Abstract
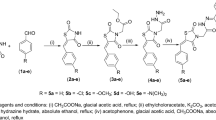
A series of indole-2-carboxylic acid linked 3-phenyl-2-alkoxy propanoic acids has been designed and synthesized employing Suzuki coupling reaction. All the six targeted compounds (7a–f) were evaluated for their in vivo antidiabetic activities using rat model while using rosiglitazone as standard drug and streptozotocin was used to induce diabetes. After 4 weeks duration of the experiment, it was shown that all the compounds exhibited significant lowering of blood glucose level (mg/dl) and decrease in body weight gain (g) as compared to the standard drug. Furthermore, molecular docking study was carried out with peroxisome proliferator-activated receptor γ (PPARγ) protein, which showed good affinity of the compounds at the active site. Compound 7b was considered as promising candidate of this series.





Similar content being viewed by others
References
Bajare S, Anthony J, Nair A, Marita R, Damre A, Patel D, Rao C, Sivaramakrishnan H, Deka N (2012) Synthesis of N-(5-chloro-6-(quinolin-3-yloxy)pyridin-3-yl)benzenesulfonamide derivatives as non-TZD peroxisome proliferator-activated receptor γ (PPARγ) agonist. Eur J Med Chem 58:355–360
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Matin A, Gavande N, Kim MS, Yang NX, Salam NK, Hanrahan JR, Roubin RH, Hibbs DE (2009) 7-Hydroxy-benzopyran-4-one derivatives: A novel pharmacophore of peroxisome proliferator-activated Receptor α and -γ (PPARα and γ) dual agonist. J Med Chem 52:6835–6850
Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR (2007) Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15:1258–1271
De Groot JC, Weidner C, Krausze J, Kawamoto K, Frank C, Schroeder FC, Sauer S, Büssow K (2013) Structural characterization of amorfrutins bound to the peroxisome proliferator-activated receptor γ. J Med Chem 56:1535–1543
Einstein M, Akiyama TE, Castriota GA, Wang CF, McKeever B, Mosley RT, Becker JW, Moller DE, Meinke PT, Wood HB, Berger JP (2008) The differential interactions of peroxisome proliferator-activated receptor γ ligands with Tyr473 is a physical basis for their unique biological activities. Mol Pharmacol 73:62–74
Epple R, Cow C, Xie Y, Azimioara M, Russo R, Wang X, Wityak J, Karanewsky DS, Tuntland T, Nguyen-Tran VTB, Ngo CC, Huang D, Saez E, Spalding T, Gerken A, Iskandar M, Seidel HM, Tian SS (2010) Novel bisaryl substituted thiazoles and oxazoles as highly potent and selective peroxisome proliferator-activated receptor δ agonists. J Med Chem 53:77–105
Guasch L, Sala E, Valls C, Blay M, Mulero M, Arola L, Pujadas G, Garcia-Vallve S (2011) Structural insights for the design of new PPARgamma partial agonists with high binding affinity and low transactivation activity. J Comput Aided Mol Des 25:717–728
Hopkins CR, O’Neil SV, Laufersweiler MC, Wang Y, Pokross M, Mekel M, Evdokimov A, Walter R, Kontoyianni M, Petrey ME, Sabatakos G, Roesgen JT, Richardson E, Demuth TP (2006) Design and synthesis of novel N-sulfonyl-2-indole carboxamides as potent PPAR-gamma binding agents with potential application to the treatment of osteoporosis. Bioorg Med Chem Lett 16:5659–5663
Kroker AJ, Bruning JB (2015) Review of the structural and dynamic mechanisms of PPARγ partial agonism. PPAR Res 2015:1–15
Kuhad A, Chopra K (2008) Effect of sesamol on diabetes-associated cognitive decline in rats. Exp Brain Res 185:411–420
Kuhad A, Sethi R, Chopra K (2008) Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci 83:128–134
Liu W, Lau F, Liu K, Wood HB, Zhou G, Chen Y, Li Y, Akiyama TE, Castriota G, Einstein M, Wang C, McCann ME, Doebber TW, Wu M, Chang CH, McNamara L, McKeever B, Mosley RT, Berger JP, Meinke PT (2011) Benzimidazolones: A new class of selective peroxisome proliferator-activated receptor γ (PPARγ) Modulators. J Med Chem 53:8541–8554
Pirat C, Farce A, Lebegue N, Renault N, Furman C, Millet R, Yous S, Speca S, Berthelot P, Desreumaux P, Chavatte P (2012) Targeting peroxisome proliferator-activated receptors (PPARs): Development of modulators. J Med Chem 55:4027–4061
Seto S, Okada K, Kiyota K, Isogai S, Iwago M, Shinozaki T, Kitamura Y, Kohno Y, Murakami K (2010) Design, synthesis, and structure-activity relationship studies of Novel 2,4,6-trisubstituted-5-pyrimidinecarboxylic acids as peroxisome proliferator-activated receptor γ (PPARγ) partial agonists with comparable antidiabetic efficacy to rosiglitazone. J Med Chem 53:5012–5024
Sybyl 7.3 (2006) Tripos Inc. 1699 Hanley Road, St. Louis 63144, USA
Verma RK, Ghosh P, Kumar V, Wadhwa LK (2012a) The application of comparative molecular field analysis for the design of α-anilino substituted-3-phenyl propanoic acids as novel PPARα/γ dual ligands. Med Chem Res 21:2873–2884
Verma RK, Kumar V, Ghosh P, Wadhwa LK (2012b) Heterocyclyl linked anilines and benzaldehydes as precursors for biologically significant new chemical entities. J Chem Sci 124:1063–1069
Verma RK, Kumar V, Ghosh P, Wadhwa LK (2013a) 3D-QSAR study of tyrosine and propanoic acid derivatives as PPARα/γ dual agonists using CoMSIA. Med Chem Res 22:287–302
Verma RK, Mall R, Ghosh P, Kumar V (2013b) Design and synthesis of benzimidazole-linked meta-substituted benzylidenes/benzyls as biologically significant new chemical entities. Synth Commun 43:1882–1895
Verma RK, Mall R, Singh A (2015) Indolyl linked meta-substituted benzylidene-based novel PPAR ligands: synthetic and docking studies. Med Chem Res 24:1396–1407
Verma RK, Mall R, Singh A (2016) Novel indole derivatives as anti-diabetic agents and method thereof. IN Patent 201611030034, filled 2 September 2016
Acknowledgements
Authors are thankful to the Punjabi University, Patiala authorities for providing the necessary research facilities. We are also grateful to Manish Kumar and Anoop Patyal of Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, respectively, for extending the facilities for spectral analysis of the compounds reported in this paper. One of the authors (A.S.) is thankful to UGC, New Delhi for awarding the UGC-BSR Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Singh, A., Verma, R.K., Kuhad, A. et al. Novel indole-2-carboxylic acid linked 3-phenyl-2-alkoxy propanoic acids: Synthesis, molecular docking and in vivo antidiabetic studies. Med Chem Res 26, 745–759 (2017). https://doi.org/10.1007/s00044-017-1791-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1791-3