Abstract
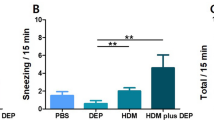
Air pollutant exposure leads to and exacerbates respiratory diseases. Particulate Matter (PM) is a major deleterious factor in the pathophysiology of asthma. Nonetheless, studies on the effects and mechanisms of exposure in the early life of mice remain unresolved. This study aimed to investigate changes in allergic phenotypes and effects on allergen-specific memory T cells resulting from co-exposure of mice in the early life to PM and house dust mites (HDM) and to explore the role of interleukin-23 (IL-23) in this process. PM and low-dose HDM were administered intranasally in 4-day-old C57BL/6 mice. After confirming an increase in IL-23 expression in mouse lung tissues, changes in the asthma phenotype and lung effector/memory Th2 or Th17 cells were evaluated after intranasal administration of anti-IL-23 antibody (Ab) during co-exposure to PM and HDM. Evaluation was performed up to 7 weeks after the last administration. Co-exposure to PM and low-dose HDM resulted in increases in airway hyperresponsiveness (AHR), eosinophils, neutrophils, and persistent Th2/Th17 effector/memory cells, which were all inhibited by anti-IL-23 Ab administration. When low-dose HDM was administered twice after a 7-week rest, mice exposed to PM and HDM during the previous early life period exhibited re-increases AHR, eosinophil count, HDM-specific IgG1, and effector/memory Th2 and Th17 cell populations. However, anti-IL-23 Ab administration during the early life period resulted in inhibition. Co-exposure to PM and low-dose HDM reinforced the allergic phenotypes and allergen-specific memory responses in early life of mice. During this process, IL-23 contributes to the enhancement of effector/memory Th2/Th17 cells and allergic phenotypes.
Graphical Abstract
Exposure of Particulate Matter (PM) and a low dose of HDM in mice of early life period induces the expression of IL-23 from CD11c + cells and airway epithelial cells, AHR, eosinophils, neutrophils, effector/memory Th2 and Th17 cells in lung tissue. These changes decreased when anti-IL-23 Ab was administered. When low-dose HDM was administered twice after 7 weeks of rest, mice exposed to PM and HDM during the previous early life period had re-increases in allergic responses, effector/memory Th2, and Th17 cells. The administration of anti-IL-23 Ab in the previous early life period showed their inhibition. HDM, house dust mite; AHR, airway hyperresponsiveness; anti-IL-23 Ab; anti-IL-23 antibody

Key messages
-
PM-induced IL-23 expression, allergic responses in HDMinstilled mice of early life period.
-
PM-induced effector/memory Th2/Th17 cells in HDMinstilled mice of early life period.
-
Inhibition of IL-23 reduced the increase in allergic responses.
-
Inhibition of IL-23 reduced the increase in allergic responses.
-
After the resting period, HDM administration showed re-increase in allergic responses.
-
Inhibition of IL-23 reduced the HDM-recall allergic responses.








Similar content being viewed by others
Data availability
All data have been reported.
Abbreviations
- PM:
-
Particulate Matter
- HDM:
-
House dust mite
- Dp:
-
Dermatophagoides pteronyssinus
- IL-23:
-
Interleukin-23
- Th2:
-
T helper 2
- Th17:
-
T helper 17
- Epcam:
-
Epithelial cell adhesion molecule
- DC:
-
Dendritic cell
- BMDC:
-
Bone marrow dendritic cell
- Anti IL-23:
-
Anti IL-23 antibody
References
Takizawa H (2015) Impacts of particulate air pollution on asthma: current understanding and future perspectives. Recent Pat Inflamm Allergy Drug Discov 9:128–135. https://doi.org/10.2174/1872213x09666150623110714
Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, Heijink IH (2022) Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev 31. https://doi.org/10.1183/16000617.0112-2021
Jacquemin B, Kauffmann F, Pin I, Le Moual N, Bousquet J, Gormand F, Just J, Nadif R, Pison C, Vervloet D et al (2012) Air pollution and asthma control in the epidemiological study on the genetics and environment of asthma. J Epidemiol Community Health 66:796–802. https://doi.org/10.1136/jech.2010.130229
Brandt EB, Bolcas PE, Ruff BP, Khurana Hershey GK (2020) IL33 contributes to diesel pollution-mediated increase in experimental asthma severity. Allergy 75:2254–2266. https://doi.org/10.1111/all.14181
Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B (2007) Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 29:879–888. https://doi.org/10.1183/09031936.00083406
McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D et al (2006) Traffic, susceptibility, and childhood asthma. Environ Health Perspect 114:766–772. https://doi.org/10.1289/ehp.8594
Jung CR, Chen WT, Tang YH, Hwang BF (2019) Fine particulate matter exposure during pregnancy and infancy and incident asthma. J Allergy Clin Immunol 143(2254–2262):e2255. https://doi.org/10.1016/j.jaci.2019.03.024
Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, LeMasters GK, Bernstein DI, Lockey JE, LeCras TD et al (2015) Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol 136(295–303):e297. https://doi.org/10.1016/j.jaci.2014.11.043
Sewell GW, Kaser A (2022) Interleukin-23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. J Crohns Colitis 16: ii3-ii19. https://doi.org/10.1093/ecco-jcc/jjac034
Chan TC, Hawkes JE, Krueger JG (2018) Interleukin 23 in the skin: role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther Adv Chronic Dis 9:111–119. https://doi.org/10.1177/2040622318759282
Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, Guggino G (2021) Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol 12:637829. https://doi.org/10.3389/fimmu.2021.637829
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y et al (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 178:1023–1032. https://doi.org/10.1164/rccm.200801-086OC
Alyasin S, Amin R, Fazel A, Karimi MH, Nabavizadeh SH, Esmaeilzadeh H, Babaei M (2017) IL-23 Gene and protein expression in childhood asthma. Iran J Immunol 14:73–80
Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H (2013) CD4(+)CD62L(+) central memory T cells can be converted to Foxp3(+) T cells. PLoS ONE 8:e77322. https://doi.org/10.1371/journal.pone.0077322
Sallusto F, Lanzavecchia A (2001) Exploring pathways for memory T cell generation. J Clin Invest 108:805–806. https://doi.org/10.1172/JCI14005
Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, Porth K, Boniface K, Mattson J, Basham B et al (2013) Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep 3:1378–1388. https://doi.org/10.1016/j.celrep.2013.03.035
Lee HS, Park HW (2022) IL-23 plays a significant role in the augmentation of particulate matter-mediated allergic airway inflammation. J Cell Mol Med 26:4506–4519. https://doi.org/10.1111/jcmm.17475
Nakajima Y, Chamoto K, Oura T, Honjo T (2021) Critical role of the CD44(low)CD62L(low) CD8(+) T cell subset in restoring antitumor immunity in aged mice. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2103730118
Maroof A (2001) Generation of murine bone-marrow-derived dendritic cells. Methods Mol Med 64:191–198. https://doi.org/10.1385/1-59259-150-7:191
Basaraba RJ (2008) Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinb) 88(Suppl 1):S35-47. https://doi.org/10.1016/S1472-9792(08)70035-0
Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M et al (2016) Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 126:3279–3295. https://doi.org/10.1172/JCI85664
Bosnjak B, Kazemi S, Altenburger LM, Mokrovic G, Epstein MM (2019) Th2-T(RMs) maintain life-long allergic memory in experimental asthma in mice. Front Immunol 10:840. https://doi.org/10.3389/fimmu.2019.00840
Mueller SN, Gebhardt T, Carbone FR, Heath WR (2013) Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31:137–161. https://doi.org/10.1146/annurev-immunol-032712-095954
Achakulwisut P, Brauer M, Hystad P, Anenberg SC (2019) Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO(2) pollution: estimates from global datasets. Lancet Planet Health 3:e166–e178. https://doi.org/10.1016/S2542-5196(19)30046-4
Fuertes E, Heinrich J (2015) The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization. Allergy 70:1350–1351. https://doi.org/10.1111/all.12611
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R et al (2017) Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389:1907–1918. https://doi.org/10.1016/S0140-6736(17)30505-6
Ghio AJ (2008) Mechanism of asthmatic exacerbation by ambient air pollution particles. Expert Rev Respir Med 2:109–118. https://doi.org/10.1586/17476348.2.1.109
Steiner S, Bisig C, Petri-Fink A, Rothen-Rutishauser B (2016) Diesel exhaust: current knowledge of adverse effects and underlying cellular mechanisms. Arch Toxicol 90:1541–1553. https://doi.org/10.1007/s00204-016-1736-5
De Grove KC, Provoost S, Brusselle GG, Joos GF, Maes T (2018) Insights in particulate matter-induced allergic airway inflammation: focus on the epithelium. Clin Exp Allergy 48:773–786. https://doi.org/10.1111/cea.13178
Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD (2013) Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy 43:1406–1418. https://doi.org/10.1111/cea.12200
Sallusto F, Lanzavecchia A, Araki K, Ahmed R (2010) From vaccines to memory and back. Immunity 33:451–463. https://doi.org/10.1016/j.immuni.2010.10.008
Lloyd CM, Hessel EM (2010) Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 10:838–848. https://doi.org/10.1038/nri2870
Wang Q, Du J, Zhu J, Yang X, Zhou B (2015) Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol 135(781–791):e783. https://doi.org/10.1016/j.jaci.2014.09.015
Kitajima M, Kubo M, Ziegler SF, Suzuki H (2020) Critical role of TSLP receptor on CD4 T cells for exacerbation of skin inflammation. J Immunol 205:27–35. https://doi.org/10.4049/jimmunol.1900758
Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, Saloga J (2010) Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology 129:437–445. https://doi.org/10.1111/j.1365-2567.2009.03199.x
Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F et al (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol 168:5699–5708. https://doi.org/10.4049/jimmunol.168.11.5699
Floss DM, Mrotzek S, Klocker T, Schroder J, Grotzinger J, Rose-John S, Scheller J (2013) Identification of canonical tyrosine-dependent and non-canonical tyrosine-independent STAT3 activation sites in the intracellular domain of the interleukin 23 receptor. J Biol Chem 288:19386–19400. https://doi.org/10.1074/jbc.M112.432153
Nakajima H, Hirose K (2010) Role of IL-23 and Th17 cells in airway inflammation in asthma. Immune Netw 10:1–4. https://doi.org/10.4110/in.2010.10.1.1
Peng J, Yang XO, Chang SH, Yang J, Dong C (2010) IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res 20:62–71. https://doi.org/10.1038/cr.2009.128
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K et al (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725. https://doi.org/10.1016/s1074-7613(00)00070-4
Glosson-Byers NL, Sehra S, Kaplan MH (2014) STAT4 is required for IL-23 responsiveness in Th17 memory cells and NKT cells. JAKSTAT 3:e955393. https://doi.org/10.4161/21623988.2014.955393
Ray A, Kolls JK (2017) Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol 38:942–954. https://doi.org/10.1016/j.it.2017.07.003
Lee HS, Park DE, Lee JW, Kim HN, Song WJ, Park HW, Cho SH (2019) Critical role of interleukin-23 in development of asthma promoted by cigarette smoke. J Mol Med (Berl) 97:937–949. https://doi.org/10.1007/s00109-019-01768-y
Wu AY, Peebles RS (2023) The emerging role of IL-23 in asthma and its clinical implications. Expert Rev Clin Immunol 19:1–5. https://doi.org/10.1080/1744666X.2023.2125380
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2019R1A2C4070214) and the SNUH Research Fund (grant no. 04–2022-0690).
Author information
Authors and Affiliations
Contributions
PHW: designed the experiments, analyzed the data, constructed the figures, drafted the manuscript. LHS: designed the experiments, performed experiments, performed the assays, analyzed the data, constructed the figures, drafted the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Resources at Seoul National University Hospital.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, HW., Lee, H.S. IL-23 contributes to Particulate Matter induced allergic asthma in the early life of mice and promotes asthma susceptibility. J Mol Med 102, 129–142 (2024). https://doi.org/10.1007/s00109-023-02393-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02393-6