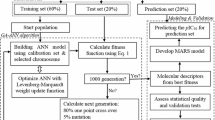
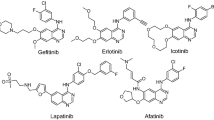
Abstract
Many compounds have been proposed and tested as human epidermal growth factor receptor (EGFR) inhibitors for cancer treatment. Recently, new survival mechanisms of cancer cells have been discovered with the consequent resistance to therapy, which makes it necessary to search for new anticancer drugs. Here we perform a quantitative structure-activity relationship (QSAR) study on 290 compounds reported in the literature as EGFR inhibitors to analyze the molecular properties that may influence their activity. A large number of nonconformational descriptors (17,974) were explored including molecular descriptors, flexible molecular descriptors, and combination of both. To avoid ambiguities derived from the existence of several conformational states, only constitutional and topological molecular descriptors have been considered. The models were validated through Y-randomization, cross-validation, and mean absolute error criteria. A simple model involving flexible descriptors shows the best predictive performance and suggests that the presence of multiple aromatic rings and amino groups in a compound structure may increase its EGFR inhibitory activity.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- QSAR:
-
Quantitative structure-activity relationship
- U.S. FDA:
-
United State Food and Drug Administration
- IC50 :
-
The inhibitory activity was expressed as the concentration of the test compound that inhibited the activity of EGFR by 50%
- pIC50 :
-
The logarithmic molar IC50 values
- SMILES:
-
Molecular-input line-entry system
- SR:
-
Structural representation
- HSG:
-
Hydrogen-suppressed graph
- HFG:
-
Hydrogen-filled graph
- GAO:
-
Graph of atomic orbitals
- SA:
-
Structural attributes
- DCW:
-
Defined flexible descriptor
- CW:
-
Correlation weights
- MC:
-
Monte Carlo simulation
- T:
-
Threshold value
- BSM:
-
Balanced subsets method (BSM)
- k-MCA:
-
k-means cluster analysis
- RM:
-
Replacement method
- MLR:
-
Multivariable linear regression
- Loo:
-
Leave-one-out cross-validation
- \(R_{{\rm{Loo}}}^2\) :
-
Loo variance
- MAE:
-
Mean absolute error
- AD:
-
Applicability domain
- h i :
-
Calculated leverage value
- h * :
-
Warning leverage value
- S Val :
-
Standard deviation in the validation set
- F:
-
Fisher parameter
- o(2.5S) :
-
Number of outlier compounds in the training set
References
Arteaga CL, Engelman JA (2014) ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25:282–303
Barber TD, Vogelstein B, Kinzler KW, Velculescu VE (2004) Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med 351:2883
Bathini R, Sivan SK, Fatima S, Manga V (2016) Molecular docking, MM/GBSA and 3D-QSAR studies on EGFR inhibitors. J Chem Sci 128:1163–1173
Cai X, Zhai H-X, Wang J, Forrester J, Qu H, Yin L, Lai C-J, Bao R, Qian C (2010) Discovery of 7-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a potent multi-acting HDAC, EGFR, and HER2 inhibitor for the treatment of cancer. J Med Chem 53:2000–2009
Chauhan J, Dhanda S, Singla D (2014) The open source drug discovery; Agarwal, SM; Raghava, GPS QSAR-based models for designing quinazoline/imidazothiazoles/pyrazolopyrimidines based inhibitors against wild and mutant EGFR. PLoS ONE 9:e101079
Curran MP (2010) Lapatinib: in postmenopausal women with hormone receptor-positive, HER2-positive metastatic breast cancer. Drugs 70:1411–1422
Dassault Systèmes Biovia (2017) Discovery Studio Modeling Environment. https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/visualization.html Accessed 28 July 2018.
Duchowicz PR, Castro EA, Fernández FM (2006) Alternative algorithm for the search of an optimal set of descriptors in QSAR-QSPR studies. MATCH Commun Math Comput Chem 55:179–192
Duchowicz PR, Comelli NC, Ortiz EV, Castro EA (2012) QSAR study for carcinogenicity in a large set of organic compounds. Curr Drug Saf 7:282–288
Duchowicz PR, Fioressi SE, Castro E, Wróbel K, Ibezim NE, Bacelo DE (2017) Conformation‐independent QSAR study on human epidermal growth factor receptor‐2 (HER2) inhibitors. ChemistrySelect 2:3725–3731
Eriksson L, Jaworska J, Worth AP, Cronin MT, Mcdowell RM, Gramatica P (2003) Methods for reliability and uncertainty assessment and for applicability evaluations of classification-and regression-based QSARs. Environ Health Perspect 111:1361
Faghih-Mirzaei E, Sabouri S, Zeidabadinejad L, Abdolahramazani S, Abaszadeh M, Khodadadi A, Shamsadinipour M, Jafari M, Pirhadi S (2019) Metronidazole aryloxy, carboxy and azole derivatives: synthesis, anti-tumor activity, QSAR, molecular docking and dynamics studies. Bioorg Med Chem 27:305–314
Fink BE, Vite GD, Mastalerz H, Kadow JF, Kim S-H, Leavitt KJ, Du K, Crews D, Mitt T, Wong TW (2005) New dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett 15:4774–4779
Fink BE, Norris D, Mastalerz H, Chen P, Goyal B, Zhao Y, Kim S-H, Vite GD, Lee FY, Zhang H (2011) Novel pyrrolo [2, 1-f][1, 2, 4] triazin-4-amines: Dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett 21:781–785
Gaber AA, Bayoumi AH, El-Morsy AM, Sherbiny FF, Mehany AB, Eissa IH (2018) Design, synthesis and anticancer evaluation of 1H-pyrazolo [3, 4-d] pyrimidine derivatives as potent EGFRWT and EGFRT790M inhibitors and apoptosis inducers. Bioorg Chem 80:375–395
Gadaleta D, Mangiatordi GF, Catto M, Carotti A, Nicolotti O (2016) Applicability domain for QSAR models: where theory meets reality. Int J Quant Struct-Prop Relat 1:45–63
Gazit A, Osherov N, Posner I, Yaish P, Poradosu E, Gilon C, Levitzki A (1991) Tyrphostins. II. heterocyclic and. alpha.-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J Med Chem 34:1896–1907
Gazit A, Osherov N, Posner I, Bar-Sinai A, Gilon C, Levitzki A (1993) Tyrphostins. 3. Structure-activity relationship studies of alpha-substituted benzylidenemalononitrile 5-S-aryltyrphostins. J Med Chem 36:3556–3564
Golbraikh A, Tropsha A (2002) Beware ofq2! J Mol Graph Model 20:269–276
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26:694–701
Gupta A, Bhunia S, Balaramnavar V, Saxena A (2011) Pharmacophore modelling, molecular docking and virtual screening for EGFR (HER 1) tyrosine kinase inhibitors. SAR QSAR Environ Res 22:239–263
Hong H, Xie Q, Ge W, Qian F, Fang H, Shi L, Su Z, Perkins R, Tong W (2008) Mold2, molecular descriptors from 2D structures for chemoinformatics and toxicoinformatics. J Chem Inf Model 48:1337–1344
Jutten B, Keulers TG, Schaaf MB, Savelkouls K, Theys J, Span PN, Vooijs MA, Bussink J, Rouschop KM (2013) EGFR overexpressing cells and tumors are dependent on autophagy for growth and survival. Radiother Oncol 108:479–483
Kalyankrishna S, Grandis JR (2006) Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 24:2666–2672
Kroep JR, Linn SC, Boven E, Bloemendal HJ, Baas J, Mandjes IA, Van Den Bosch J, Smit WM, De Graaf H, Schroder CP, Vermeulen GJ, Hop WC, Nortier JW (2010) Lapatinib: clinical benefit in patients with HER 2-positive advanced breast cancer. Neth J Med 68:371–376
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134
Levitzki A, Gazit A (1995) Tyrosine kinase inhibition: an approach to drug development. Science 267:1782–1788
Li H-Q, Yan T, Yang Y, Shi L, Zhou C-F, Zhu H-L (2010) Synthesis and structure–activity relationships of N-benzyl-N-(X-2-hydroxybenzyl)-N′-phenylureas and thioureas as antitumor agents. Bioorg Med Chem 18:305–313
Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, Mills GB, Hortobagyi GN, Mendelsohn J, Hung MC, Fan Z (2010) Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 18:423–435
Lv P-C, Zhou C-F, Chen J, Liu P-G, Wang K-R, Mao W-J, Li H-Q, Yang Y, Xiong J, Zhu H-L (2010) Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 kinase inhibitors. Bioorg Med Chem 18:314–319
Marzaro G, Chilin A, Guiotto A, Uriarte E, Brun P, Castagliuolo I, Tonus F, González-Díaz H (2011) Using the TOPS-MODE approach to fit multi-target QSAR models for tyrosine kinases inhibitors. Eur J Med Chem 46:2185–2192
Mastalerz H, Chang M, Chen P, Dextraze P, Fink BE, Gavai A, Goyal B, Han WC, Johnson W, Langley D, Lee FY, Marathe P, Mathur A, Oppenheimer S, Ruediger E, Tarrant J, Tokarski JS, Vite GD, Vyas DM, Wong H, Wong TW, Zhang H, Zhang G (2007) New C-5 substituted pyrrolotriazine dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett 17:2036–2042
Mastalerz H, Chang M, Chen P, Fink BE, Gavai A, Han W-C, Johnson W, Langley D, Lee FY, Leavitt K (2007) 5-((4-Aminopiperidin-1-yl) methyl) pyrrolotriazine dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett 17:4947–4954
Mastalerz H, Chang M, Gavai A, Johnson W, Langley D, Lee FY, Marathe P, Mathur A, Oppenheimer S, Tarrant J, Tokarski JS, Vite GD, Vyas DM, Wong H, Wong TW, Zhang H, Zhang G (2007) Novel C-5 aminomethyl pyrrolotriazine dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg Med Chem Lett 17:2828–2833
Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Nandi S, Bagchi MC (2010) 3D-QSAR and molecular docking studies of 4-anilinoquinazoline derivatives: a rational approach to anticancer drug design. Mol Diversity 14:27–38
Noolvi MN, Patel HM (2010) 3d QSAR studies on a series of quinazoline derrivatives as tyrosine kinase (egfr) inhibitor: the k-nearest neighbor molecular field analysis approach. J Basic Clin Pharm 1:153
Orman JS, Perry CM (2007) Trastuzumab. Drugs 67:2781–2789
Pohlmann PR, Mayer IA, Mernaugh R (2009) Resistance to trastuzumab in breast cancer. Clin Cancer Res 15:7479–7491
Rojas C, Tripaldi P, Duchowicz PR (2016) A new QSPR study on relative sweetness. Int J Quant Struct-Prop Relat 1:78–93
Roy K (2007) On some aspects of validation of predictive quantitative structure–activity relationship models. Expert Opin Drug Discov 2:1567–1577
Roy K, Kar S, Ambure P (2015) On a simple approach for determining applicability domain of QSAR models. Chemom Intell Lab Syst 145:22–29
Roy K, Das RN, Ambure P, Aher RB (2016) Be aware of error measures. Further studies on validation of predictive QSAR models. Chemom Intell Lab Syst 152:18–33
Ruslin R, Amelia R, Yamin Y, Megantara S, Wu C, Arba M (2019) 3D-QSAR, molecular docking, and dynamics simulation of quinazoline–phosphoramidate mustard conjugates as EGFR inhibitor. J Appl Pharm Sci 9:089–097
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Shinde MG, Modi SJ, Kulkarni VM (2017) QSAR and molecular docking of phthalazine derivatives as epidermal growth factor receptor (EGFR) inhibitors. J Appl Pharm Sci 7:181–191
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR in cancer. Mol Oncol 12:3–20
Singh H, Singh S, Singla D, Agarwal SM, Raghava GP (2015) QSAR based model for discriminating EGFR inhibitors and non-inhibitors using random forest. Biol Direct 10:10
Singh H, Kumar R, Singh S, Chaudhary K, Gautam A, Raghava GP (2016) Prediction of anticancer molecules using hybrid model developed on molecules screened against NCI-60 cancer cell lines. BMC Cancer 16:77
Sun X-Q, Chen L, Li Y-Z, Li W-H, Liu G-X, Tu Y-Q, Tang Y (2014) Structure-based ensemble-QSAR model: a novel approach to the study of the EGFR tyrosine kinase and its inhibitors. Acta Pharm Sin 35:301
Talevi A, Bellera CL, Di Ianni M, Duchowicz PR, Bruno-Blanch LE, Castro EA (2012) An integrated drug development approach applying topological descriptors. Curr Comput Aided Drug Des 8:172–181
Tan X, Lambert PF, Rapraeger AC, Anderson RA (2016) Stress-induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol 26:352–366
The Mathworks I (2018) MATLAB 7.0 and Statistics Toolbox 7.1. http://www.mathworks.com Accessed 29 Mar 2019
Toropova A, Toropov A, Martyanov S, Benfenati E, Gini G, Leszczynska D, Leszczynski J (2012) CORAL: QSAR modeling of toxicity of organic chemicals towards Daphnia magna. Chemom Intell Lab Syst 110:177–181
U.S. Environmental Protection Agency (2016) Estimation Programs Interface Suite. https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface Accessed 6 June 2019
Ueno NT, Zhang D (2011) Targeting EGFR in triple negative breast cancer. J Cancer 2:324
Verma RP, Hansch C (2005) An approach toward the problem of outliers in QSAR. Bioorg Med Chem 13:4597–4621
Walker F, Abramowitz L, Benabderrahmane D, Duval X, Descatoire V, Hénin D, Lehy T, Aparicio T (2009) Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum Pathol 40:1517–1527
Wold S, Eriksson L, Clementi S (1995) Statistical validation of QSAR results. In: van de Waterbeemd H (ed) Chemometric methods in molecular design. Wiley-VCH, Weinheim, p 309–338
Wu P, Nielsen TE, Clausen MH (2016) Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today 21:5–10
Xu G, Abad MC, Connolly PJ, Neeper MP, Struble GT, Springer BA, Emanuel SL, Pandey N, Gruninger RH, Adams M (2008) 4-Amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones as potent ErbB-2/EGFR dual kinase inhibitors. Bioorg Med Chem Lett 18:4615–4619
Xu G, Searle LL, Hughes TV, Beck AK, Connolly PJ, Abad MC, Neeper MP, Struble GT, Springer BA, Emanuel SL (2008) Discovery of novel 4-amino-6-arylaminopyrimidine-5-carbaldehyde oximes as dual inhibitors of EGFR and ErbB-2 protein tyrosine kinases. Bioorg Med Chem Lett 18:3495–3499
Yap CW (2011) PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32:1466–1474
Acknowledgements
We are grateful for financial support provided by the National Research Council of Argentina (CONICET) project PIP11220130100311 and to the Ministerio de Ciencia, Tecnología e Innovación Productiva for access to electronic library facilities. SEF, DEB, and PRD are members of the scientific researcher career of CONICET.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Fioressi, S.E., Bacelo, D.E. & Duchowicz, P.R. QSAR study of human epidermal growth factor receptor (EGFR) inhibitors: conformation-independent models. Med Chem Res 28, 2079–2087 (2019). https://doi.org/10.1007/s00044-019-02437-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02437-y