Abstract
Esophageal manometry has traditionally been utilized for respiratory physiology research, but clinicians have recently found numerous applications within the intensive care unit. Esophageal pressure (PEs) is a surrogate for pleural pressures (PPl), and the difference between airway pressure (PAO) and PEs provides a good estimate for the pressure across the lung also known as the transpulmonary pressure (PL). Differentiating the effects of mechanical ventilation and spontaneous breathing on the respiratory system, chest wall, and across the lung allows for improved personalization in clinical decision making. Measuring PL in acute respiratory distress syndrome (ARDS) may help set positive end expiratory pressure (PEEP) to prevent derecruitment and atelectrauma, while assuring peak pressures do not cause over distension during tidal breathing and recruitment maneuvers. Monitoring PEs allows improved insight into patient–ventilator interactions and may help in decisions to adjust sedation and paralytics to correct dyssynchrony. Intrinsic PEEP (auto-PEEP) may be monitored using esophageal manometry, which may also improve patient comfort and synchrony with the ventilator. Finally, during weaning, PEs may be used to better predict weaning success and allow for rapid intervention during failure. Improved consistency in definition and terminology and further outcomes research is needed to encourage more widespread adoption; however, with clear clinical benefit and increased ease of use, it appears time to reintroduce basic physiology into personalized ventilator management in the intensive care unit.
Zusammenfassung
Traditionell wurde die Ösophagusmanometrie in Studien zur Atmungsphysiologie eingesetzt, in den zurückliegenden Jahren hat sie zunehmend Eingang in die Intensivmedizin gefunden. Der Ösophagusdruck (PEs) ist ein Surrogatparameter für den Pleuradruck (PPl). Die Differenz zwischen Atemwegsdruck (PAO) und PEs ist ein guter Schätzwert für den Druck im Lungengewebe, auch transpulmonaler Druck (PL) genannt. Die Differenzierung der Effekte von Beatmung und Spontanatmung auf den Atmungsapparat, die Brustwand und die Lunge erlaubt eine individualisiertere klinische Entscheidungsfindung. Die Messung des PL beim „acute respiratory distress syndrome“ (ARDS) könnte die optimierte Einstellung des positiven endexspiratorischen Drucks (PEEP) zur Vermeidung einer Derekrutierung und damit der möglichen Entstehung eines Atelektraumas erleichtern, während sie gleichzeitig sicherstellt, dass die Spitzendrücke bei Ruheatmung und bei Rekrutierungsmanövern keine Überblähung verursachen. Die Überwachung des PEs ermöglicht bessere Einblicke in die Interaktionen zwischen Patient und Beatmungsgerät und könnte bei der Entscheidung über die Anpassung von Sedierung und Relaxierung zur Synchronisierung von Patient und Beatmungsgerät helfen. Der intrinsische PEEP (Auto-PEEP) kann mithilfe der Ösophagusmanometrie überwacht werden, auch damit könnten der Patientenkomfort und die Synchronizität mit dem Beatmungsgerät verbessert werden. Zuletzt kann der PEs während des Weanings herangezogen werden, um den Weaning-Erfolg besser vorhersagen zu können und im Versagensfall ein schnelles Einschreiten zu ermöglichen. Die Vereinheitlichung von Definitionen und Terminologie sowie weitere Outcome-Forschung sind erforderlich, um eine breitere Anwendung zu erreichen. Angesichts des klaren klinischen Nutzens und der vereinfachten Anwendung erscheint es an der Zeit, Grundlagen der Physiologie für ein personalisiertes Beatmungsmanagement auf der Intensivstation nutzbar zu machen.
Similar content being viewed by others
Introduction
The use of esophageal manometry in respiratory physiology has formed the foundation of current understanding of pulmonary pathophysiology [1,2,3]. Esophageal manometry simplifies the estimation of pleural pressures, allowing physiologists and clinicians to differentiate between the effects of the chest wall (including the rib cage, diaphragm, and abdomen) and the lung itself [1,2,3]. Despite the extensive use of esophageal manometry in research, clinical adoption has been less universal. In the era of personalized medicine however, clinicians have gained interest in esophageal manometry to better titrate care to the unique physiology of a patient. Additionally, clinical application has become straightforward with multiple balloon catheter options and elegantly designed ventilators. This article reviews the use of transpulmonary pressure monitoring in clinical applications within the intensive care unit.
Basic concepts and definitions
Pressure definitions
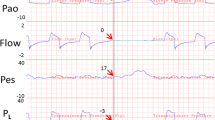
The pressure across the respiratory system (PRS) is defined as the difference between the pressure at the airway (PAO) and body surface (PBS) (PRS = PAO − PBS) [2]. The pressure difference across the lung is called the transpulmonary pressure (PL) and is the difference between PAO and pleural pressure (PPl) (PL = PAO − PPl) [2]. Finally the pressure across the chest wall (PCW) is defined as the PPl minus PBS (PCW = PPl − PBS) [2]. Esophageal balloon pressures (PEs) represent central thorax pressures and may underestimate PPL in dependent lung regions and overestimate PPl in less dependent regions. The correlation between PEs and PPl was initially described in upright, spontaneously breathing patients. When moved into the supine position, the balloon sits directly under the weight of the mediastinum, and abdominal contents push upwards against the diaphragm raising the measured value of PES. However, knowledge of the effects of position [1, 4], lung disease asymmetry, chest wall distortion [5], and other factors remains limited. Traditionally, roughly 3–7 cmH2O of additional pressure is thought to be due to positioning when correcting the PEs to estimate the PPL [4] with the supine position causing increased pressure from a decrease in lung volume and the shift in mediastinal weight. Indeed, a recent study comparing ex vivo measurements pre-lung transplant with in vivo measurements of the same lungs post-transplant found PPl was ~5 cmH2O different from PEs [6]. Despite these minor limitations, PEs in animal and human studies has been thought to represent a reasonable estimate for average PPl and may be used in substitution to easily calculate the PL for clinical and research use ([5]; Fig. 1).
Pressure and volume tracings from a patient with elevated pleural pressures. a Airway pressure (Pao) measuring total respiratory system pressure. End-inspiratory hold pressure (plateau) and end-expiratory pressure hold (PEEPtotal) are shown. Respiratory system driving pressure (∆PRS) was calculated as the plateau pressure minus the PEEPtotal. b Esophageal pressure (Pes) estimates the trans-chest wall pressure. Chest wall driving pressure (∆PCW) was calculated as the end-inspiratory hold Pes minus end-expiratory hold Pes. c Transpulmonary pressure (PL) was calculated as Pao minus Pes. Transpulmonary driving pressure (∆PL), also known as the cyclical stress, was calculated as the end-inspiratory hold PL minus the end-expiratory hold PL. d Lung volumes during tidal breathing and during expiratory and inspiratory holds. e Pressure–volume (P–V) curves during tidal breathing with P–V measurements following respiratory system pressures (Pao), transpulmonary pressures (PL), and chest wall pressures (Pes). Dotted lines represent the static compliance of the respiratory system and lung as measured by the slope between end-inspiratory holds and end-expiratory holds (stars on the graphs). Arrows indicate the direction of inspiration and expiration (from [24])
“Elastance-based” transpulmonary pressure calculation
With concerns that high PEs and consequently negative PL values were incompatible with an open lung, an alternative calculation of PL has been introduced [7,8,9,10,11,12]. This alternative is the “elastance-based” method of calculating PL (PL = PAO * lung elastance[EL]/respiratory system elastance[ERS]). This method uses ∆PEs during tidal breathing to estimate ∆PL and then calculate EL as ∆PL/tidal volume. The elastance-based technique assumes pleural pressure at end expiration to be zero when airway pressure is zero, and there is ongoing debate as to which technique is ideal [13]. The elastance-based definition measures the ∆PL during tidal breathing (the cyclical stress), but does not account for the baseline PL, which may vary widely. PPl and PALV may be substantially increased above atmospheric pressure at end expiration secondary to small airway collapse in obese patients or alveolar flooding in acute respiratory distress syndrome (ARDS )[13]. Indeed, the authors’ own unpublished data studying obese patients using slow-flow pressure–volume (PV) loops and trials of zero positive end expiratory pressure (PEEP) emphasizes this point (Fig. 2). As illustrated in the figure, there is no evidence of flow or volume gain from the start of the PV loop (when airway pressures are zero) until the airway pressures have increased sufficiently to overcome the elevated pleural pressure. In these same patients (in whom there is no evidence of obstruction) while on zero PEEP, we measured substantial intrinsic positive end-expiratory pressure (PEEPi) during expiratory breath holds that is roughly equal to both the measured PEs during holds and the airway pressure required to overcome pleural pressure on the PV loop. These findings appear to be the result of elevated PPl causing airway collapse (Fig. 2). In the sickest patients where accuracy in clinical decision making is paramount, using the absolute transpulmonary pressure or the elastance-based method in clinical practice may lead clinicians to set very different PEEP on the ventilator with unclear outcomes [13, 14].
a Pressure–volume loop of study patient. There was no flow or volume increase until roughly 7.5 cmH2O airway pressure. In order to generate flow, the airway opening pressure must be greater than the pressure within the lungs, which appears to be roughly 7–7.5 cmH2O. This pressure within the lungs appears to be secondary to elevated pleural pressures. b In the same patient, wave forms were recorded with zero PEEP, which shows that during end-expiratory occlusion, the PEEPi is roughly 7.5 cmH2O, secondary to elevated esophageal pressure of roughly 9.5 cmH2O causing airway collapse. The patient had normal lungs at baseline without COPD or asthma with normal resistance
Esophageal manometry in clinical practice
Using PES to prevent lung collapse in ARDS
Esophageal manometry is frequently utilized to titrate PEEP in patients with ARDS. Critically ill patients often have elevated pleural pressures secondary to pulmonary and chest wall edema, pleural effusions, and abdominal distension [15,16,17,18]. In a heterogeneous lung, these elevated pleural pressures may cause regions of lung collapse leading to decreased aeration, worsened oxygenation, and worsened compliance with decreased functional lung size secondary to closed airways and flooded lung units. This collapsing pressure can be detected and measured as an elevated PEs and negative PL. In 2008, Talmor and colleagues published a single-center randomized controlled trial testing a ventilator strategy of titrating PEEP to achieve a positive PL [18]. Titrating PEEP to a positive PL may have prevented significant end-expiratory collapsing pressures, increased the size of functional lung, reduced cyclical opening and closing of the lung (atelectrauma), improved oxygenation, and prevented ventilator-induced lung injury [18]. This strategy resulted in significantly higher applied PEEP, improved oxygenation and compliance, and a strong trend towards improved survival and shorter duration of mechanical ventilation compared with standard low tidal volume lung protective ARDSnet ventilation strategies [18]. An animal study using surfactant-depleted rats agreed with the rationale behind this study, finding that a strategy targeting positive end-expiratory PL maintained lung volumes, improved compliance, reduced hypoxemia and pulmonary edema, and decreased pro-inflammatory mediator release as well as histological signs of ventilation-induced lung injury (VILI) [19]. The newer multi-center randomized controlled trial uses higher PEEP in control patients to better replicate current clinical practices [20]. Determining the “best PEEP” for a given patient has remained challenging with no clear best strategy or technique, and esophageal manometry provides an easy and straightforward solution.
The elastance-based method for PL estimation has also been used to set PEEP and guide treatment. In a report by Grasso and colleagues in patients with severe ARDS due to influenza [21], clinicians used the elastance-based method to avoid ECMO in seven patients with severe hypoxemia, raising PEEP beyond levels that had previously been considered safe using elastance-based transpulmonary pressure.
PES use to limit cyclical and total stress to prevent VILI and overdistension
The clinical importance of respiratory system driving pressure was suggested in a large retrospective analysis performed by Amato et al., finding that driving pressure may the best predictor for mortality in patients with ARDS [22]. The authors’ group proposed that the most important aspect of these findings may be in limiting the distending pressures or cyclical stress across the lungs (the transpulmonary driving pressure [∆PL]; [23]). We tested this hypothesis retrospectively and found that survivors had decreased transpulmonary driving pressure [24]. We know that a ∆PL of 20 cmH2O raises a healthy lung into its total lung capacity and that continuous ventilation with these volumes in animal models leads to lethal VILI [25]. As inhomogeneous lung injury can dramatically increase local stress (more than doubling local pressure) [26, 27], it has been suggested that ∆PL be kept below 10–12 cmH2O to prevent lung injury [28]. Additionally, it is suggested that total lung stress (cyclical stress, ∆PL, added to end-expiratory PL) be limited to less than 20–25 cmH2O to decrease overdistension and prevent VILI by limiting total strain to <2 [28, 29]. With widely variable chest wall pressures and elastance, we cannot predict whether we are reaching these thresholds of cyclic and total stress without the use of an esophageal balloon. Although we need further research to corroborate these assumptions, strong circumstantial evidence suggests that the use of esophageal balloons to calculate ∆PL, can improve patient safety and personalization of ventilator settings.
Assessment of ventilator synchrony and spontaneous efforts
Ventilator dyssynchrony has gained increased attention as these episodes can be associated with worsened hypoxemia, increased workload on the respiratory muscles, discomfort, cardiovascular compromise[30], as well as increased ICU stay[31] and mortality [32]. There is also increasing concern that dyssynchrony may cause large transpulmonary pressure swings and inappropriately large tidal volumes that may be especially harmful in critically ill patients that are receiving lung-protective ventilation [33]. While some dyssynchrony is obvious while measuring PAO alone, the type and severity of an event is often missed or underestimated if not measuring PL (Fig. 3).
a Reverse triggering due to entrainment where the initial breath triggers a reflex diaphragm contraction triggering a second breath. The dotted line shows the diaphragm contraction causing a drop in esophageal pressure which triggers the stacked breath. As shown, breath stacking can lead to significantly higher lung volumes than set on the ventilator. b Double triggering often occurs in patients with increased respiratory effort. The inspiratory effort (as seen by the esophageal pressure) continues beyond the delivery of the initial breath (red arrows to dotted lines), triggering a second breath on top of the first. This patient also shows evidence of expiratory effort (blue arrows) which may result in derecruitment. Comparing panel a and b, it would be difficult to differentiate reverse triggering from double triggering without esophageal pressure monitoring as the airway waveforms appear quite similar
Double triggering and reverse triggering are commonly observed phenomenon that can be distinguished by using esophageal manometry to measure swings in PEs [33]. Double triggering occurs when a patient has a respiratory drive/effort greater than the support delivered on the ventilator. The patient has persistent inspiratory effort at the termination of the ventilator-delivered breath which triggers an immediate second breath delivered by the machine. Alternatively, reverse triggering (due to entrainment) occurs as a reflexive diaphragm contraction in response to a ventilator-delivered breath, which triggers a second breath prior to exhalation of the initial breath ([34]; Fig. 3). Double triggering and reverse triggering both lead to significantly larger tidal volumes, large transpulmonary pressure swings and peak pressures, as well as incorrect plateau pressure measurements. The importance of this differentiation using PEs monitoring is emphasized as double triggering improves with increased sedation, while, in contrast, reverse triggering may actually improve by decreasing sedation levels. As such, the use of an esophageal balloon may allow for improved sedation titration or even help in the decision to paralyze a critically ill patient with severe hypoxemia.
While spontaneous breathing may be well tolerated in patients with less severe lung injury, in severe ARDS, these efforts may be harmful, which has been suggested from the survival benefit of paralytics [35, 36]. The combination of large inspiratory efforts added to the ventilator-assisted breaths may lead to large swings in PL and/or larger than desired tidal volumes leading to overdistension. Similarly, active expiratory efforts may counter the benefits from PEEP [36], leading to derecruitment, lung collapse, and worsened oxygenation. Again, without esophageal pressure monitoring, detecting this clinically may be quite challenging.
PL monitoring during recruitment maneuvers
Recruitment maneuvers are commonly used to reopen lung and maintain aeration promoting improved oxygenation and pulmonary mechanics. Although several techniques are used for recruitment, the general concept involves increasing and holding airway pressures. Grasso et al. found that half of ARDS patients were “responders” in terms of oxygenation after recruitment, while non-responders were found to have higher chest-wall elastance [37]. They postulated that pressure dissipated by the chest wall caused decreased transpulmonary pressure and impaired recruitment [37]. Although this suggests that sufficient PL is needed for recruitment, elevated PL during recruitment maneuvers may also cause overdistension. Therefore, it was hypothesized that with widely variable chest wall mechanics, recruitment maneuvers targeting airway pressures may result in unpredictable PL causing either under-recruitment or overdistension. Indeed, results from a recent retrospective analysis by the authors suggest that PL above 20 cmH2O may lead to overdistension, increase lung stress [38]; Fig. 4). This analysis is currently being replicated, but initial results suggest that performing recruitment maneuvers without transpulmonary pressure monitoring may be unsuccessful or even potentially harmful.
Airway pressure (dark blue) and transpulmonary pressure (light blue) during standard recruitment maneuvers in 28 patients with esophageal balloons. Transpulmonary pressure (PL) was calculated as the airway pressure minus the esophageal pressure. The figure illustrates a possible “optimal” window for transpulmonary pressure during recruitment where lung elastance improved from baseline, suggesting a successful recruitment, but this window needs further testing in larger numbers. Above 20 cmH2O, lung elastance during recruitment worsened from baseline, suggesting an upper safety limit above which overdistension may be caused (modified from [38])
Intrinsic PEEP adjustment
Auto-PEEP, or intrinsic PEEP (PEEPi), is the additional pressure in the lungs above the airway pressure at end expiration and may be caused by incomplete emptying of gas from premature termination of the expiratory phase [39]. PEEPi is commonly seen secondary to flow limitation in patients with COPD, asthma, or obesity [39]. This additional pressure above airway pressure can lead to patient dyssynchrony, missed breaths, dyspnea, increased work of breathing, and patient discomfort. In severe cases, PEEPi can lead to breath stacking and dynamic hyperinflation with hemodynamic compromise [39]. Patients spontaneously breathing on the ventilator must overcome the PEEPi before the alveolar pressure drop is sufficient to move air inwards and trigger a breath. Overcoming PEEPi requires additional muscle work with every breath, which can be a significant cause for dyspnea and muscle fatigue. While measuring airway pressure during end-expiratory breath hold gives an estimate of the PEEPi in deeply sedated patients, the most accurate method is to use esophageal manometry, which can also be used in spontaneously breathing patients [40, 41]. With a balloon in place, Pes is measured at end expiration with PEEPi calculated as the drop in PEs from the contraction of the inspiratory muscles until inspiratory flow starts [40]. Improved detection and awareness of PEEPi with an esophageal balloon may facilitate prompt correction (ventilator adjustment or medications targeting obstruction), improving dyssynchrony, dyspnea, and work of breathing.
Weaning from the ventilator
During weaning from the ventilator, there is a gradual reduction in ventilator support and increased reliance upon patient effort to generate ventilation. Weaning and extubation failure have been found to be associated with progressive increases in the esophageal pressure-time product, while weaning success has been associated with its stability [42, 43]. Esophageal pressure monitoring may allow for better prediction of weaning success compared with the rapid shallow breathing index [43]. Furthermore, large swings in Pes during weaning might allow for more rapid awareness of problems and possible correction with treatments such as bronchodilators or diuretics.
Conclusion
Despite its clear clinical utility, there are several barriers to the widespread clinical adoption of esophageal manometry. Firstly, the lack of consistent terminology and the use of alternative definitions and calculations of PL and PEs in the clinical and research literature may lead to the incorrect application of principles with variable and perhaps harmful outcomes and discourage widespread adoption [13]. Secondly, although esophageal balloon use is easily learned, the proper technique in placement and interpretation is required for successful use. Lastly, the clinical use of esophageal manometry is relatively new in the intensive care unit and further research into its application is needed.
In summary, regardless of these barriers and the current lack of its widespread clinical use, esophageal manometry is easy to use and has extensive application within the intensive care unit. Clinicians monitoring transpulmonary and chest wall pressures have a better understanding of a patient’s unique physiology from the time of intubation through extubation. Transpulmonary pressure monitoring early after intubation may allow optimization of PEEP to prevent lung collapse and optimize recruitment, and measuring cyclical and total transpulmonary stress may prevent ventilator-induced lung injury or overdistension during tidal breathing and recruitment maneuvers. Monitoring esophageal pressures may then allow detection of dyssynchrony and respiratory efforts, helping guide the decision to change sedation or paralyze the patient, and may improve patient tolerance of spontaneous breathing by better matching intrinsic PEEP. During the weaning phase, monitoring transpulmonary pressure may improve understanding of weaning failure and help to more quickly correct the underlying problems. With these numerous benefits and the increased ease of use, it appears time to reintroduce basic physiology into clinical care to allow a more personalized approach to critically ill patients.
References
Mead J, Gaenslear EA (1959) Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 14:81–83
Mead J (1961) Mechanical properties of lungs. Physiol Rev 41:281–330
Dornhost AC, Leathart GL (1952) A method of assessing the mechanical properties of lungs and air-passages. Lancet 6736(52):92151–X
Washko GR, O’Donnell CR, Loring SH (2006) Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol 100:753–758
Pecchiari M, Loring SH, D’Angelo E (2013) Esophageal pressure as an estimate of average pleural pressure with lung or chest distortion in rats. Respir Physiol Neurobiol 186:229–235. https://doi.org/10.1016/j.resp.2013.02.006
Terragni P, Mascia L, Fanelli V, Biondi-Zoccai G, Ranieri VM (2017) Accuracy of esophageal pressure to assess transpulmonary pressure during mechanical ventilation. Intensive Care Med 43:142–143. https://doi.org/10.1007/s00134-016-4589-8
Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D (2003) Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl 47:15s–25s
Gattinoni L, Carlesso E, Caironi P (2012) Stress and strain within the lung. Curr Opin Crit Care 18:42–47. https://doi.org/10.1097/MCC.0b013e32834f17d9
Chiumello D, Cressoni M, Colombo A et al (2014) The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 40:1670–1678. https://doi.org/10.1007/s00134-014-3415-4
Chiumello D, Guerin C (2015) Understanding the setting of PEEP from esophageal pressure in patients with ARDS. Intensive Care Med 41:1465–1467. https://doi.org/10.1007/s00134-015-3776-3
Chiumello D, Cressoni M, Carlesso E et al (2014) Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 42:252–264. https://doi.org/10.1097/CCM.0b013e3182a6384f
Chiumello D, Carlesso E, Cadringher P et al (2008) Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355. https://doi.org/10.1164/rccm.200710-1589OC
Loring SH, Topulos GP, Hubmayr RD (2016) Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med 194:1452–1457. https://doi.org/10.1164/rccm.201512-2448CP
Gulati G, Novero A, Loring SH, Talmor D (2013) Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med 41:1951–1957. https://doi.org/10.1097/CCM.0b013e31828a3de5
Behazin N, Jones SB, Cohen RI, Loring SH (2010) Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 198(5):212–218. https://doi.org/10.1152/japplphysiol.91356.2008
Loring SH, O’Donnell CR, Behazin N et al (2010) Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol 198(5):515–522. https://doi.org/10.1152/japplphysiol.00835.2009
Talmor D, Sarge T, O’Donnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH (2006) Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 34:1389–1394. https://doi.org/10.1097/01.CCM.0000215515.49001.A2
Talmor D, Sarge T, Malhotra A et al (2008) Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359:2095–2104. https://doi.org/10.1056/NEJMoa0708638
Loring SH, Pecchiari M, Valle DP, Monaco A, Gentile G, D’Angelo E (2010) Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med 38:2358–2364. https://doi.org/10.1097/CCM.0b013e3181fa02b8
Fish E, Novack V, Banner-Goodspeed VM, Sarge T, Loring S, Talmor D (2014) The Esophageal Pressure-Guided Ventilation 2 (EPVent2) trial protocol: a multicentre, randomised clinical trial of mechanical ventilation guided by transpulmonary pressure. BMJ Open 4(006356):e6356–2014. https://doi.org/10.1136/bmjopen-2014-006356
Grasso S, Terragni P, Birocco A et al (2012) ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 38:395–403. https://doi.org/10.1007/s00134-012-2490-7
Amato MB, Meade MO, Slutsky AS et al (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755. https://doi.org/10.1056/NEJMsa1410639
Loring SH, Malhotra A (2015) Driving pressure and respiratory mechanics in ARDS. N Engl J Med 372:776–777. https://doi.org/10.1056/NEJMe1414218
Baedorf Kassis E, Loring SH, Talmor D (2016) Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 42:1206–1213. https://doi.org/10.1007/s00134-016-4403-7
Protti A, Cressoni M, Santini A et al (2011) Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 183:1354–1362. https://doi.org/10.1164/rccm.201010-1757OC
Mead J, Takishima T, Leith D (1970) Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28:596–608
Cressoni M, Cadringher P, Chiurazzi C et al (2014) Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 189:149–158. https://doi.org/10.1164/rccm.201308-1567OC
Mauri T, Yoshida T, Bellani G et al (2016) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 42:1360–1373. https://doi.org/10.1007/s00134-016-4400-x
Hess DR (2014) Respiratory mechanics in mechanically ventilated patients. Respir Care 59:1773–1794. https://doi.org/10.4187/respcare.03410
Messina A, Colombo D, Cammarota G et al (2015) Patient-ventilator asynchrony affects pulse pressure variation prediction of fluid responsiveness. J Crit Care 30:1067–1071. https://doi.org/10.1016/j.jcrc.2015.06.010
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522. https://doi.org/10.1007/s00134-006-0301-8
Blanch L, Villagra A, Sales B et al (2015) Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 41:633–641. https://doi.org/10.1007/s00134-015-3692-6
Beitler JR, Sands SA, Loring SH et al (2016) Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med 42:1427–1436. https://doi.org/10.1007/s00134-016-4423-3
Akoumianaki E, Lyazidi A, Rey N et al (2013) Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest 143:927–938. https://doi.org/10.1378/chest.12-2412
Papazian L, Forel JM, Gacouin A et al (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363:1107–1116. https://doi.org/10.1056/NEJMoa1005372
Guervilly C, Bisbal M, Forel JM et al (2017) Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med 43:408–418. https://doi.org/10.1007/s00134-016-4653-4
Grasso S, Mascia L, Del Turco M et al (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Baedorf Kassis E, Loring S, Talmor D (2017) Recruitment maneuvers: using transpulmonary pressure to help goldilocks. Intensive Care Med. https://doi.org/10.1007/s00134-017-4784-2
Marini JJ (2011) Dynamic hyperinflation and auto-positive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 184:756–762. https://doi.org/10.1164/rccm.201102-0226PP
Brochard L (2002) Intrinsic (or auto-) positive end-expiratory pressure during spontaneous or assisted ventilation. Intensive Care Med 28:1552–1554
Lessard MR, Lofaso F, Brochard L (1995) Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients. Am J Respir Crit Care Med 151:562–569. https://doi.org/10.1164/ajrccm.151.2.7842221
Jubran A, Tobin MJ (1997) Passive mechanics of lung and chest wall in patients who failed or succeeded in trials of weaning. Am J Respir Crit Care Med 155:916–921. https://doi.org/10.1164/ajrccm.155.3.9117026
Jubran A, Grant BJ, Laghi F, Parthasarathy S, Tobin MJ (2005) Weaning prediction: esophageal pressure monitoring complements readiness testing. Am J Respir Crit Care Med 171:1252–1259
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Baedorf Kassis, S. H. Loring, and D. Talmor declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
Redaktion
M. Quintel, Göttingen
L. Gattinoni, Milan
Rights and permissions
About this article
Cite this article
Baedorf Kassis, E., Loring, S.H. & Talmor, D. Esophageal pressure: research or clinical tool?. Med Klin Intensivmed Notfmed 113 (Suppl 1), 13–20 (2018). https://doi.org/10.1007/s00063-017-0372-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-017-0372-z