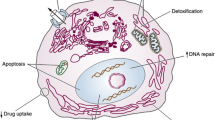
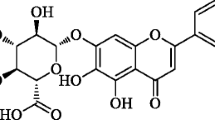
Abstract
Numerous antitumor drugs exhibited excellent anti-proliferation activities, discovered from natural products. Oridonin is isolated from a traditional Chinese medicine Isodon rubescens, and it exhibit various biological functions including anticancer, anti-inflammation, antibacterial, and so on. The anticancer mechanisms of oridonin include apoptosis, autophagy, cell cycle arrest, and so on. However, clinical research and application of oridonin has been hampered, due to the poor pharmaceutical property. So many studies on oridonin have been explored in the purpose of improve the pharmaceutical property, including investigation of the mechanism of action in depth and structural modification. In this review, we summarized oridonin and oridonin derivatives as potential anticancer agents, and detailed discussion on biological properties, mechanism of actions and potential for overcoming therapeutic resistance.























Similar content being viewed by others
References
Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–30. https://doi.org/10.1002/cncr.33587
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660
Liu X, Xu J, Zhou J, Shen Q. Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance. Genes Dis 2021;8:448–62. https://doi.org/10.1016/j.gendis.2020.06.010
Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S, et al. Natural product-based nanoformulations for cancer therapy: opportunities and challenges. Semin Cancer Biol. 2021;69:5–23. https://doi.org/10.1016/j.semcancer.2019.08.014
Karaman Mayack B, Sippl W, Ntie-Kang F. Natural products as modulators of sirtuins. Molecules. 2020;25(14):3287. https://doi.org/10.3390/molecules25143287.
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–614. https://doi.org/10.1016/j.biotechadv.2015.08.001
Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–8. https://doi.org/10.1038/nrc723
Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61. https://doi.org/10.1021/acs.jnatprod.5b01055
Fujita E, Fujita T, Katayama H, Shibuya M, Shingu T. Terpenoids. Part XV. Structure and absolute configuration of oridonin isolated from isodon japonicus and isodon trichocarpus. J Chem Soc C. 1970;12:1674–81. https://doi.org/10.1039/j39700001674
Kadota S, Basnet P, Ishii E, Tamura T, Namba T. Antibacterial activity of trichorabdal A from Rabdosia trichocarpa against Helicobacter pylori. Zentralbl Bakteriol. 1997;286:63–7. https://doi.org/10.1016/s0934-8840(97)80076-x
Chen RY, Xu B, Chen SF, Chen SS, Zhang T, Ren J, et al. Effect of oridonin-mediated hallmark changes on inflammatory pathways in human pancreatic cancer (BxPC-3) cells. World J Gastroenterol. 2014;20:14895–903. https://doi.org/10.3748/wjg.v20.i40.14895
Fujita E, Nagao Y, Node M, Kaneko K, Nakazawa S, Kuroda H. Antitumor activity of the Isodon diterpenoids: structural requirements for the activity. Experientia. 1976;32:203–6. https://doi.org/10.1007/BF01937766
Bohanon FJ, Wang X, Ding C, Ding Y, Radhakrishnan GL, Rastellini C, et al. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res. 2014;190:55–63. https://doi.org/10.1016/j.jss.2014.03.036
Hu AP, Du JM, Li JY, Liu JW. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm Res. 2008;57:163–70. https://doi.org/10.1007/s00011-007-7193-0
Xu Y, Xue Y, Wang Y, Feng D, Lin S, Xu L. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int Immunopharmacol. 2009;9:360–5. https://doi.org/10.1016/j.intimp.2009.01.002
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27 https://doi.org/10.1186/1749-8546-6-27
Ma Z, Hu C, Zhang Y. Therapeutic effect of Rabdosia rubescens aqueous extract on chronic pharyngitis and its safety. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:170–3. https://doi.org/10.3969/j.issn.1672-7347.2011.02.014
Zhou M, Yi Y, Hong L. Oridonin Ameliorates Lipopolysaccharide-Induced Endometritis in Mice via Inhibition of the TLR-4/NF-kappaBpathway. Inflammation. 2019;42:81–90. https://doi.org/10.1007/s10753-018-0874-8
Li J, Bao L, Zha D, Zhang L, Gao P, Zhang J, et al. Oridonin protects against the inflammatory response in diabetic nephropathy by inhibiting the TLR4/p38-MAPK and TLR4/NF-kappaB signaling pathways. Int Immunopharmacol. 2018;55:9–19. https://doi.org/10.1016/j.intimp.2017.11.040
Huang W, Huang M, Ouyang H, Peng J, Liang J. Oridonin inhibits vascular inflammation by blocking NF-kappaB and MAPK activation. Eur J Pharm. 2018;826:133–9. https://doi.org/10.1016/j.ejphar.2018.02.044
Zhao G, Zhang T, Ma X, Jiang K, Wu H, Qiu C, et al. Oridonin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-induced RAW264.7 cells and acute lung injury. Oncotarget. 2017;8:68153–64. https://doi.org/10.18632/oncotarget.19249
Deng Y, Chen C, Yu H, Diao H, Shi C, Wang Y, et al. Oridonin ameliorates lipopolysaccharide/D-galactosamine-induced acute liver injury in mice via inhibition of apoptosis. Am J Transl Res. 2017;9:4271–9.
Liu QQ, Wang HL, Chen K, Wang SB, Xu Y, Ye Q, et al. Oridonin derivative ameliorates experimental colitis by inhibiting activated T-cells and translocation of nuclear factor-kappa B. J Dig Dis. 2016;17:104–12. https://doi.org/10.1111/1751-2980.12314
Zhao YJ, Lv H, Xu PB, Zhu MM, Liu Y, Miao CH, et al. Protective effects of oridonin on the sepsis in mice. Kaohsiung J Med Sci. 2016;32:452–7. https://doi.org/10.1016/j.kjms.2016.07.013
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550 https://doi.org/10.1038/s41467-018-04947-6
Wang S, Zhang Y, Saas P, Wang H, Xu Y, Chen K, et al. Oridonin’s therapeutic effect: suppressing Th1/Th17 simultaneously in a mouse model of Crohn’s disease. J Gastroenterol Hepatol. 2015;30:504–12. https://doi.org/10.1111/jgh.12710
Wu QJ, Zheng XC, Wang T, Zhang TY. Effects of dietary supplementation with oridonin on the growth performance, relative organ weight, lymphocyte proliferation, and cytokine concentration in broiler chickens. BMC Vet Res. 2018;14:34 https://doi.org/10.1186/s12917-018-1359-6
Wu QJ, Zheng XC, Wang T, Zhang TY. Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys in the spleens of broiler chickens challenged with Salmonella pullorum. Res Vet Sci. 2018;119:262–7. https://doi.org/10.1016/j.rvsc.2018.07.008
Wang J, Li F, Ding J, Tian G, Jiang M, Gao Z, et al. Investigation of the antiasthmatic activity of Oridonin on a mouse model of asthma. Mol Med Rep. 2016;14:2000–6. https://doi.org/10.3892/mmr.2016.5485
Shang CH, Zhang QQ, Zhou JH. Oridonin inhibits cell proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2016;39:873–80. https://doi.org/10.1007/s10753-016-0318-2
Zhou L, Sun L, Wu H, Zhang L, Chen M, Liu J, et al. Oridonin ameliorates lupus-like symptoms of MRL(lpr/lpr) mice by inhibition of B-cell activating factor (BAFF). Eur J Pharm. 2013;715:230–7. https://doi.org/10.1016/j.ejphar.2013.05.016
Liu J, Huang R, Lin D, Wu X, Peng J, Lin Q, et al. Apoptotic effect of oridonin on NB4 cells and its mechanism. Leuk Lymphoma. 2005;46:593–7. https://doi.org/10.1080/10428190400019800
Wang MY, Lin C, Zhang TM. Cytokinetic effects of oridonin on leukemia L1210 cells. Zhongguo Yao Li Xue Bao. 1985;6:195–8.
Ma YC, Ke Y, Zi X, Zhao W, Shi XJ, Liu HM. Jaridonin, a novel ent-kaurene diterpenoid from Isodon rubescens, inducing apoptosis via production of reactive oxygen species in esophageal cancer cells. Curr Cancer Drug Targets. 2013;13:611–24. https://doi.org/10.2174/15680096113139990030
Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induced A375-S2 cell apoptosis via bax-regulated caspase pathway activation, dependent on the cytochrome c/caspase-9 apoptosome. J Asian Nat Prod Res. 2004;6:127–38. https://doi.org/10.1080/1028602031000147375
Li XT, Lin C, Li PY. Characteristics of the cytostatic effects of oridonin in vitro. Zhongguo Yao Li Xue Bao. 1986;7:361–3.
He Z, Xiao X, Li S, Guo Y, Huang Q, Shi X, et al. Oridonin induces apoptosis and reverses drug resistance in cisplatin resistant human gastric cancer cells. Oncol Lett. 2017;14:2499–504. https://doi.org/10.3892/ol.2017.6421
Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–64. https://doi.org/10.1248/bpb.30.859
Chen S, Cooper M, Jones M, Madhuri TK, Wade J, Bachelor A, et al. Combined activity of oridonin and wogonin in advanced-stage ovarian cancer cells: sensitivity of ovarian cancer cells to phyto-active chemicals. Cell Biol Toxicol. 2011;27:133–47. https://doi.org/10.1007/s10565-010-9176-0
Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol Res. 2006;35:104–10. https://doi.org/10.1016/j.hepres.2006.03.007
Xu T, Jin F, Wu K, Ye Z, Li N. Oridonin enhances in vitro anticancer effects of lentinan in SMMC-7721 human hepatoma cells through apoptotic genes. Exp Ther Med. 2017;14:5129–34. https://doi.org/10.3892/etm.2017.5168
Liu JJ, Huang RW, Lin DJ, Peng J, Wu XY, Pan XL, et al. Anti-proliferative effects of oridonin on SPC-A-1 cells and its mechanism of action. J Int Med Res. 2004;32:617–25. https://doi.org/10.1177/147323000403200606
Zheng M, Zhu Z, Zhao Y, Yao D, Wu M, Sun G. Oridonin promotes G2/M arrest in A549 cells by facilitating ATM activation. Mol Med Rep. 2017;15:375–9. https://doi.org/10.3892/mmr.2016.6008
Bi E, Liu D, Li Y, Mao X, Wang A, Wang J. Oridonin induces growth inhibition and apoptosis in human gastric carcinoma cells by enhancement of p53 expression and function. Braz J Med Biol Res. 2018;51:e7599 https://doi.org/10.1590/1414-431X20187599
Xia R, Chen SX, Qin Q, Chen Y, Zhang WW, Zhu RR, et al. Oridonin Suppresses Proliferation of Human Ovarian Cancer Cells via Blockage of mTOR Signaling. Asian Pac J cancer Prev: APJCP. 2016;17:667–71. https://doi.org/10.7314/apjcp.2016.17.2.667
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S, Ikejima T. Fas/FasL signaling allows extracelluar-signal regulated kinase to regulate cytochrome c release in oridonin-induced apoptotic U937 cells. Biol Pharm Bull. 2006;29:1873–9. https://doi.org/10.1248/bpb.29.1873
Cui Q, Yu JH, Wu JN, Tashiro S, Onodera S, Minami M, et al. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharm Sin. 2007;28:1057–66. https://doi.org/10.1111/j.1745-7254.2007.00588.x
Zhang CL, Wu LJ, Zuo HJ, Tashiro S, Onodera S, Ikejima T. Cytochrome c release from oridonin-treated apoptotic A375-S2 cells is dependent on p53 and extracellular signal-regulated kinase activation. J Pharm Sci. 2004;96:155–63. https://doi.org/10.1254/jphs.fpj04008x
Sartippour MR, Seeram NP, Heber D, Hardy M, Norris A, Lu Q, et al. Rabdosia rubescens inhibits breast cancer growth and angiogenesis. Int J Oncol. 2005;26:121–7.
Lu Y, Sun Y, Zhu J, Yu L, Jiang X, Zhang J, et al. Oridonin exerts anticancer effect on osteosarcoma by activating PPAR-gamma and inhibiting Nrf2 pathway. Cell Death Dis. 2018;9:15 https://doi.org/10.1038/s41419-017-0031-6
Liu JJ, Wu XY, Lul HL, Pan XL, Peng J, Huang RW. Anti-proliferation effect of oridonin on HL-60 cells and its mechanism. Chin Med Sci J. 2004;19:134–7.
Liu J, Huang R, Lin D, Wu X, Chen F. Apoptotic effect of oridonin on NB4 cells and its mechanism. Chin Med Sci J. 2004;19:134.
Liu JJ, Huang RW, Lin DJ, Wu XY, Peng J, Pan XL, et al. Antiproliferation effects of oridonin on HPB-ALL cells and its mechanisms of action. Am J Hematol. 2010;81:86–94.
Xu W, Sun J, Zhang T, Ma B, He Z. Determination of equilibrium solubility of oridonin and its apparent oil/water partition coefficient by HPLC. J Shenyang Pharm Univ. 2007(04):220-222. https://doi.org/10.3969/j.issn.1006-2858.2007.04.008
Xu W, Sun J, Zhang TT, Ma B, Cui SM, Chen DW, et al. Pharmacokinetic behaviors and oral bioavailability of oridonin in rat plasma. Acta Pharm Sin. 2006;27:1642–6. https://doi.org/10.1111/j.1745-7254.2006.00440.x
Ding C, Zhang Y, Chen H, Yang Z, Wild C, Chu L, et al. Novel nitrogen-enriched oridonin analogues with thiazole-fused A-ring: protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J Med Chem. 2013;56:5048–58. https://doi.org/10.1021/jm400367n
Xu J, Yang J, Ran Q, Wang L, Liu J, Wang Z, et al. Synthesis and biological evaluation of novel 1-O- and 14-O-derivatives of oridonin as potential anticancer drug candidates. Bioorg Med Chem Lett. 2008;18:4741–4. https://doi.org/10.1016/j.bmcl.2008.06.097
Wang L, Ran Q, Da-Hong LI, Yao HQ, Zhang YH, Yuan ST, et al. Synthesis and anti-tumor activity of 14-O-derivatives of natural oridonin. Chin J Nat Med 2011;9:194–8.
Li DH, Wang L, Cai H, Jiang BW, Zhang YH, Sun YJ, et al. Synthesis of novel furozan-based nitric oxide-releasing derivatives of 1-oxo-oridonin with anti-proliferative activity. Chin J Nat Med 2012;10:471–6. https://doi.org/10.3724/Sp.J.1009.2012.00471
Sun P, Wu G, Qiu Z, Chen Y. Preparation of L-alanine-(14-oridonin) ester trifluoroacetate for treatment of cancer. Chinese Patent. 2014:CN 104017000A.
Shen J, Zhang D, Zhao Z, Jia L, Zheng D, Liu G, et al. Synthesis, characterization, in vitro and in vivo evaluation of PEGylated oridonin conjugates. Int J Pharm. 2013;456:80–6. https://doi.org/10.1016/j.ijpharm.2013.08.014
Combes S, Barbier P, Douillard S, McLeer-Florin A, Bourgarel-Rey V, Pierson JT, et al. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. Part 2. J Med Chem. 2011;54:3153–62. https://doi.org/10.1021/jm901826e
Xu S, Pei L, Wang C, Zhang YK, Li D, Yao H, et al. Novel hybrids of natural oridonin-bearing nitrogen mustards as potential anticancer drug candidates. ACS Med Chem Lett. 2014;5:797–802. https://doi.org/10.1021/ml500141f
Xu S, Wang G, Lin Y, Zhang Y, Pei L, Yao H, et al. Novel anticancer oridonin derivatives possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: Design, synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem Lett. 2016;26:2795–800. https://doi.org/10.1016/j.bmcl.2016.04.068
Xu S, Yao H, Luo S, Zhang YK, Yang DH, Li D, et al. A novel potent anticancer compound optimized from a natural oridonin scaffold induces apoptosis and cell cycle arrest through the mitochondrial pathway. J Med Chem. 2017;60:1449–68. https://doi.org/10.1021/acs.jmedchem.6b01652
Shen QK, Deng H, Wang SB, Tian YS, Quan ZS. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: a novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur J Med Chem. 2019;173:15–31. https://doi.org/10.1016/j.ejmech.2019.04.005
Luo D, Yi Y, Peng K, Liu T, Yang J, Liu S, et al. Oridonin derivatives as potential anticancer drug candidates triggering apoptosis through mitochondrial pathway in the liver cancer cells. Eur J Med Chem. 2019;178:365–79. https://doi.org/10.1016/j.ejmech.2019.06.006
Li H, Mu J, Sun J, Xu S, Liu W, Xu F, et al. Hydrogen sulfide releasing oridonin derivatives induce apoptosis through extrinsic and intrinsic pathways. Eur J Med Chem. 2020;187:111978 https://doi.org/10.1016/j.ejmech.2019.111978
Yao H, Xie S, Ma X, Liu J, Wu H, Lin A, et al. Identification of a Potent Oridonin Analogue for Treatment of Triple-Negative Breast Cancer. J Med Chem. 2020;63:8157–78. https://doi.org/10.1021/acs.jmedchem.0c00408
Ding C, Zhang Y, Chen H, Wild C, Wang T, White MA, et al. Overcoming synthetic challenges of oridonin a-ring structural diversification: regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position. Org Lett. 2013;15:3718–21.
Shen X, Zhao L, Chen P, Gong Y, Liu D, Zhang X, et al. A thiazole-derived oridonin analogue exhibits antitumor activity by directly and allosterically inhibiting STAT3. J Biol Chem. 2019;294:17471–86. https://doi.org/10.1074/jbc.RA119.009801
Ding C, Zhang Y, Chen H, Yang Z, Wild C, Ye N, et al. Oridonin ring A-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J Med Chem. 2013;56:8814–25. https://doi.org/10.1021/jm401248x
Ding C, Wang L, Chen H, Wild C, Ye N, Ding Y, et al. ent-Kaurane-based regio- and stereoselective inverse electron demand hetero-Diels-Alder reactions: synthesis of dihydropyran-fused diterpenoids. Org Biomol Chem. 2014;12:8442–52. https://doi.org/10.1039/c4ob01040j
Huang SX, Xiao WL, Li LM, Li SH, Zhou Y, Ding LS, et al. Bisrubescensins A-C: three new dimeric ent-kauranoids isolated from isodon rubescens. Org Lett. 2006;8:1157–60. https://doi.org/10.1021/ol0531379
Ding Y, Ding C, Ye N, Liu Z, Wold EA, Chen H, et al. Discovery and development of natural product oridonin-inspired anticancer agents. Eur J Med Chem. 2016;122:102–17. https://doi.org/10.1016/j.ejmech.2016.06.015
Shen QK, Chen ZA, Zhang HJ, Li JL, Liu CF, Gong GH, et al. Design and synthesis of novel oridonin analogues as potent anticancer agents. J Enzym Inhib Med Chem. 2018;33:324–33. https://doi.org/10.1080/14756366.2017.1419219
Ding Y, Li D, Ding C, Wang P, Liu Z, Wold EA, et al. Regio- and stereospecific synthesis of oridonin d-ring aziridinated analogues for the treatment of triple-negative breast cancer via mediated irreversible covalent warheads. J Med Chem. 2018;61:2737–52. https://doi.org/10.1021/acs.jmedchem.7b01514
Li D, Wang H, Ding Y, Zhang Z, Zheng Z, Dong J, et al. Targeting the NRF-2/RHOA/ROCK signaling pathway with a novel aziridonin, YD0514, to suppress breast cancer progression and lung metastasis. Cancer Lett. 2018;424:97–108. https://doi.org/10.1016/j.canlet.2018.03.029
Wang L, Li D, Xu S, Cai H, Yao H, Zhang Y, et al. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur J Med Chem. 2012;52:242–50. https://doi.org/10.1016/j.ejmech.2012.03.024
Li D, Cai H, Jiang B, Liu G, Wang Y, Wang L, et al. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur J Med Chem. 2013;59:322–8. https://doi.org/10.1016/j.ejmech.2012.11.002
Xu S, Yao H, Hu M, Li D, Zhu Z, Xie W, et al. 6,7-Seco-ent-Kauranoids derived from oridonin as potential anticancer agents. J Nat Prod. 2017;80:2391–8. https://doi.org/10.1021/acs.jnatprod.7b00057
Li D, Xu S, Cai H, Pei L, Wang L, Wu X, et al. Library construction and biological evaluation of enmein-type diterpenoid analogues as potential anticancer agents. Chem Med Chem. 2013;8:812–8. https://doi.org/10.1002/cmdc.201200559
Li D, Xu S, Cai H, Pei L, Zhang H, Wang L, et al. Enmein-type diterpenoid analogs from natural kaurene-type oridonin: Synthesis and their antitumor biological evaluation. Eur J Med Chem. 2013;64:215–21. https://doi.org/10.1016/j.ejmech.2013.04.012
Hu X, Bai Z, Qiao J, Li H, Xu S, Wang X, et al. Effective enmein-type mimics of clinical candidate HAO472: Design, synthesis and biological evaluation. Eur J Med Chem. 2019;171:169–79. https://doi.org/10.1016/j.ejmech.2019.03.046
Antoni F, Bernhardt G. Derivatives of nitrogen mustard anticancer agents with improved cytotoxicity. Arch Pharm (Weinh). 2021;354:e2000366 https://doi.org/10.1002/ardp.202000366
Yao H, Liu J, Xu S, Zhu Z, Xu J. The structural modification of natural products for novel drug discovery. Expert Opin Drug Disco. 2017;12:121–40. https://doi.org/10.1080/17460441.2016.1272757
Wang S, Dong G, Sheng C. Structural simplification of natural products. Chem Rev. 2019;119:4180–220. https://doi.org/10.1021/acs.chemrev.8b00504
Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–71. https://doi.org/10.1158/1535-7163.MCT-06-0174
Guan C, Waxman DJ. Role of cellular glutathione and glutathione S-transferase in the expression of alkylating agent cytotoxicity in human breast cancer cells. Biochem Pharm. 1994;47:1079.
Kumar D, Sharma P, Singh H, Nepali K, Gupta GK, Jain SK, et al. The value of pyrans as anticancer scaffolds in medicinal chemistry. Rsc Adv. 2017;7:36977–99. https://doi.org/10.1039/c7ra05441f
Sutanto F, Konstantinidou M, Domling A. Covalent inhibitors: a rational approach to drug discovery. RSC Med Chem. 2020;11:876–84. https://doi.org/10.1039/d0md00154f
Luo X, Pu JX, Xiao WL, Zhao Y, Gao XM, Li XN, et al. Cytotoxic ent-kaurane diterpenoids from Isodon rubescens var. lushiensis. J Nat Prod. 2010;73:1112–6. https://doi.org/10.1021/np100110u
Zou J, Du X, Pang G, Shi YM, Wang WG, Zhan R, et al. Ternifolide A, a new diterpenoid possessing a rare macrolide motif from Isodon ternifolius. Org Lett. 2012;14:3210–3. https://doi.org/10.1021/ol3013205
Li X, Pu JX, Weng ZY, Zhao Y, Zhao Y, Xiao WL, et al. 6,7-seco-ent-kaurane diterpenoids from Isodon sculponeatus with cytotoxic activity. Chem Biodivers. 2010;7:2888–96. https://doi.org/10.1002/cbdv.200900302
Gong J, Lin G, Sun W, Li CC, Yang Z. Total synthesis of (+/-) maoecrystal V. J Am Chem Soc. 2010;132:16745–6. https://doi.org/10.1021/ja108907x
Sidoryk K, Switalska M, Wietrzyk J, Jaromin A, Pietka-Ottlik M, Cmoch P, et al. Synthesis and biological evaluation of new amino acid and dipeptide derivatives of neocryptolepine as anticancer agents. J Med Chem. 2012;55:5077–87. https://doi.org/10.1021/jm300468t
Krecmerova M. Amino acid ester prodrugs of nucleoside and nucleotide antivirals. Mini Rev Med Chem. 2017;17:818–33. https://doi.org/10.2174/1389557517666170216151601
Tao L, Li Y, Guo X, Dong L, Liu L, Wang Q, et al. Synthesis and anti-CVB3 activity of 4-amino acid derivative substituted pyrimidine nucleoside analogues. Bioorg Med Chem Lett. 2020;30:126770 https://doi.org/10.1016/j.bmcl.2019.126770
Ma S, Tan W, Du B, Liu W, Li W, Che D, et al. Oridonin effectively reverses cisplatin drug resistance in human ovarian cancer cells via induction of cell apoptosis and inhibition of matrix metalloproteinase expression. Mol Med Rep. 2016;13:3342–8. https://doi.org/10.3892/mmr.2016.4897
Zhang Y, Wang L, Zi Y, Zhang L, Guo Y, Huang Y. Oridonin effectively reverses the drug resistance of cisplatin involving induction of cell apoptosis and inhibition of MMP expression in human acute myeloid leukemia cells. Saudi J Biol Sci. 2017;24:678–86. https://doi.org/10.1016/j.sjbs.2017.01.042
Weng H, Huang H, Dong B, Zhao P, Zhou H, Qu L. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Cancer Res. 2014;74:4409–19. https://doi.org/10.1158/0008-5472.CAN-13-1748
Zheng W, Zhou CY, Zhu XQ, Wang XJ, Li ZY, Chen XC, et al. Oridonin enhances the cytotoxicity of 5-FU in renal carcinoma cells by inducting necroptotic death. Biomed Pharmacother. 2018;106:175–82. https://doi.org/10.1016/j.biopha.2018.06.111
Zhang D, Zhou Q, Huang D, He L, Zhang H, Hu B, et al. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 2019;513:594–601. https://doi.org/10.1016/j.bbrc.2019.04.011
Wang B, Shen C, Li Y, Zhang T, Huang H, Ren J, et al. Oridonin overcomes the gemcitabine resistant PANC-1/Gem cells by regulating GST pi and LRP/1 ERK/JNK signalling. Onco Targets Ther. 2019;12:5751–65. https://doi.org/10.2147/OTT.S208924
Xiao X, He Z, Cao W, Cai F, Zhang L, Huang Q, et al. Oridonin inhibits gefitinib-resistant lung cancer cells by suppressing EGFR/ERK/MMP-12 and CIP2A/Akt signaling pathways. Int J Oncol. 2016;48:2608–18. https://doi.org/10.3892/ijo.2016.3488
Huang H, Weng H, Dong B, Zhao P, Zhou H, Qu L. Oridonin triggers chaperon-mediated proteasomal degradation of BCR-ABL in leukemia. Sci Rep. 2017;7:41525 https://doi.org/10.1038/srep41525
Shan QQ, Guo Y, Gong YP, Lin J, Wang YS. Anti-Leukemia Effect and Mechanism of Oridonin on Imatinib-Sensitive and Imatinib-Resistant K562 Cells. Zhongguo shi yan xue ye xue za zhi. 2017;25:1378–83. https://doi.org/10.7534/j.issn.1009-2137.2017.05.017
Acknowledgements
This work was supported by the National Natural Sciences Foundations of China (No. 81703541 and U2004123 for Sai-Yang Zhang and No. 81673322 for Yan-Bing Zhang) and China Postdoctoral Science Foundation (No. 2018M632812 for Sai-Yang Zhang). Henan Association of Science and Technology (No. 2020HYTP056 for Sai-Yang Zhang, China) and Science and Technology Department of Henan Province (No. 20202310144, for Sai-Yang Zhang, China). The open fund of state key laboratory of Pharmaceutical Biotechnology, Nan-jing University, China (Grant no. KF-GN-202101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, YF., Liu, XJ., Pang, XJ. et al. Recent progress of oridonin and its derivatives for cancer therapy and drug resistance. Med Chem Res 30, 1795–1821 (2021). https://doi.org/10.1007/s00044-021-02779-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02779-6