Abstract
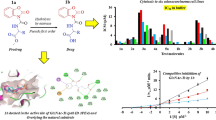
In this study, Mannich bases 2–8, 2-(3-or-5-aminomethyl-4-hydroxy-3-or-5-methoxybenzylidene)indan-1-one, were designed and synthesized starting from 2-(4-hydroxy-3-methoxybenzylidene)indan-1-one, 1. Synthesized compounds were tested against several tumor cell lines and non-tumor cells to evaluate the cytotoxicities of the compounds and to test whether the sequential cytotoxicity hypothesis works on the studied compounds. The data obtained from cytotoxicity tests pointed out that sequential cytotoxicity hypothesis worked on compounds 3 and 4 since they had higher potency selectivity expression values. The leader compound of the present study is compound 4, 2-(3-dipropylaminomethyl-4-hydroxy-5-methoxy-benzylidene)-indan-1-one, since it has the highest potency selectivity expression value among the compounds studied. This molecule can be the leader compound for further studies and designes.



Similar content being viewed by others
References
Bilginer S, Gul HI, Mete E, Das U, Sakagami H, Umemura N, Dimmock JR (2013) 1-(3-aminomethyl-4-hydroxyphenyl)-3-pyridinyl-2-propen-1-ones: a novel group of tumour-selective cytotoxins. J Enzyme Inhib Med Chem 28:974–980
Das S, Das U, Michel D, Gorecki DK, Dimmock JR (2013) Novel 3,5-bis(arylidene)-4-piperidone dimers: potent cytotoxins against colon cancer cells. Eur J Med Chem 64:321–328
Das U, Sharma RK, Dimmock JR (2009) 1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets. Curr Med Chem 16:2001–2020
Dimmock JR, Kumar P, Allen TM, Kao GY, Halleran S, Balzarini J, de Clercq E (1997) Synthesis and cytotoxic evaluation of some carbohydrazones and thiocarbohydrazones of various unsaturated ketones and related Mannich bases. Pharmazie 52:182–186
Gul HI, Calis U, Vepsalainen J (2004) Synthesis of some mono-Mannich bases and corresponding azine derivatives and evaluation of their anticonvulsant activity. Arzneimittelforschung 54:359–364
Gul HI, Gul M, Erciyas E (2003) Toxicity of some bis Mannich bases and corresponding piperidinols in the brine shrimp (Artemia salina) bioassay. J Appl Toxicol 23:53–57
Gul HI, Gul M, Hanninen O (2002) Cytotoxic activities of some mono and bis Mannich bases derived from acetophenone in brine shrimp bioassay. Arzneimittelforschung Drug Res 52:840–843
Gul HI, Ojanen T, Vepsalainen J, Gul M, Erciyas E, Hanninen O (2001a) Antifungal activity of some mono, bis and quaternary Mannich bases derived from acetophenone. Arzneimittelforschung 51:72–75
Gul HI, Sahin F, Gul M, Ozturk S, Yerdelen KO (2005a) Evaluation of antimicrobial activities of several mannich bases and their derivatives. Arch Pharm 338:335–338
Gul HI, Suleyman H, Gul M (2009) Evaluation of the anti-inflammatory activity of N,N’-bis(3-dimethylamino-1-phenyl-propylidene) hydrazine dihydrochloride. Pharm Biol 47:968–972
Gul HI, Tugrak M, Sakagami H (2015) Synthesis of some acrylophenones with N-methylpiperazine and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 31(1):147–151
Gul HI, Yerdelen KO, Das U, Gul M, Pandit B, Li PK, Dimmock JR (2008) Synthesis and cytotoxicity of novel 3-aryl-1-(3’-dibenzylaminomethyl-4’-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull 56:1675–1681
Gul HI, Yerdelen KO, Gul M, Das U, Pandit B, Li PK, Secen H, Sahin F (2007) Synthesis of 4’-hydroxy-3’-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm 340:195–201
Gul M, Atalay M, Gul HI, Nakao C, Lappalainen J, Hanninen O (2005b) The effects of some Mannich bases on heat shock proteins HSC70 and GRP75, and thioredoxin and glutaredoxin levels in Jurkat cells. Toxicol In Vitro 19:573–580
Gul M, Gul HI, Das U, Hanninen O (2005c) Biological evaluation and structure-activity relationships of bis-(3-aryl-3-oxo-propyl)-methylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-methyl-4-piperidinol hydrochlorides as potential cytotoxic agents and their alkylating ability towards cellular glutathione in human leukemic T cells. Arzneimittelforschung 55:332–337
Gul M, Gul HI, Vepsalainen J, Erciyas E, Hanninen O (2001b) Effect of acetophenone derived Mannich bases on cellular glutathione level in Jurkat cells. A possible mechanism of action. Arzneimittelforschung 51:679–682
Hou XL, Kong Y, Wang ML, Yan AX (2015) QSAR Studies on the bioactivity of plasmodium falciparum glucose-6-phosphate dehydrogenase inhibitors. Lett Drug Des Discov 12:728–735
Jedhe GS, Paul D, Gonnade RG, Santra MK, Hamel E, Nguyen TL, Sanjayan GJ (2013) Correlation of hydrogen-bonding propensity and anticancer profile of tetrazole-tethered combretastatin analogues. Bioorg Med Chem Lett 23:4680–4684
Motohashi N, Wakabayashi H, Kurihara T, Fukushima H, Yamada T, Kawase M, Sohara Y, Tani S, Shirataki Y, Sakagami H, Satoh K, Nakashima H, Molnar A, Spengler G, Gyemant N, Ugocsai K, Molnar J (2004) Biological activity of barbados cherry (acerola fruits, fruit of Malpighia emarginata DC) extracts and fractions. Phytother Res 18:212–223
Neighbors JD, Salnikova MS, Beutler JA, Wiemer DF (2006) Synthesis and structure-activity studies of schweinfurthin B analogs: evidence for the importance of a D-ring hydrogen bond donor in expression of differential cytotoxicity. Bioorg Med Chem 14:1771–1784
Pradeep PS, Shrungesh Kumar TO, Prashantha N, Mahadevan KM (2015) Synthesis, in vitro antibacterial, toxicity and molecular docking anticancer activity of novel N-[(2-chloroquinolin-3-yl) methylidene]-2-aniline Schiff Bases. Int J Curr Pharm Res 7:37–46
Suleyman H, Gul HI, Gul M, Alkan M, Gocer F (2007) Anti-inflammatory activity of bis(3-aryl-3-oxo-propyl)methylamine hydrochloride in rat. Biol Pharm Bull 30:63–67
Tugrak M, Gul HI, Sakagami H (2015a) Synthesis and cytotoxicities of 2-[4-hydroxy-(3,5-bis-aminomethyl)-benzylidene]-indan-1-ones. Lett Drug Des Discov 12:806–812
Tugrak M, Yamali C, Sakagami H, Gul HI (2015b) Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 31(5):818–823
Yerdelen KO, Gul HI, Sakagami H, Umemura N (2015a) Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem 30:383–388
Yerdelen KO, Gul HI, Sakagami H, Umemura N, Sukuroglu M (2015b) Synthesis and cytotoxic activities of a curcumin analogue and its bis-Mannich derivatives. Lett Drug Des Discov 12:643–649
Acknowledgements
This research work was supported by Ataturk University Research Found (Project No. BAP: 2011/289, 2012/75), Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tugrak, M., Gul, H.I., Sakagami, H. et al. Synthesis and anticancer properties of mono Mannich bases containing vanillin moiety. Med Chem Res 26, 1528–1534 (2017). https://doi.org/10.1007/s00044-017-1833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1833-x