Abstract
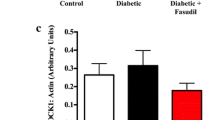
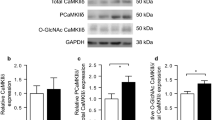
Previous study showed inhibition of RhoA and Rho kinase (ROCK) activity with fasudil could alleviate diabetes-induced cardiac dysfunction partially due to improvement of myocardial fibrosis. However, the effect of fasudil on intracellular calcium cycling and actin remodeling, both of which are important to regulate excitation-contract coupling, is still not fully elucidated. In this study, a diabetic cardiomyopathy model was induced by a single intraperitoneal injection of streptozotocin (STZ) in male Sprague Dawley rats. Diabetic rats were treated with fasudil or placebo for 8 weeks. We found that long-term administration of fasudil, a specific Rho kinase inhibitor, significantly ameliorated diabetes-induced contractile dysfunction both at cellular and whole organ levels. Fasudil-treated rats displayed improved diastolic intracellular calcium ([Ca2+]i) removal and rescued expression of protein responsible for [Ca2+]i clearance. Furthermore, our study indicated that fasudil treatment normalized the phosphorylation of the PKCβ2/Akt pathway in the diabetic heart, which might be the underlying mechanism accounting for the protective effect of fasudil on [Ca2+]i clearance. In addition, compared to the diabetes group, fasudil also normalized the G/F-actin ratio by preventing cofilin phosphorylation and promoted F-actin organization, suggesting a beneficial effect on actin remodeling. These findings indicate the protective effect of fasudil against diabetes-induced cardiac dysfunction via modulation of Ca2+ handling and actin remodeling. Overactivation of RhoA/ROCK plays a key role in the development of DCM. Inhibition of ROCK activity with fasudil improved [Ca2+]i removal in diabetic cardiomyocytes. Fasudil normalized the G/F-actin ratio and promoted F-actin organization. ROCK may be an excellent therapeutic target for the treatment of DCM.
Key message
-
Overactivation of RhoA/ROCK plays a key role in the development of DCM.
-
Inhibition of ROCK activity with fasudil improved [Ca2+]i removal in diabetic cardiomyocytes.
-
Fasudil normalized the G/F-actin ratio and promoted F-actin organization.
-
ROCK may be an excellent therapeutic target for the treatment of DCM.





Similar content being viewed by others
References
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–149
Bayeva M, Sawicki KT, Ardehali H (2013) Taking diabetes to heart—deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J Am Heart Assoc 2:e000433
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635
Hamid SA, Bower HS, Baxter GF (2007) Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol 292:H2598–H2606
Li Y, Zhu W, Tao J, Xin P, Liu M, Li J, Wei M (2012) Fasudil protects the heart against ischemia-reperfusion injury by attenuating endoplasmic reticulum stress and modulating SERCA activity: the differential role for PI3K/Akt and JAK2/STAT3 signaling pathways. PLoS One 7:e48115
Molina-Sanchez P, Chevre R, Rius C, Fuster JJ, Andres V (2015) Loss of p 27 phosphorylation at Ser10 accelerates early atherogenesis by promoting leukocyte recruitment via RhoA/ROCK. J Mol Cell Cardiol 84:84–94
Phrommintikul A, Tran L, Kompa A, Wang B, Adrahtas A, Cantwell D, Kelly DJ, Krum H (2008) Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol 294:H1804–H1814
Guan SJ, Ma ZH, YL W, Zhang JP, Liang F, Weiss JW, Guo QY, Wang JY, Ji ES, Chu L (2012) Long-term administration of fasudil improves cardiomyopathy in streptozotocin-induced diabetic rats. Food Chem Toxicol 50:1874–1882
Guo R, Su Y, Yan J, Sun H, Wu J, Liu W, Xu Y (2015) Fasudil improves short-term echocardiographic parameters of diastolic function in patients with type 2 diabetes with preserved left ventricular ejection fraction: a pilot study. Heart Vessel 30:89–97
Lin G, Craig GP, Zhang L, Yuen VG, Allard M, McNeill JH, MacLeod KM (2007) Acute inhibition of Rho-kinase improves cardiac contractile function in streptozotocin-diabetic rats. Cardiovasc Res 75:51–58
Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA (2002) Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283:H1398–H1408
Zhao SM, Wang YL, Guo CY, Chen JL, YQ W (2014) Progressive decay of Ca2+ homeostasis in the development of diabetic cardiomyopathy. Cardiovasc Diabetol 13:75
Zhang L, Cannell MB, Phillips AR, Cooper GJ, Ward ML (2008) Altered calcium homeostasis does not explain the contractile deficit of diabetic cardiomyopathy. Diabetes 57:2158–2166
Zhang L, Ward ML, Phillips AR, Zhang S, Kennedy J, Barry B, Cannell MB, Cooper GJ (2013) Protection of the heart by treatment with a divalent-copper-selective chelator reveals a novel mechanism underlying cardiomyopathy in diabetic rats. Cardiovasc Diabetol 12:123
Yang Y, Lu X, Rong X, Jiang W, Lai D, Ma Y, Zhou K, Fu G, Xu S (2015) Inhibition of the mevalonate pathway ameliorates anoxia-induced down-regulation of FKBP12.6 and intracellular calcium handling dysfunction in H9c2 cells. J Mol Cell Cardiol 80:166–174
Ding D, Du Y, Qiu Z, Yan S, Chen F, Wang M, Yang S, Zhou Y, Hu X, Deng Y, et al. (2016) Vaccination against type 1 angiotensin receptor prevents streptozotocin-induced diabetic nephropathy. J Mol Med 94:207–218
Lai D, Xu L, Cheng J, Guilbert AB, Lim HJ, Fu G, Wang Y (2013) Stretch current-induced abnormal impulses in CaMKIIdelta knockout mouse ventricular myocytes. J Cardiovasc Electrophysiol 24:457–463
Cheng J, Xu L, Lai D, Guilbert A, Lim HJ, Keskanokwong T, Wang Y (2012) CaMKII inhibition in heart failure, beneficial, harmful, or both. Am J Physiol Heart Circ Physiol 302:H1454–H1465
Li L, Chu G, Kranias EG, Bers DM (1998) Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Phys 274:H1335–H1347
Lei S, Li H, Xu J, Liu Y, Gao X, Wang J, Ng KF, Lau WB, Ma XL, Rodrigues B, et al. (2013) Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 62:2318–2328
Lin G, Brownsey RW, Macleod KM (2014) Complex regulation of PKCbeta2 and PDK-1/AKT by ROCK2 in diabetic heart. PLoS One 9: e86520
Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN (2010) Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 107:294–304
Soliman H, Craig GP, Nagareddy P, Yuen VG, Lin G, Kumar U, McNeill JH, Macleod KM (2008) Role of inducible nitric oxide synthase in induction of RhoA expression in hearts from diabetic rats. Cardiovasc Res 79:322–330
Waddingham MT, Edgley AJ, Astolfo A, Inagaki T, Fujii Y, CK D, Zhan DY, Tsuchimochi H, Yagi N, Kelly DJ, et al. (2015) Chronic Rho-kinase inhibition improves left ventricular contractile dysfunction in early type-1 diabetes by increasing myosin cross-bridge extension. Cardiovasc Diabetol 14:92
Soliman H, Gador A, YH L, Lin G, Bankar G, MacLeod KM (2012) Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKC 2. Am J Physiol Heart Circ Physiol 303:H989–H1000
Guo R, Liu B, Zhou S, Zhang B, Xu Y (2013) The protective effect of fasudil on the structure and function of cardiac mitochondria from rats with type 2 diabetes induced by streptozotocin with a high-fat diet is mediated by the attenuation of oxidative stress. Biomed Res Int 2013:430791
Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, et al. (2009) Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 58:215–226
Pearson JT, Jenkins MJ, Edgley AJ, Sonobe T, Joshi M, Waddingham MT, Fujii Y, Schwenke DO, Tsuchimochi H, Yoshimoto M, et al. (2013) Acute Rho-kinase inhibition improves coronary dysfunction in vivo, in the early diabetic microcirculation. Cardiovasc Diabetol 12:111
Tada M, Ohmori F, Yamada M, Abe H (1979) Mechanism of the stimulation of Ca2+-dependent ATPase of cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. Role of the 22,000-dalton protein. J Biol Chem 254:319–326
Tada M, Yamada M, Ohmori F, Kuzuya T, Inui M, Abe H (1980) Transient state kinetic studies of Ca2-dependent ATPase and calcium transport by cardiac sarcoplasmic reticulum. Effect of cyclic AMP-dependent protein kinase-catalyzed phosphorylation of phospholamban. J Biol Chem 255:1985–1992
MacLennan DH, Kranias EG (2003) Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4:566–577
Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. (2013) Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502:372–376
Pereira L, Ruiz-Hurtado G, Rueda A, Mercadier JJ, Benitah JP, Gomez AM (2014) Calcium signaling in diabetic cardiomyocytes. Cell Calcium 56:372–380
Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, Santonastasi M, Bellacosa A, Brown JH, Condorelli G (2009) Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem 284:28180–28187
Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, Feramisco JR, Hammond HK (2008) Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem 283:33527–33535
Yuan B, Wan P, Chu D, Nie J, Cao Y, Luo W, Lu S, Chen J, Yang Z (2014) A cardiomyocyte-specific Wdr1 knockout demonstrates essential functional roles for actin disassembly during myocardial growth and maintenance in mice. Am J Pathol 184:1967–1980
Davani EY, Dorscheid DR, Lee CH, van Breemen C, Walley KR (2004) Novel regulatory mechanism of cardiomyocyte contractility involving ICAM-1 and the cytoskeleton. Am J Physiol Heart Circ Physiol 287:H1013–H1022
Tate M, Robinson E, Green BD, McDermott BJ, Grieve DJ (2016) Exendin-4 attenuates adverse cardiac remodelling in streptozocin-induced diabetes via specific actions on infiltrating macrophages. Basic Res Cardiol 111:1
Li CJ, Lv L, Li H, DM Y (2012) Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 11:73
Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA (2012) Alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res 96:265–275
Lagna G, MM K, Nguyen PH, Neuman NA, Davis BN, Hata A (2007) Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem 282:37244–37255
Witt W, Buttner P, Jannasch A, Matschke K, Waldow T (2014) Reversal of myofibroblastic activation by polyunsaturated fatty acids in valvular interstitial cells from aortic valves. Role of RhoA/G-actin/MRTF signalling. J Mol Cell Cardiol 74:127–138
Acknowledgments
We sincerely thank Lu Zhang at Zhejiang University for her excellent technical assistance for confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant support
This work was supported by grants from the National Natural Science Foundation of China (No. 81100159 and 81100167), Medical and Health Science Program of Zhejiang Province (No. 201477370 and 201646246), Natural Science Foundation of Zhejiang Province (No. LY15H020003), and Health Science and Technology Plan of Hangzhou City (No. 2013A28).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dongwu Lai and Jing Gao contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1007/s00109-016-1476-2.
Electronic supplementary material
ESM 1
(PDF 496 kb)
Rights and permissions
About this article
Cite this article
Lai, D., Gao, J., Bi, X. et al. The Rho kinase inhibitor, fasudil, ameliorates diabetes-induced cardiac dysfunction by improving calcium clearance and actin remodeling. J Mol Med 95, 155–165 (2017). https://doi.org/10.1007/s00109-016-1469-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1469-1