by the morphology of sound-producing organs than temporal parameters (Montealegre-Z et al. 2011; Robillard et al. 2013). Hence, within-individual variations in the frequency of calls are expected to be much lower than in the temporal parameters.
Conclusions
Based on the experiments using 11 individual crickets, we used a sophisticated statistical approach to the behavioural assay and demonstrated how variations in cricket size, age, body condition, and temperature can lead to the plasticity of calling behaviours and call parameters in lebinthine crickets for the first time. We offer a few plausible explanations for the phenotypic plasticity of calling behaviours and call parameters of L. luae. But this study also raises some open questions or new hypotheses for further research (e.g., the precise physiological and energetic requirements of producing different parts of the calling songs and the impacts of fine-scale microclimates on acoustic communication).
References
Bailey JD, King AJ, Codling EA, Short AM, Johns GI, Fürtbauer I (2021) “Micropersonality” traits and their implications for behavioral and movement ecology research. Ecol Evol 11(7):3264–3273. https://doi.org/10.1002/ece3.7275
Baroga-Barbecho JB, Tan MK, Yap SA, Robillard T (2020) Taxonomic study of Lebinthus Stål, 1877 (Orthoptera: Gryllidae: Eneopterinae) with description of six new species in the Philippines. Zootaxa 4816(4):401–438. https://doi.org/10.11646/zootaxa.4816.4.1
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B (2014) lme4: linear mixed-effects models using Eigen and S4 (Version 1.1–7)
Beckers OM (2020) Phenotypic plasticity related to temperature induces song variation in the field cricket Gryllus rubens. Ethology 126(8):781–790. https://doi.org/10.1111/eth.13035
Benavides-Lopez JL, ter Hofstede H, Robillard T (2020) Novel system of communication in crickets originated at the same time as bat echolocation and includes male-male multimodal communication. Sci Nat 107(1):1–6. https://doi.org/10.1007/s00114-020-1666-1
Bennet-Clark HC (1989) Songs and the physics of sound production. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and Neurobiology. Cornell University Press, Ithaca, N.Y, U.S.A., pp 227–261
Bennet-Clark HC (1998) Size and scale effects as constraints in insect sound communication. Philos Trans R Soc B 353:407–419. https://doi.org/10.1098/rstb.1998.0219
Bennet-Clark HC (2003) Wing resonances in the Australian field cricket Teleogryllus oceanicus. J Exp Biol 206(9):1479–1496. https://doi.org/10.1242/jeb.00281
Bentsen CL, Hunt J, Jennions MD, Brooks R (2006) Complex multivariate sexual selection on male acoustic signaling in a wild population of Teleogryllus commodus. Am Nat 167(4):102–116. https://doi.org/10.1086/501376
Bertram SM (2000) The influence of age and size on temporal mate signalling behaviour. Anim Behav 60:333e339. https://doi.org/10.1006/anbe.2000.1473
Bertram SM, Thomson IR, Auguste B, Dawson JW, Darveau CA (2011) Variation in cricket acoustic mate attraction signalling explained by body morphology and metabolic differences. Anim Behav 82(6):1255–1261. https://doi.org/10.1016/j.anbehav.2011.08.021
Brandt EE, Kelley JP, Elias DO (2018) Temperature alters multimodal signaling and mating success in an ectotherm. Behav Ecol Sociobiol 72(12):1–14. https://doi.org/10.1007/s00265-018-2620-5
Brown WD, Wideman J, Andrade MCB, Mason AC, Gwynne DT (1996) Female choice for an indicator of male size in the song of the black-horned tree cricket, Oecanthus nigricornis (Orthoptera: Gryllidae: Oecanthinae). Evolution 50:2400–2411. https://doi.org/10.1111/j.1558-5646.1996.tb03627.x
Brown WD, Smith AT, Moskalik B, Gabriel J (2006) Aggressive contests in house crickets: size, motivation and the information content of aggressive songs. Anim Behav 72(1):225–233. https://doi.org/10.1016/j.anbehav.2006.01.012
Brumm H (2004) The impact of environmental noise on song amplitude in a territorial bird. J Anim Ecol 73(3):434–440. https://doi.org/10.1111/j.0021-8790.2004.00814.x
Cade WH, Wyatt DR (1984) Factors affecting calling behaviour in field crickets, Teleogryllus and Gryllus (age, weight, density, and parasites). Behaviour 88:61–75
Callander S, Kahn AT, Hunt J, Backwell PR, Jennions MD (2013) The effect of competitors on calling effort and life span in male field crickets. Behav Ecol 24(5):1251–1259. https://doi.org/10.1093/beheco/art059
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85(1):139–169. https://doi.org/10.1111/j.1469-185X.2009.00097.x
Ciceran M, Murray AM, Rowell G (1994) Natural variation in the temporal patterning of calling song structure in the field cricket Gryllus pennsylvanicus: effects of temperature, age, mass, time of day, and nearest neighbour. Can J Zool 72(1):38–42. https://doi.org/10.1139/z94-006
Deb R, Bhattacharya M, Balakrishnan R (2012) Females of a tree cricket prefer larger males but not the lower frequency male calls that indicate large body size. Anim Behav 84(1):137–149. https://doi.org/10.1016/j.anbehav.2012.04.020
Drayton JM, Milner RN, Hunt J, Jennions MD (2010) Inbreeding and advertisement calling in the cricket Teleogryllus commodus: laboratory and field experiments. Evol: Int J Org Evol 64(10):3069–3083. https://doi.org/10.1111/j.1558-5646.2010.01053.x
Gordo O, Brotons L, Herrando S, Gargallo G (2021) Rapid behavioural response of urban birds to COVID-19 lockdown. Proc R Soc B 288(1946):20202513. https://doi.org/10.1098/rspb.2020.2513
Greenfield MD, Medlock C (2007) Temperature coupling as an emergent property: parallel thermal effects on male song and female response do not contribute to species recognition in an acoustic moth. Evolution 61(7):1590–1599. https://doi.org/10.1111/j.1558-5646.2007.00140.x
Gross K, Pasinelli G, Kunc HP (2010) Behavioural plasticity allows short-term adjustment to a novel environment. Am Nat 176(4):456–464. https://doi.org/10.1086/655428
Harrison SJ, Thomson IR, Grant CM, Bertram SM (2013) Calling, courtship, and condition in the fall field cricket Gryllus Pennsylvanicus. Plos One 8(3):e60356. https://doi.org/10.1371/journal.pone.0060356
Hedrick AV (2000) Crickets with extravagant mating songs compensate for predation risk with extra caution. Proceedings of the Royal Society of London. Series B: Biol Sci 267(1444):671–675. https://doi.org/10.1098/rspb.2000.1054
Hedrick AV, Kortet R (2006) Hiding behaviour in two cricket populations that differ in predation pressure. Anim Behav 72(5):1111–1118. https://doi.org/10.1016/j.anbehav.2006.03.018
Hedrick A, Perez D, Lichti N, Yew J (2002) Temperature preferences of male field crickets (Gryllus integer) alter their mating calls. J Comp Physiol A 188(10):799–805. https://doi.org/10.1007/s00359-002-0368-9
Hill PS (1998) Environmental and social influences on calling effort in the prairie mole cricket (Gryllotalpa major). Behav Ecol 9(1):101–108. https://doi.org/10.1093/beheco/9.1.101
Hoskin CJ, James S, Grigg GC (2009) Ecology and taxonomy-driven deviations in the frog call–body size relationship across the diverse Australian frog fauna. J Zool 278(1):36–41. https://doi.org/10.1111/j.1469-7998.2009.00550.x
Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiere LF (2004) High-quality male field crickets invest heavily in sexual display but die young. Nature 432 1024e1027. https://doi.org/10.1038/nature03084
Jacot A, Scheuber H, Brinkhof MWG (2007) The effect of age on a sexually selected acoustic display. Ethology 113:615–620. https://doi.org/10.1111/j.1439-0310.2007.01360.x
Koch UT, Elliott CJ, Schäffner KH, Kleindienst HU (1988) The mechanics of stridulation of the cricket Gryllus campestris. J Comp Physiol A 162(2):213–223. https://doi.org/10.1007/BF00606086
Koehler J, Jansen M, Rodriguez A, Kok PJ, Toledo LF, Emmrich M, Glaw F, Haddad CFB, Rödel MO, Vences M (2017) The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251(1):1–124. https://doi.org/10.11646/zootaxa.4251.1.1
Kolluru GR (1999) Variation and repeatability of calling behaviour in crickets subject to a phonotactic parasitoid fly. J Insect Behav 12(5):611–626. https://doi.org/10.1023/A:1020923602780
Kortet R, Hedrick ANN (2007) A behavioural syndrome in the field cricket Gryllus integer: intrasexual aggression is correlated with activity in a novel environment. Biol J Lin Soc 91(3):475–482. https://doi.org/10.1111/j.1095-8312.2007.00812.x
Kotiaho JS (2001) Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76(3):365–376. https://doi.org/10.1017/s1464793101005711
Lewkiewicz DA, Zuk M (2004) Latency to resume calling after disturbance in the field cricket, Teleogryllus oceanicus, corresponds to population-level differences in parasitism risk. Behav Ecol Sociobiol 55(6):569–573. https://doi.org/10.1007/s00265-003-0745-6
Martin SD, Gray DA, Cade WH (2000) Fine-scale temperature effects on cricket calling song. Can J Zool 78(5):706–712. https://doi.org/10.1139/z99-262
Moiroux J, Abram PK, Louâpre P, Barrette M, Brodeur J, Boivin G (2016) Influence of temperature on patch residence time in parasitoids: physiological and behavioural mechanisms. Sci Nat 103(3–4):32. https://doi.org/10.1007/s00114-016-1357-0
Montealegre-Z F, Jonsson T, Robert D (2011) Sound radiation and wing mechanics in stridulating field crickets (Orthoptera: Gryllidae). J Exp Biol 214:2105–2117. https://doi.org/10.1242/jeb.056283
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109(965):17–34
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85(4):935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Nandi D, Balakrishnan R (2013) Call intensity is a repeatable and dominant acoustic feature determining male call attractiveness in a field cricket. Anim Behav 86(5):1003–1012. https://doi.org/10.1016/j.anbehav.2013.09.003
Nityananda V, Balakrishnan R (2008) Leaders and followers in katydid choruses in the field: call intensity, spacing and consistency. Anim Behav 76(3):723–735. https://doi.org/10.1016/j.anbehav.2008.04.015
O'Dea RE, Noble D, Nakagawa S (2020) Unifying individual differences in personality, predictability, and plasticity: a practical guide. https://doi.org/10.32942/osf.io/bnugw
Pincebourde S, Suppo C (2016) The vulnerability of tropical ectotherms to warming is modulated by the microclimatic heterogeneity. Integr Comp Biol 56(1):85–97. https://doi.org/10.1093/icb/icw014
Pincebourde S, Woods HA (2020) There is plenty of room at the bottom: microclimates drive insect vulnerability to climate change. Curr Opin Insect Sci 41:63–70
Pincebourde S, Murdock CC, Vickers M, Sears MW (2016) Fine-scale microclimatic variation can shape the responses of organisms to global change in both natural and urban environments. Integr Comp Biol 56(1):45–61. https://doi.org/10.1093/icb/icw016
Prestwich KN, Lenihan KM, Martin DM (2000) The control of carrier frequency in cricket calls: a refutation of the subalaretegminal resonance/ auditory feedback model. J Exp Biol 203:585–596
R Core Team (2022) The R Stats Package. R statistical functions. R 4.2.0. https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html
Robillard T, Tan MK (2013) A taxonomic review of common but little known crickets from Singapore and the Philippines (Insecta: Orthoptera: Eneopterinae). Raffles Bull Zool 61(2):705–725
Robillard T, Montealegre-Z F, Desutter-Grandcolas L, Grandcolas P, Robert D (2013) Mechanisms of high-frequency song generation in brachypterous crickets and the role of ghost frequencies. J Exp Biol 216(11):2001–2011. https://doi.org/10.1242/jeb.083964
Simmons LW (1988) The calling song of the field cricket, Gryllus bimaculatus (De Geer): constraints on transmission and its role in intermale competition and female choice. Anim Behav 36(2):380–394. https://doi.org/10.1016/S0003-3472(88)80009-5
Simmons LW (1995) Correlates of male quality in the field cricket, Gryllus campestris L: age, size, and symmetry determine pairing success in field populations. Behav Ecol 6:376–381. https://doi.org/10.1093/beheco/6.4.376
Simmons LW, Ritchie MG (1996) Symmetry in the songs of crickets. Proc R Soc B 263:1305–1311
Simmons LW, Zuk M (1992) Variability in call structure and pairing success of male field crickets, Gryllus bimaculatus: the effects of age, size and parasite load. Anim Behav 44(6):1145–1152. https://doi.org/10.1016/S0003-3472(05)80326-4
Singh R, Jain M (2020) Variation in call types, calling activity patterns and relationship between call frequency and body size in a field cricket, Acanthogryllus asiaticus. Bioacoustics 30:284–302. https://doi.org/10.1080/09524622.2020.1720817
Singh R, Prathibha P, Jain M (2020) Effect of temperature on life-history traits and mating calls of a field cricket, Acanthogryllus asiaticus. J Therm Biol 93:102740. https://doi.org/10.1016/j.jtherbio.2020.102740
Slabbekoorn H (2013) Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim Behav 85(5):1089–1099. https://doi.org/10.1016/j.anbehav.2013.01.021
Snell-Rood EC (2013) An overview of the evolutionary causes and consequences of behavioural plasticity. Anim Behav 85(5):1004–1011. https://doi.org/10.1016/j.anbehav.2012.12.031
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Sueur J, Windmill JF, Robert D (2010) Sound emission and reception tuning in three cicada species sharing the same habitat. J Acoust Soc Am 127(3):1681–1688. https://doi.org/10.1121/1.3291036
Symes LB, Martinson SJ, Kernan CE, ter Hofstede HM (2020) Sheep in wolves’ clothing: prey rely on proactive defences when predator and non-predator cues are similar. Proc R Soc B 287(1933):20201212. https://doi.org/10.1098/rspb.2020.1212
Tan MK (2020) Soundscape of urban-tolerant crickets (Orthoptera: Gryllidae, Trigonidiidae) in a tropical city Singapore. Bioacoustics 30(4):469–486. https://doi.org/10.1080/09524622.2020.1813627
Tan MK, Robillard T (2021) Highly diversified circadian rhythms in the calling activity of eneopterine crickets (Orthoptera: Grylloidea: Gryllidae) from Southeast Asia. Bioacoustics. https://doi.org/10.1080/09524622.2021.1973562
Tan MK, Malem J, Legendre F, Dong J, Baroga-Barbecho JB, Yap SA, Wahab RA, Japir R, Chung AYC, Robillard T (2021) Phylogeny, systematics and evolution of calling songs of the Lebinthini crickets (Orthoptera, Grylloidea, Eneopterinae), with description of two new genera. Syst Entomol 46(4):1060–1087. https://doi.org/10.1111/syen.12510
Tan MK, Schöneich S, Robillard T (2021) Inter-individual differences of calling and exploratory behaviour in a lebinthine cricket species hint at different mate-finding strategies. Behaviour. https://doi.org/10.1163/1568539X-bja10141
Tan MK, Robillard T (2021a) Population divergence in the acoustic properties of crickets during the COVID-19 pandemic. Ecology (The Scientific Naturalist):e03323. https://doi.org/10.1002/ecy.3323
ter Hofstede HM, Schöneich S, Robillard T, Hedwig B (2015) Evolution of a communication system by sensory exploitation of startle behaviour. Curr Biol 25(24):3245–3252. https://doi.org/10.1016/j.cub.2015.10.064
Tuckerman JF, Gwynne DT, Morris GK (1993) Reliable acoustic cues for female mate preference in a katydid (Scudderia curvicauda, Orthoptera: Tettigoniidae). Behav Ecol 4:106–113. https://doi.org/10.1093/beheco/4.2.106
Ulagaraj SM, Walker TJ (1973) Phonotaxis of crickets in flight: attraction of male and female crickets to male calling songs. Sci 182(4118):1278–1279. https://doi.org/10.1126/science.182.4118.1278
Verburgt L, Ferreira M, Ferguson JWH (2011) Male field cricket song reflects age, allowing females to prefer young males. Anim Behav 81(1):19e29. https://doi.org/10.1016/j.anbehav.2010.09.010
Walker TJ (1962) Factors responsible for intraspecific variation in the calling songs of crickets. Evolution 16:407–428. https://doi.org/10.1111/j.1558-5646.1962.tb03234.x
Witte K, Farris HE, Ryan MJ, Wilczynski W (2005) How cricket frog females deal with a noisy world: habitat-related differences in auditory tuning. Behav Ecol 16(3):571–579. https://doi.org/10.1093/beheco/ari032
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73(4):415–438
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7:636–645
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–4
Acknowledgements
The authors thank Alaina Eckert for working on the language and writing of the manuscript.
Funding
The work of ARB at MNHN was supported by the European Union Erasmus + Programme for traineeships. The work of MKT was supported by the Fyssen Foundation Postdoctoral Fellowship and the Wildlife Reserves Singapore Conservation Fund. Permission for the collection of crickets was granted by the National Parks Board of Singapore (NP/RP18-064).
Author information
Authors and Affiliations
Contributions
Alberto Rodríguez Ballesteros: methodology, investigation, data curation, writing; Ming Kai Tan: conceptualisation, methodology, formal analysis, writing, visualisation, supervision; Tony Robillard: conceptualisation, methodology, writing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Dany Azar
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ballesteros, A.R., Tan, M.K. & Robillard, T. Phenotypic plasticity of acoustic traits in high-frequency lebinthine crickets (Orthoptera: Eneopterinae: Lebinthina). Sci Nat 109, 29 (2022). https://doi.org/10.1007/s00114-022-01800-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-022-01800-1