Abstract
The interpretation of the outputs of acoustic tomography is often altered by different physical and mechanical parameters. Detailed information on the relationships between static mechanical properties and dynamic parameters of intact and degraded green wood can improve the results of this device-supported method used for tree stability assessment. This research presents a graphic and statistic comparison of acoustic tomography outputs with the laboratory assessed material parameters. The analysis was based on the relationship between the dynamic and static mechanical parameters of four cross-sections from two living tree stems. The occurrence of seven white and soft rot fungi was taken into consideration. The influence of density (\(\uprho\)) on stress-wave propagation (\(v\)) was proved. A strong correlation between the dynamic moduli of elasticity (\({E}_{dyn}\)) and compressive strength (\(\upsigma\)) is reported. A higher heterogeneity of wood degradation among the cross-section can lead to an underestimation of the defect during AT assessment. The dynamic modulus of elasticity \({E}_{dyn}\) was less influenced than \(v\) by the heterogeneity of degraded wood. Therefore, \({E}_{dyn}\) can be used to form a better interpretation of acoustic tomography assessment of standing beech trees. Due to the complexity of the topic, further investigation of previously mentioned relationships is still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The need to preserve mature trees colonised by wood decay fungi is increasing with the rising awareness of the value of trees in the urban environment (Sterken 2005). In natural woodlands, wood decay fungi rarely affect the mechanical behaviour of standing trees (Luchi et al. 2016; Boddy 2021). Nevertheless, trees outside the forest ecosystem are more often exposed to various negative abiotic and biotic factors (Ordóñez-Barona et al. 2018), and the balanced relationship between wood decay fungi and host trees is altered by stressful anthropogenic conditions (Deflorio 2006). Therefore, fungi colonisation can irreversibly compromise the host tree’s stability through the degradation of external intact sound-wood layers, causing the collapse of the tree’s structure (Schwarze et al. 2004). In many cases, wood decay is not detectable by visual assessment, and the use of advanced risk assessment methods is necessary (Koeser et al. 2017). These methods include acoustic tomography (AT), which detects defects in a cross-section of the assessed stem, using the velocity of stress-wave propagation, and creates a spatial (2D/3D) estimation of the defect (Turpening et al. 1999; Liang et al. 2008; Wang 2013). Stress-wave propagation velocity is higher in sound wood than in degraded wood (Divos and Szalai 2002). In addition to the matrix of measured velocities among all sensors, the main result comprises an image reconstruction obtained from the interpolation of the measured velocities (Feng et al. 2014; Du et al. 2018), which shows the theoretical integrity of the wood on a cross-section at the chosen height on the stem (Maurer et al. 2006). Because of its low cost, portability and low invasiveness, this technique attracts the attention of many professionals in the arboriculture sector (Du et al., 2018). By comparing visual assessments of decay in cross-sections with tomography results, Gilbert and Smiley (2004) proved that the average accuracy of degraded wood detection was approximately 89%. Ostrovský et al. (2017) assessed the accuracy and reliability of the acoustic tomography technique for detecting internal structural defects and discovered that irregularity of the cross-section shape does not affect the final accuracy of the tomographic assessment. With a reliable representation of cavities and degraded wood, and a high correlation between the dynamic parameters and the static mechanical properties (Chauhan and Sethy 2016), AT can predict the loss of the load-bearing capacity of trees with internal defects (Burcham et al. 2019). However, due to a wide range of factors that can influence stress-wave propagation velocities, such as the natural heterogeneity in standing trees (Palma et al. 2018), moisture content (Divos and Divos 2005; Montero et al. 2015; Kumar et al. 2016) and wood density (de Oliveira and Sales 2006; Baar et al. 2016), results of AT can be altered (Socco et al. 2004). AT outputs can also vary according to the used device (Cristini et al. 2021). Regarding the presence of wood-decaying fungi on standing trees (Schmidt 2006; Guglielmo et al. 2012; Zhou 2014), one crucial factor to consider is wood degradation caused by fungal enzymatic activity (Schwarze et al. 1995). Fungal degradation of wood is generally described by a loss of mass (Witomski et al. 2016), where most mechanical properties are influenced by wood density, including green wood (Niklas and Spatz 2010). Nevertheless, there can be a decrease in strength even without an observable loss of mass (Brischke et al. 2008). For example, with a small weight loss (up to 5%), a sharp decrease in strength (35–50%) can occur (Wilcox 1978). Curling et al. (2002) showed that the ratio of strength to weight loss was 4:1 on average. Humar et al. (2008) reported changes in the modulus of elasticity of Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). These changes are affected by wood decay fungi causing brown rot and blue stain, where the colonised wood showed a slight increase in the modulus of elasticity. Yang et al. (2017) tested the static bending and stress wave propagation properties of Elliot pine (Pinus elliotii) wood samples artificially inoculated with white and brown rot fungi, which showed a significant correlation between the static and dynamic bending moduli of elasticity (MOE and MOED). Bader et al. (2012) investigated changes in the longitudinal elastic moduli and stiffness data for all anatomical directions of Scots pine (P. sylvestris) sapwood that was degraded by Gloeophyllum trabeum and Trametes versicolor for up to 28 weeks. Schwarze et al. (1995) investigated the acoustic and mechanical properties of artificially inoculated samples with various wood-decaying fungi, which showed that the classic relationship between density, modulus of elasticity and sound propagation in sound wood does not apply to degraded wood. Deflorio et al. (2008) investigated changes in the acoustic properties of wooden bodies after 2, 16 and 27 months of exposure to various wood decay fungi occurring on living trees. In some cases, involving more advanced stages of decomposition, an increase in the speed of sound propagation in wood was detected. Nevertheless, the mechanical properties of degraded wood in standing trees, in relation to results obtained from non-destructive testing, still need further investigation. This study presents a description of the relationship between the dynamic and static mechanical parameters of the green intact and degraded wood of standing beech trees in relation to AT results taking into consideration the spatial distribution of fungal colonisation.
2 Methods
2.1 In-situ measurement
Two standing beech trees (Fagus sylvatica L.), with diameters at breast height of 34 cm and 84 cm, located on the ground of the Training Forest Enterprise, Masarykův les Křtiny, of Mendel University in Brno in the forest district of Bilovice, 236 msl (CZ), were chosen as experimental specimens. Each tree was colonised on the stem by wood-decaying fungi with fruit bodies present (Flammulina velutipes, Inonotus cuticularis, Auricularia mesenterica). Both trees were assessed with the acoustic tomograph, ArborSonic® (Fakopp Enterprise Bt.), at two different heights, A and B, and in the proximity (0–20 cm) of fungal fruit bodies (Fig. 1).
The geometry of the cross-sections was determined by the triangulation method using the ArborSonic® device. After the measurement, the trees were felled (each tree on a different day). Two cross-sections (15 cm and 2 cm thick) were removed from each position (A and B) and transported immediately for laboratory processing. To preserve the moisture content (\(w\)) during the transport, all cross-sections were hermetically stored in plastic bags. The aim was to process the measured sections during the same day to conserve the original \(w\) corresponding to green wood.
2.2 Laboratory measurement and parameters computation
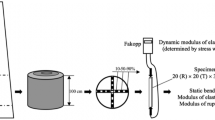
Thicker cross-sections (150 mm) were divided by a regular 50 × 50 mm grid into cells (Fig. 2a/b) less than an hour after felling. The velocities of stress-wave propagation were calculated using the times of signal transmission, measured with the Time of Flight (ToF) device (MicroSecond Timer®—Fakopp Enterprise Bt.), and the distances between the two US10 sensors for each cell in longitudinal (\({v}_{l}\)), radial (\({v}_{r}\)) and tangential (\({v}_{t}\)) directions. Afterwards, each sample was reduced to a standard size (20 × 20 × 30 mm). All samples used for compressive testing were orthotropic. Static testing was carried out on a universal testing machine (ZWICK® Z050) (Fig. 2c), where compressive stress (\({\upsigma }_{c}\)) parallel to grain was obtained. For each tree, the whole process took approximately 4 h.
Using the grid that was used for dividing cross-sections, average interpolated velocities using tomographic measurement were obtained for each cell from tomograms in the Arborsonic 3D software (Fakopp Enterprise Bt.). Compressive strain parallel to grain \(({\upepsilon }_{c}\)) was evaluated by a full-field optical displacement measurement based on the Digital Image Correlation (DIC) technique. Two cameras were used to acquire the images (AVT Stingray Copper F504B, cell size: 3.45 µm, resolution: 5 MPx, image-capture frequency: 2 fps). Images were processed in Mercury sw (Sobriety Ltd), where the DIC technique allows the measurement of strain for an average deformation of a selected area on the sample surface (Fig. 3). The selected area covered the entire sample surface excluding the top and bottom zones close to the compression plates (1/8 of the sample high), where, according to Brabec et al. (2015), deformations are not representative.
The moisture content (\(w\)), green density (\({\uprho }_{w}\)) and conventional density (\({\uprho }_{c}\)) were calculated from dimensions and dry/wet masses of specimens used for compressive testing. Dynamic moduli of elasticity (\({E}_{dyn,r}\) and \({E}_{dyn,t}\)) were calculated from the measured velocities (\({v}_{r}\) and \({v}_{t}\)) and \({\rho }_{w}\) according to the equation
According to the above equation, dynamic moduli of elasticity (\({E}_{dyn,tomo}\)) were calculated from velocities obtained from the tomograms and \({\uprho }_{w}\) of the cell at the same position. The static moduli of elasticity (\(E\)) were established using a least-squares method fitting data from the zone of linear elastic behaviour from the stress–strain diagram of each sample. The data have been processed using MATLAB® (The MathWorks, Inc.). Longitudinal compressive strength (\({\upsigma }_{c}\)) was calculated as the maximal measured stress during static mechanical testing. All the obtained physical and mechanical parameters of the tested samples were used to create image reconstructions of the assessed cross-sections in MATLAB®. These reconstructions were then compared with the results of the in-situ AT assessments (2.1). The relationship between the measured parameters was statistically investigated in MATLAB® (Spearman’s correlation coefficient (\(\mathrm{\alpha }=\) 0.05) and linear regression).
2.3 Fungal isolation and identification
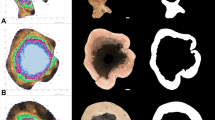
Thinner cross-sections (2 cm thick) were divided into different zones according to the present fungal interaction lines (Fig. 4a). From each zone, 10 small wooden samples (approximately 5 × 5 mm) were sterilised in ethanol and sodium hypochlorite. After superficial sterilisation, samples were placed in Petri dishes (five samples each) containing sterilised malt extract agar (Fig. 4b). Petri dishes were incubated at 17ºC and checked daily for the growth of fungal cultures. Each new culture was subcultured to a new Petri dish with malt extract agar (Fig. 4c). Obtained isolates were morphologically studied (colony morphology and microscopic investigation) and identified using fungal DNA barcoding. The 2-mm piece of mycelium of the freshly grown culture was put into 20 µl of dilution buffer (component of Phire Plant Direct PCR Master Mix, Thermo Fisher Scientific). The solution was used as a template for a PCR reaction using primer pair ITS1/ITS4 or LR0R/LR6 targeting the ITS or LSU region of the ribosomal RNA gene during standard procedure (Tomšovský 2012). The PCR results were sequenced using the Sanger sequencing method by Eurofins Genomics (Ebersberg, Germany) and identified by a comparison of the similarity between DNA sequences (BLAST, blast.ncbi.nlm.nih.gov/Blast.cgi; UNITE, unite.ut.ee).
3 Results and discussion
3.1 Fungal isolation and identification
The relevant wood-decaying fungi that were isolated from sampled trees were Inonotus cuticularis and Flammulina velutipes from tree number 1 and Kretzschmaria deusta, Fomitiporella cavicola, Auricularia mesenterica, Pholiota adiposa and Neonectria coccinea agg. from tree number 2. During isolation and identification, other species were found (Thrichoderma sp., Pseudorotium ovale, Pezicula sp., Valsa ambiens and Eutypella quaternata). Another culture isolated from the intact peripheric zones of both trees was Biscogniuaxia nummularia, a common endophyte of European beech, found living in standing trees’ tissues without development (Luchi et al. 2016). Tree number 1 exhibits a lower diversity of fungal species compared to tree number 2 (Fig. 5). Species detected from the cultures correspond to the fruit bodies found on the stem of tree number 1 before its felling (Flammulina velutipes at the stem base, and Inonotus cuticularis at 3 m high). Considering the isolated fungi as wound colonisers (Boddy 2021), mechanical damage of the stem at the same height of the fruit bodies could have served as an entrance point for spores and aided their proliferation.
Tree number 2 had a higher fungal diversity in both its horizontal and vertical distribution (Fig. 5). The tree presented old damage, probably caused by frost, on the southwest side of the stem stretching from the base to 10 m up, which was covered by Auricularia mesenterica fruit bodies (Fig. 1). No other wood-decaying fungi were identified during the visual evaluation before felling. The presence of Kretzschmaria deusta close to the stem base is not exceptional because Fagus sylvatica L. is one of the main host species (Schwarze et al. 2004), and decay can be localised at the base of the stem and/or root system (Guglielmo et al. 2012). The only culture isolated on both cross-sections is Fomitiporella cavicola, which usually inhabits the cavities of standing trees (Zhou 2014). The life cycle of this rare species is not sufficiently known.
3.2 Physical and mechanical properties of the examined cross-sections
According to Spearman’s correlation coefficients (\(Sc\)) from Table 1, the moduli \({E}_{l}\), \({E}_{dyn,r}\) and \({E}_{dyn,t}\) all show a significant relationship with \({\sigma }_{c}\) (\(Sc\) between 0.43 and 0.68) (Table 1). Even if relevant coefficients were calculated for all the moduli of elasticity, values calculated for \({E}_{dyn,r}\) and \({E}_{dyn,t}\) create a closer graphic representation to \({\sigma }_{c}\) (Fig. 5). Radial and tangential velocities (\({v}_{r,t}\)) were proved to have a relevant correlation with \({\sigma }_{c}\) in all cross-sections (\(Sc\) between 0.42 and 0.61). On the other hand, \({\rho }_{w}\) showed a relevant correlation with \({\sigma }_{c}\) for tree number 1 (\(Sc\) between 0.44 and 0.58), but for tree number 2 the relationship was weaker (\(Sc\) between 0.12 and 0.21). The same trend applies to the coefficients between \({v}_{r}\), \({v}_{t}\) and \({\uprho }_{c}\) (\(S{c}_{tree no.1}\) between 0.42 and 0.77) and tree number 2 (\(S{c}_{tree no.2}\) between 0.03 and 0.4). These results can be explained by the higher heterogeneity among the cross-sections of tree number 2, caused by a larger area and a more complex fungal colonization structure (Fig. 5). Nevertheless, the weaker correlation between \({\rho }_{w}\) and \({\sigma }_{c}\) does not influence the relationship between \({E}_{dyn,r,t}\) and \({\sigma }_{c}.\)
The low correlation coefficients for cross-section 1B between \({v}_{r}\), \({v}_{t}\) and \(w\) (\(Sc\) 0.15; 0.05) are caused by the higher \(w\) in the central degraded part (Fig. 6), from which the wood-decaying fungus Inonotus cuticularis was isolated (Fig. 5). Nevertheless, the higher \(\mathrm{w}\) of the degraded part did not alter the results of the acoustic measurements, which were able to identify degradation during the field and laboratory assessment (according to the relevant correlations between \({E}_{dyn,r}\), \({E}_{dyn,t}\) and \({\upsigma }_{c}\) (Table 1)). The weaker correlation between \({v}_{r}\), \({v}_{t}\) and \(w\) for tree number 2 can be caused by a higher heterogeneity of the measured samples. For two cross-sections (1B, 2A) there is a non-significant negative correlation (Table 1). Considering that the average velocity of stress-wave propagation in water at room temperature is approximately 1500 m/s (Kumar et al., 2016), and the average \({v}_{r}\) measured for all cross-sections are above this value (minimum, median and maximum are 674 m/s, 1689 m/s and 1926 m/s, respectively), high \(w\) can have a negative influence on stress-wave propagation in the radial direction. On the other hand, \({v}_{t}\) average values are lower than 1500 m/s (minimum, median and maximum are 576 m/s, 1220 m/s and 1685 m/s, respectively), resulting in a stronger positive relationship, where an increase in \(w\) corresponds to higher stress-wave propagation velocity in the tangential direction. The results (Table 1) propose that \({\uprho }_{c}\) has a stronger influence on stress-wave propagation in the radial and tangential directions than \(w\) does, which did not influence the localisation of degraded wood in cross-section 1B (Fig. 6). The differences between the measured velocities support the relationship proposed by Palma et al. (2018), who reported the tangential velocities of the measured cross-sections were approximately 70–80% of the radial ones. In the cross-sections from tree number 2, AT did not correctly represent the strength distribution across the section (Fig. 6). AT’s underestimation of the decayed area in the cross-section 2A (Fig. 5) can be caused by the presence of Kretzschmaria deusta, which has been proved to influence the mechanical parameters of wood without altering acoustic properties (Schwarze et al. 1995). According to the previous statement, AT measurement can be negatively influenced by the higher heterogeneity of wood degradation process (Figs. 5 and 6), causing a misinterpretation of the condition of the assessed cross-section. Such characteristic can lead to wrong deductions, influencing the result of the device-supported assessment of the tree stability. The crack shown in cross-section 2B was previously occupied by highly degraded wood due to mechanical deterioration caused by wood ants, which was lost during manipulation. Considering all the cross-sections in Fig. 5, \({E}_{dyn}\) represents the strength distribution more closely than \(v\) does, which reveals the high influence of \({\uprho }_{\mathrm{w}}\) on strength representation and supports the relationship described by Niklas and Spatz (2010), who presented a strong positive correlation between green wood density and compressive strength parallel to the grain. Lower \({\rho }_{c}\) in the central parts of cross-sections 1A/B shown in Fig. 6 could also be caused by the presence of juvenile wood. Nevertheless, F. sylvatica, as a deciduous tree with diffuse-porous wood structure, does not present relevant differences in \({\rho }_{c}\) between juvenile and mature wood (Bouriaud et al., 2004; Gryc et al., 2008). Therefore, lower density in the central parts of cross-sections 1A/B was caused by fungal degradation.
As shown in Fig. 7, \({\mathrm{E}}_{\mathrm{dyn},\mathrm{l}}\) from all the cross-sections proved to have a stronger relationship with \({\upsigma }_{c}\)(\(\mathrm{Sc} = 0.66\)) rather than \(E\) (\(\mathrm{Sc} = 0.21\)). According to the coefficients of determination (\({r}^{2}\)), \({\upsigma }_{c}\) can be predicted from \({E}_{dyn,l}\) (\({r}^{2}= 0.65\)). On the other hand, the linear regression between \({E}_{dyn,l}\) and \(E\) is not relevant (\({r}^{2}=0.05\)). This result contradicts results presented by Chauhan and Sethy (2016), who produced a stronger correlation between \({E}_{dyn}\) and \(E\) than between \({E}_{dyn}\) and \({\sigma }_{c}\). However, their study did not consider degraded wood. A low correlation between \(E\) and \({E}_{dyn,l}\) does not correspond to the results presented by Yang et al. (2017), who proved there was a strong relationship between MOE and MOED of intact and degraded bending samples after white and brown-rot decay (\(r\) between 0.66 and 0.80). The presented and compared results suggest that different testing approaches (compressive test parallel to the grain vs bending test) can lead to relationships between dynamic and static moduli of elasticity having a different significance.
Measured stress-wave velocities from the acoustic field and laboratory measurements (expressed as \({v}_{r}\) and \({v}_{t}\) average) have similar trends (Fig. 8). Because of the current methods used for image reconstructions (Feng et al. 2014; Du et al. 2018), velocities obtained from tomographic measurements come from the interpolation of previously measured velocities (point-to-point). This can explain the lower variability of tomograph values when compared to those acquired from laboratory assessments. Considering the coincidence of peaks shown in Fig. 8, values obtained from the interpolation of velocities using field measurements can be considered representative. According to this result, AT assessment can create a reliable stress-wave velocity map (image reconstruction) corresponding to detailed laboratory acoustic assessments.
According to Fig. 9, a strong correlation between \({E}_{dyn,r}\) and \({\upsigma }_{\mathrm{c}}\) was proved based on data obtained from all the assessed cross-sections (\(\mathrm{Sc}=0.77\)). This is relatively stronger than the correlation between \({v}_{r}\) and \({\upsigma }_{c}\). By calculating \({E}_{dyn}\) from AT velocities and applying the \({\uprho }_{\mathrm{w}}\) measured at each grid cell, a significant increase in Spearman’s correlation coefficient is shown (\(Sc=0.24\) for \({v}_{tomo}\) and \({\upsigma }_{c}\), \(Sc=0.48\)) for \({E}_{dyn,tomo}\) and \({\upsigma }_{c}\). According to \({r}^{2}\) values, the linear regression obtained from \({E}_{dyn,tomo}\) is more reliable than the one calculated from AT stress-wave velocities (Fig. 9). This high correlation suggests that the influence of the different degradation in each cross-section, caused by the presence of different wood-decaying fungi, is attenuated by \({\uprho }_{\mathrm{w}}\) being an input parameter for the calculation of \({E}_{dyn}\).
4 Conclusion
The results of this study proved relationships between the physical, mechanical and acoustic/dynamic parameters of intact and degraded green wood in beech trees.
The following conclusive statements can be drawn from the results:
-
According to statistical analysis, velocities obtained from AT measurements have similar trends to velocities measured during laboratory assessments and can be considered representative.
-
Heterogeneity of differently degraded wood (e.g., soft rot caused by Kretzschmaria deusta) can influence the velocities of stress-wave propagation, leading to an underestimation of the condition of the assessed cross-section during AT assessment.
-
A strong relationship exists between \({E}_{dyn}\) and \({\upsigma }_{c}\) (\(Sc\) between 0.43 and 0.68) and was proved for all cross-sections
-
The modulus \({E}_{dyn}\) is not influenced as much as \(v\) by the heterogeneity of wood degradation.
-
According to the comparison of image reconstructions, \({E}_{dyn}\) created a more reliable representation of the bearing capacity of the assessed cross-section than \(v\).
-
The conventional density \({\uprho }_{c}\) was proved to have a more significant influence on \({v}_{r}\) and \({v}_{t}\) than on \(\mathrm{w}.\) The correlations of both physical factors with \({v}_{r}\) and \({v}_{t}\) decreased with a higher heterogeneity of the assessed cross-sections of tree number 2.
-
In some cases (cross-sections 1B and 2A), high \(w\) values can lead to a reduction in stress-wave velocities in the radial direction (\({v}_{r}\)).
-
The modulus \({E}_{dyn,l}\) has a closer correlation to \({\upsigma }_{c}\) (\(\mathrm{r}=0.66\)) than to \(E\) (\(\mathrm{r}=0.21\)).
-
Even if the environmental conditions for both assessed trees were mostly identical, the structure of fungal colonies would differ between specimens and vertically among one tree.
Based on the distributions of the investigated material parameters in this study, the knowledge of the absolute values of \({\rho }_{w}\) used for the calculation of \({E}_{dyn}\) can be used to better estimate the presence of degraded wood in standing beech trees during acoustic tomography, which can lead to a more detailed estimation of tree stability during non-destructive device-supported assessment. Obtained results could be applied to tree species with the same wood structure (diffuse porosity). Nevertheless, due to the complexity of performed tests and limited number of tested trees the research is based on, further investigation between different wood and fungal species is still needed.
References
Baar J, Tippner J, Gryc V (2016) Wood anatomy and acoustic properties of selected tropical hardwoods. IAWA J 37:69–83. https://doi.org/10.1163/22941932-20160121
Bader TK, Hofstetter K, Alfredsen G, Bollmus S (2012) Changes in microstructure and stiffness of Scots pine (Pinus sylvestris L.) sapwood degraded by Gloeophyllum trabeum and Trametes versicolor Part II: anisotropic stiffness properties. Holzforschung 66:199–206. https://doi.org/10.1515/HF.2011.153
Boddy L (2021) Fungi and trees—their complex relationships. In: Arboricultural Association, Stonehouse GL10 3DL. ISBN: 978–0–900978–70–8
Bouriaud O, Bréda N, le Moguédec G, Nepveu G (2004) Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees Struct Funct 18(3):264–276. https://doi.org/10.1007/s00468-003-0303-x
Brabec M, Tippner J, Sebera V, Milch J, Rademacher P (2015) Standard and non-standard deformation behaviour of European beech and Norway spruce during compression. Holzforschung 69(9):1107–1116. https://doi.org/10.1515/hf-2014-0231
Brischke C, Welzbacher CR, Huckfeldt T (2008) Influence of fungal decay by different basidiomycetes on the structural integrity of Norway spruce wood. Holz Roh-Werkst 66:433–438. https://doi.org/10.1007/s00107-008-0257-1
Burcham DC, Brazee NJ, Marra RE, Kane B (2019) Can sonic tomography predict loss in load-bearing capacity for trees with internal defects? A comparison of sonic tomograms with destructive measurements. Trees Struct Funct. https://doi.org/10.1007/s00468-018-01808-z
Chauhan S, Sethy A (2016) Differences in dynamic modulus of elasticity determined by three vibration methods and their relationship with static modulus of elasticity. Maderas Cienc y Tecnol 18:373–382. https://doi.org/10.4067/S0718-221X2016005000034
Cristini V, Tippner J, Vojáčková B, Paulić V (2021) Comparison of variability in results of acoustic tomographs in pedunculate oak (Quercus robur L.). BioResources 16: 3046–3058. https://doi.org/10.15376/biores.16.2.3046-3058
Curling SF, Clausen CA, Winandy JE (2002) Relationships between mechanical properties, weight loss and chemical composition of wood during incipient brown-rot decay. For Prod J 52:34–39
Deflorio G (2006) Wood decay dynamics in the sapwood of trees—in vitro and in vivo studies on the role of the wood substrate in decay developement. Cuvillier, Gottingen, Germany (ISBN 3-86537-939-7)
Deflorio G, Johnson C, Fink S, Schwarze FWMR (2008) Decay development in living sapwood of coniferous and deciduous trees inoculated with six wood decay fungi. For Ecol Manage 255:2373–2383. https://doi.org/10.1016/j.foreco.2007.12.040
de Oliveira FGR, Sales A (2006) Relationship between density and ultrasonic velocity in Brazilian tropical woods. Bioresour Technol 97:2443–2446. https://doi.org/10.1016/j.biortech.2005.04.050
Divos F, Divos P (2005) Resolution of stress wave based acoustic tomography. In: 14th International Symposium on Nondestructive Testing of Wood. Shaker, Eberswalde
Divos E, Szalai L (2002) Tree evaluation by acoustic tomography. In: Proc. of the 13th International Symp. on Non Destructive Testing of Wood, Aug. 19–21, Berkeley, CA. pp. 251–256.
Du X, Li J, Feng H, Chen S (2018) Image reconstruction of internal defects in wood based on segmented propagation rays of stress waves. Appl Sci. https://doi.org/10.3390/app8101778
Feng H, Li G, Fu S, Wang X (2014) Tomographic image reconstruction using an interpolation method for tree decay detection. BioResources 9(2): 3248–3263. https://doi.org/10.15376/biores.9.2.3248-3263g
Gilbert EA, Smiley ET (2004) Picus Sonic tomography for the quantification of decay in white oak (Quercus alba) and Hickory (Carya spp.). J Arboric 30:277–280
Gryc V, Vavrčík H, Rybníček M, Přemyslovská E (2008) The relation between the microscopic structure and the wood density of European beech (Fagus sylvatica L.). J For Sci 54(4):170–175
Guglielmo F, Michelotti S, Nicolotti G, Gonthier P (2012) Population structure analysis provides insights into the infection biology and invasion strategies of Kretzschmaria deusta in trees. Fungal Ecol 5:714–725. https://doi.org/10.1016/j.funeco.2012.06.001
Humar M, Vek V, Bucar B (2008) Properties of blue-stained wood. Drv Ind 59:75–79
Koeser AK, Hauer RJ, Klein RW, Miesbauer JW (2017) Assessment of likelihood of failure using limited visual, basic, and advanced assessment techniques. Urban for Urban Green 24:71–79. https://doi.org/10.1016/j.ufug.2017.03.024
Kumar A, Pathak PP, Dass N (2016) A study of speed of sound in water. IOSR J Appl Phys 8:21–23. https://doi.org/10.9790/4861-0804022123
Liang S, Wang X, Wiedenbeck J, Cai Z, Fu F (2008) Evaluation of acoustic tomography for tree decay detection. In: 15th Int. Symp. Nondestruct. Test. Wood. 49–54.
Luchi N, Capretti P, Feducci M, Vannini A, Ceccarelli B, Vettraino AM (2016) Latent infection of biscogniauxia nummularia in fagus sylvatica: a possible bioindicator of beech health conditions. Iforest 9:49–54. https://doi.org/10.3832/ifor1436-008
Maurer H, Schubert SI, Bächle F, Clauss S, Gsell D, Dual J, Niemz P (2006) A simple anisotropy correction procedure for acoustic wood tomography. Holzforschung 60(5):567–573. https://doi.org/10.1515/HF.2006.094
Montero MJ, de La Mata J, Esteban M, Hermoso E (2015) Influence of moisture content on the wave velocity to estimate the mechanical properties of large cross-section pieces for structural use of scots pine from Spain. Maderas Cienc y Tecnol 17:407–420. https://doi.org/10.4067/S0718-221X2015005000038
Niklas KJ, Spatz HC (2010) Worldwide correlations of mechanical properties and green wood density. Am J Bot 97:1587–1594. https://doi.org/10.3732/ajb.1000150
Ordóñez-Barona C, Sabetski V, Millward AA, Urban J, Steenberg J, Grant A (2018) The influence of abiotic factors on street tree condition and mortality in a commercial-retail streetscape. Arboric Urban for 44:133–144
Ostrovský R, Kobza M, Gažo J (2017) Extensively damaged trees tested with acoustic tomography considering tree stability in urban greenery. Trees Struct Funct 31:1015–1023. https://doi.org/10.1007/s00468-017-1526-6
Palma SSA, Gonçalves R, Trinca AJ, Costa CP, Reis MN, Martins GA, (2018) Interference from knots, wave propagation direction, and effect of juvenile and reaction wood on velocities in ultrasound tomography. BioResources. 13: 2834–2845. https://doi.org/10.15376/biores.13.2.2834-2845
Schmidt O (2006) Wood and tree fungi—biology, damage, protection and use. Springer, Berlin, Heidelberg (ISBN 3-540-32138-1)
Schwarze FWMR, Lonsdale D, Mattheck C (1995) Detectability of wood decay caused by Ustulina deusta in comparison with other tree-decay fungi. Eur J for Pathol 25:327–341. https://doi.org/10.1111/j.1439-0329.1995.tb01348.x
Schwarze FWMR, Engels J, Mattheck C (2004) Fungal strategies of wood decay in trees. Springer, Berlin Heidelberg (ISBN 978-3-642-63133-7)
Socco LV, Sambuelli L, Martinis R, Cornino E, Nicolotti G (2004) Feasibility of ultrasonic tomography for nondestructive testing of decay on living trees. Res Nondestruct Eval 15:31–54. https://doi.org/10.1080/09349840490432678
Sterken P (2005) A guide for tree-stability analysis. https://www.researchgate.net/publication/242418928_A_Guide_For_Tree-Stability_Analysis
Tomšovský M (2012) Delimitation of an almost forgotten species Spongipellis litschaueri (Polyporales, Basidiomycota) and its taxonomic position within the genus. Mycol Prog 11:415–424. https://doi.org/10.1007/s11557-011-0756-z
Turpening RM, Zhu Z, Matarese JR, Lewis CE (1999) Acoustic tree and wooden member imaging apparatus (Patent No. PCT/US99/04092). Massachusetts Institute of Technology.
Wang X (2013) Acoustic measurements on trees and logs: a review and analysis. Wood Sci Technol 47:965–975. https://doi.org/10.1007/s00226-013-0552-9
Wilcox WW (1978) Review of literature on the effects of early stages of decay on wood strength. Wood Fiber Sci 9:252–257
Witomski P, Olek W, Bonarski JT (2016) Changes in strength of Scots pine wood (Pinus silvestris L.) decayed by brown rot (Coniophora puteana) and white rot (Trametes versicolor). Constr Build Mater 102:162–166. https://doi.org/10.1016/j.conbuildmat.2015.10.109
Yang Z, Jiang Z, Hse CY, Liu R (2017) Assessing the impact of wood decay fungi on the modulus of elasticity of slash pine (Pinus elliottii) by stress wave non-destructive testing. Int Biodeterior Biodegrad 117:123–127. https://doi.org/10.1016/j.ibiod.2016.12.003
Zhou LW (2014) Fomitiporella caviphila sp. nova (hymenochaetales, basidiomycota) from Eastern China, with a preliminary discussion on the taxonomy of fomitiporella. Ann Bot Fenn 51:279–284. https://doi.org/10.5735/085.051.0503
Acknowledgements
The study was supported by Mendel University in Brno (CZ), internal Ph.D. student grant project LDF VP_2020009. Optical measurements and data evaluation were supported by the Ministry of Education, Youth and Sports of the Czech Republic, project ERC CZ no. LL1909 ‘Tree Dynamics: Understanding of Mechanical Response to Loading’.
Funding
The study was supported by Mendel University in Brno (CZ), internal Ph.D. student grant project LDF VP_2020009. Optical measurements and data evaluation were supported by the Ministry of Education, Youth and Sports of the Czech Republic, project ERC CZ no. LL1909 ‘Tree Dynamics: Understanding of Mechanical Response to Loading’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cristini, V., Tippner, J., Tomšovský, M. et al. Acoustic tomography outputs in comparison to the properties of degraded wood in beech trees. Eur. J. Wood Prod. 80, 1377–1387 (2022). https://doi.org/10.1007/s00107-022-01872-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-022-01872-w