Abstract
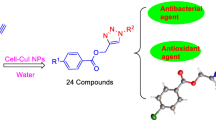
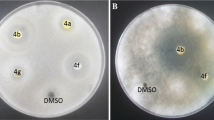
A series of 1,4-disubstituted 1,2,3-triazoles with amide-hydroxyl functionality (5a–5t) was synthesized from aliphatic alkynes (4a–4e) and aromatic bromides (3a–3d) in presence of catalytic amount of cellulose CuI nanoparticles. All the synthesized triazoles were characterized by various analytical techniques: FTIR, 1H NMR, 13C NMR and HRMS. Further, all the synthesized compounds were screened for in vitro antioxidant and antimicrobial activities. The antioxidant activity of the compound 5s was found better than other compounds. Compounds 5h and 5l exhibited good antibacterial and antifungal activity, respectively. The docking studies were performed to find out various binding interactions of protein-ligand complex. In silico ADME study was performed to evaluate their drug likeness.





Similar content being viewed by others
References
Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306 https://doi.org/10.1128/jb.178.1.306-308.1996
Isturiz RE. Optimizing antimicrobial prescribing. Int J Antimicrob Agents. 2010;36:S19–S22. https://doi.org/10.1016/S0924-8579(10)70006-6
Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front Microbiol. 2018;9:2928 https://doi.org/10.3389/fmicb.2018.02928
Guyton KZ, Kensler TW. Oxidative mechanism in carcinogenesis. Br Med Bull. 1993;49:523–44. https://doi.org/10.1093/oxfordjournals.bmb.a072628
Singh A, Fong G, Liu J, Wu YH, Chang K, Park W, et al. Synthesis and preliminary antimicrobial analysis of isatin−ferrocene and isatin−ferrocenyl chalcone conjugates. ACS Omega. 2018;3:5808–5813. https://doi.org/10.1021/acsomega.8b00553
Recnik LM, Kandioller W, Mindt TL. 1, 4-Disubstituted 1, 2, 3-triazoles as amide bond surrogates for the stabilisation of linear peptides with biological activity. Molecules. 2020;25:3576 https://doi.org/10.3390/molecules25163576
Whiting M, Muldoon J, Lin YC, Silverman SM, Lindstron W, Olson AJ, et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew Chem. 2006;118:1463–7. https://doi.org/10.1002/ange.200502161
Sampat S, Vadivelu M, Ravindran R, Perumal PT, Velkannan V, Karthikeyan K. Synthesis of 1,2,3-triazole tethered 3-hydroxy-2-oxindoles: promising corrosion inhibitors for steel in acidic medium and their anti-microbial evaluation. ChemistrySelect. 2020;5:2130–4. https://doi.org/10.1002/slct.201904320
Deswal S, Naveen, Tittal RK, Ghule VD, Lal K, Kumar A. 5-Fluoro-1H-indole-2,3-dione-triazoles- synthesis, biological activity, molecular docking, and DFT study. J Mol Struct. 2020;1209:127982 https://doi.org/10.1016/j.molstruc.2020.127982
Mazzotta S, Cebrero-Cangueiro T, Frattaruolo L, Vega-Holm M, CarreteroLedesma M, Sánchez-Céspedes J, et al. Exploration of piperazine-derived thioureas as antibacterial and anti-inflammatory agents. In vitro evaluation against clinical isolates of colistin-resistant Acinetobacter baumannii. Bioorg Med Chem Lett. 2020;30:127411 https://doi.org/10.1016/j.bmcl.2020.127411
Poonia N, Lal K, Kumar A. Design, synthesis, antimicrobial evaluation and in silico studies of symmetrical bis (urea-1,2,3-triazole) hybrids. Res Chem Intermed. 2021;47:1087–10103. https://doi.org/10.1007/s11164-020-04318-1
Moussaoui O, Bhadane R, Sghyar R, Ilas J, Hadrami EME, Chakroune S et al. Design, synthesis, in vitro and in silico characterization of new 2‐quinolone‐L‐alaninate‐1, 2, 3‐triazoles as novel antimicrobial agents. ChemMedChem. 2022;17:e202100714 https://doi.org/10.1002/cmdc.202100714
Shafie A, Mohammadi-Khanaposhtani M, Asadi M, Rahimi N, Ranjbar PR, Ghasemi JB, et al. Novel fused 1, 2, 3-triazolo-benzodiazepine derivatives as potent anticonvulsant agents: design, synthesis, in vivo, and in silico evaluations. Mol Divers. 2020;24:179–89. https://doi.org/10.1007/s11030-019-09940-9
Patil PS, Kasare SL, Haval NB, Khedkar VM, Dixit PP, Rekha EM, et al. Novel isoniazid embedded triazole derivatives: synthesis, antitubercular and antimicrobial activity evaluation. Bioorg Med Chem Lett. 2020;30:127434 https://doi.org/10.1016/j.bmcl.2020.127434
Garg A, Borah D, Trivedi P, Gogoi D, Chaliha AK, Ali AA, et al. A simple work-up-free, solvent-free approach to novel amino acid linked 1,4-disubstituted 1,2,3-triazoles as potent antituberculosis agents. ACS Omega. 2020;5:29830–29837. https://doi.org/10.1021/acsomega.0c03862
Girase PS, Dhawan S, Kumar V, Shinde SR, Palkar MB, Karpoormath R. An appraisal of anti-mycobacterial activity with structure-activity relationship of piperazine and its analogues: a review. Eur J Med Chem. 2021;210:112967 https://doi.org/10.1016/j.ejmech.2020.112967
Deswal L, Verma V, Kumar D, Kaushik CP, Kumar A, Deswal Y, et al. Synthesis and antidiabetic evaluation of benzimidazole‐tethered 1, 2, 3‐triazoles. Arch Pharm. 2020;2020:e2000090 https://doi.org/10.1002/ardp.202000090
Theeramunkong S, Thiengsusuk A, Vajragupta O, Muhamad P. Synthesis, characterization and antimalarial activity of isoquinoline derivatives. Med Chem Res 2021;30:109–19. https://doi.org/10.1007/s00044-020-02642-0
Kaushik CP, Chahal M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1, 4-disubstituted 1, 2, 3-triazoles. Monatsh Chem. 2021;152:1001–12. https://doi.org/10.1007/s00706-021-02821-8
Kandula MKR, Gundluru M, Nemallapudi BR, Gundala S, Kotha P, Zyryanov GV, et al. Synthesis, antioxidant activity, and α-glucosidase enzyme inhibition of α-aminophosphonate derivatives bearing piperazine-1,2,3-triazole moiety. J Heterocycl Chem. 2021;58:172–81. https://doi.org/10.1002/jhet.4157
Reddivari CKR, Devineni SR, Nemallapudi BR, Sravya G, Avula B, Shaik N, et al. Design, synthesis, biological evaluation and molecular docking studies of 1,4-disubstituted 1,2,3-triazoles: peg-400:h2o mediated click reaction of fluorescent organic probes under ultrasonic irradiation. Polycycl Aromat Compd. 2021. https://doi.org/10.1080/10406638.2021.1878246
Nural Y, Ozdemir S, Yalcin MS, Demir B, Atabey H, Seferoglu Z, et al. New bis-and tetrakis-1, 2, 3-triazole derivatives: synthesis, DNA cleavage, molecular docking, antimicrobial, antioxidant activity and acid dissociation constants. Bioorg Med Chem Lett. 2022;55:128453 https://doi.org/10.1016/j.bmcl.2021.128453
Shinoda K, Kanai M, Sohma Y. Design, synthesis, and properties of a chemically-tethered amyloid-# segment trimer resistant to inter-trimer mis-aggregation. J Org Chem. 2020;85:1635–43. https://doi.org/10.1021/acs.joc.9b02612
Kaushik CP, Sangwan J, Luxmi R, Kumar D, Kumar D, Das A, et al. Design, synthesis, anticancer and antioxidant activities of amide linked 1,4-disubstituted 1,2,3-triazoles. J Mol Struct. 2021;1226:129255 https://doi.org/10.1016/j.molstruc.2020.129255
Suryanarayana K, Robert AR, Kerru N, Pooventhiran T, Thomas R, Maddila S, et al. Design, synthesis, anticancer activity and molecular docking analysis of novel dinitrophenylpyrazole bearing 1, 2, 3-triazoles. J Mol Struct. 2021;1243:130865 https://doi.org/10.1016/j.molstruc.2021.130865
Begam R, Shajahan A, Vadivelu M. Synthesis of novel naphthalimide tethered 1, 2, 3-triazoles: in vitro biological evaluation and docking study of anti-inflammatory inhibitors. J Mol Struct. 2022;1254:132364 https://doi.org/10.1016/j.molstruc.2022.132364
Pertino MW, Torre AFDL, Hirschmann GS, Vega C, Rolon M, Coronel C, et al. Synthesis, trypanocidal and anti-leishmania activity of new triazole-lapachol and nor-lapachol hybrids. Bioorg Chem. 2020;103:104122 https://doi.org/10.1016/j.bioorg.2020.104122
El-Sayed WA, Khalaf HS, Mohamed SF, Hussien HA, Kutkat OM, Amr AE. Synthesis and antiviral activity of 1, 2, 3-triazole glycosides based substituted pyridine via click cycloaddition. Russ J Gen Chem. 2017;87:2444–53. https://doi.org/10.1134/S1070363217100279
Kumar H, Devaraji V, Joshi R, Jadhao M, Ahirkar P, Prasath R, et al. Antihypertensive activity of a quinoline appended chalcone derivative and its site specific binding interaction with a relevant target carrier protein. RSC Adv. 2015;5:65496–513. https://doi.org/10.1039/C5RA08778C
Cherif M, Horchani M, Ghamdi YOA, Almalki SG, Alqurashi YE, Jannet HB, et al. New pyrano-1,2,3-triazolopyrimidinone derivatives as anticholinesterase and antibacterial agents: Design, microwave-assisted synthesis and molecular docking study. J Mol Struct. 2020;1220:128685 https://doi.org/10.1016/j.molstruc.2020.128685
Igual MO, Nunes PSG, Costa RMD, Mantoani SP, Tostes RC, Carvalho I.Novel glucopyranoside C2-derived 1,2,3-triazoles displaying selective inhibition of O-GlcNAcase (OGA).Carbohydr Res.2019;471:43–55. https://doi.org/10.1016/j.carres.2018.10.007
Saghanezhad SJ, Buhamidi MM, Ebadi S, Taheri N, Sayyahi S. Entangled nanofbrous copper: an effcient and high performance nanostructured catalyst in azide-alkyne cycloaddition reaction and reduction of nitroarenes and aromatic aldehydes. React Kinet Mech Catal. 2021;133:897–911. https://doi.org/10.1007/s11144-021-02011-x
Barman K, Dutta P, Chowdhury D, Baruah PK. Green biosynthesis of copper oxide nanoparticles using waste colocasia esculenta leaves extract and their application as recyclable catalyst towards the synthesis of 1,2,3-triazoles. Bionanoscience. 2021;11:189–99. https://doi.org/10.1007/s12668-021-00826-5
Huisgen R, Szeimies G, Mobius L. 1.3-Dipolare cycloadditionen, XXXII. Kinetik der additionen organischer azide an CC-mehrfachbindungen. Chem Ber. 1967;100:2494–507. https://doi.org/10.1002/cber.19671000806
Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–64. https://doi.org/10.1021/jo011148j
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–9. https://doi.org/10.1002/1521-3773(20020715)41:14%3C2596::aid-anie2596%3E3.0.co;2-4
Kaushik CP, Luxmi R. Synthesis and antimicrobial activity of 2-(4-(Hydroxyalkyl)-1H-1,2,3- triazol-1-yl)-N-substituted propanamides. J Heterocycl Chem. 2017;54:3618 https://doi.org/10.1002/jhet.2988
Chavan PV, Pandit KS, Desai UV, Kulkarni MA, Wadgaonkar PP. Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): a new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted – 1,2,3-triazoles in water. RSC Adv. 2014;4:42137–46. https://doi.org/10.1039/C4RA05080K
Kaushik CP, Chahal M. Synthesis and antibacterial activity of benzothiazole and benzoxazole-appended substituted 1,2,3-triazoles. J Chem Sci. 2020;132:142 https://doi.org/10.1007/s12039-020-01844-8
Kaushik CP, Luxmi R, Singh D, Kumar A. Synthesis and antimicrobial evaluation of ester-linked 1,4-disubstituted 1,2,3-triazoles with a furyl/thienyl moiety. Mol Divers. 2017;21:137–45. https://doi.org/10.1007/s11030-016-9710-y
Kaushik CP, Sangwan J, Luxmi R, Kumar D, Kumar D, Das A, et al. Design, synthesis, anticancer and antioxidant activities of amide linked 1,4-disubstituted 1,2,3-triazoles. J Mol Struct. 2021;1226:129255 https://doi.org/10.1016/j.molstruc.2020.129255
Lal K, Poonia N, Rani P, Kumar A, Kumar A. Design, synthesis, antimicrobial evaluation and docking studies of urea-triazole-amide hybrids. J Mol Struct. 2020;1215:128234 https://doi.org/10.1016/j.molstruc.2020.128234
MarvinSketch 19.19.0, 2019, ChemAxon (http://www.chemaxon.com)
Trott O, Olson AJ. J Comput Chem. 2010;31:455–61.
Dassault Systemes BIOVIA, Discovery studio visualizer v17.2.0.16349, San Diego: Dassault Systèmes, 2016.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. J Comput Chem. 2004;25:1605–12.
Sharma MK, Parashar S, Chahal M, Lal K, Pandya NU, Om H. Antimicrobial and in-silico evaluation of novel chalcone and amide-linked 1,4-disubstituted 1,2,3 triazoles. J Mol Struct. 2020;1257:132632 https://doi.org/10.1016/j.molstruc.2022.132632
Acknowledgements
Authors are highly thankful to the Council of Scientific and Industrial Research (CSIR) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chahal, M., Kaushik, C.P., Luxmi, R. et al. Synthesis, antimicrobial, and antioxidant activities of disubstituted 1,2,3-triazoles with amide-hydroxyl functionality. Med Chem Res 32, 85–98 (2023). https://doi.org/10.1007/s00044-022-02993-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02993-w