Abstract
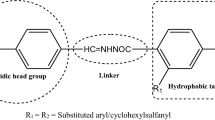
A novel series of thiazolidine-2,4-diones was designed, synthesized and investigated for anti-diabetic activity. The (2,4-dioxo-1,3-thiazolidin-5-yl)methylphenylbenzamide derivatives contain an amide linkage between the central aryl ring and the hydrophobic tail. Structures of the compounds were confirmed by spectroscopic techniques fourier transform infrared spectroscopy, 1H-nuclear magnetic resonance and elemental (C, H, N) analysis. The synthesized compounds were evaluated for their in-vivo pharmacological activity (blood glucose and triglyceride lowering activity), where compounds thiazolidinediones-C and thiazolidinediones-E showed significant effects, comparable to the standard pioglitazone. Computational studies positively substantiated the nature and interaction of the designed compounds with peroxisome proliferator-activated receptor gamma.




Similar content being viewed by others
References
Bedir A, Aliyazicioglu Y, Kahraman H, Yurdakul Z, Uysal M, Suvaci DE, Okuyucu A, Kelek MH, Alvur M (2006) Genotoxicity in rats treated with the antidiabetic agent, rosiglitazone. Environ Mol Mutagen 47:718–724
Bernardon JM, Clary L (2002) (Galerma Research and Development, Fr.) preparation of biphenyl derivatives and their use as PPARc receptor agonist. PCT Int Appl, pp 113
Cantello BCC, Cawthorne MA, Cottam GP, Duff PT, Haigh D, Hindley RM, Lister CA, Smith SA, Thurlby PL (1994) [[ö-(Heterocyclylamino) alkoxy]- benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. J Med Chem 37:3977–3985
De Fronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32:S157–S163. doi:10.2337/dc09-S302
Dumasia R, Eagle KA, Kline-Rogers E, May N, Cho L, Mukherjee D (2005) Role of PPAR- gamma agonist thiazolidinediones in treatment of pre-diabetic and diabetic individuals: a cardiovascular perspective. Curr Drug Targets Cardiovasc Haematol Disord 5:377–386
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365:1415–1428
Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu Y, Iwatsuka H, Suzuoki Z (1983) Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)-benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, ciglitazone), a new antidiabetic agent. Diabetes 32:804
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1989) Vogels text book of practical organic chemistry. John Wiley & Sons, New York
Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS (2014) In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 21(4):415–422. doi:10.1016/j.phymed.2013.10.015
Hulin B, Clark DA, Goldstein SW, McDermott RE, Dambek PJ, Kappeler WH, Lamphere CH, Lewis DM, Rizzi JP (1992) Novel thiazolidine-2,4-diones as potent euglycemic agents. J Med Chem 35(10):1853–1864
Kahn CR, Vicent D, Doria A (1996) Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu Rev Med 47:509–531
Kim D, Choi SS, Kim S, Yun BS, Yoo ID, Reddy NR, Yoon HS, Kim KT (2006) Isoliquiritigenin selectivity inhibits H(2) histamine receptor signaling. Mol Pharmacol 70:493–500
Kokil GR, Veedu RN, Ramm GA, Prins JB, Parekh HS (2015) Type 2 diabetes mellitus: limitations of conventional therapies and intervention with nucleic acid-based therapeutics. Chem Rev 115:4719–4743. doi:10.1021/cr5002832
Kopelman PG, Hitman GA (1998) Diabetes. Exploding type II. Lancet 325:19–26
Masiello P1, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G (1998) Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 47(2):224–229
Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442
Mehendale-Munj S, Ghosh R, Ramaa CS (2011) Synthesis and evaluation of the hypoglycemic and hypolipidemic activity of novel 5-benzylidene-2, 4-thiazolidinedione analogs in a type-2 diabetes model. Med Chem Res 20(5):642–647
Mlinar B, Marc J, Janez A, Pfeifer M (2007) Molecular mechanisms of insulinresistance and associated diseases. Clinica Chimica Acta 375:20–35
Muniyappa R, Lee S, Chen H, Quon MJ (2008) Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294:E15–E26
Oguchi M1, Wada K, Honma H, Tanaka A, Kaneko T, Sakakibara S, Ohsumi J, Serizawa N, Fujiwara T, Horikoshi H, Fujita T (2000) Molecular design, synthesis, and hypoglycemic activity of a series of thiazolidine-2,4-diones. J Med Chem 43:3052–3066
Organization for Economic Coeoperation and Development (OECD), OECD Guidelines (2001) Testing of Chemicals e425 (Acute Oral Toxicity up and Down Procedure). http://www.oecd.org. Accessed 23 May 2014
Parekh NM, Juddhawala KV, Rawal BM (2013) Antimicrobial activity of thiazolyl benzenesulfonamide-condensed 2,4-thiazolidinediones derivatives. Med Chem Res 22:2737–2745
Scheen AJ (2001) Thiazolidinediones and liver toxicity. Diabetes Metab 27:305
Skyler JS (2004) Diabetes mellitus: pathogenesis and treatment strategies. J Med Chem 47:4113–4117
Storr T1, Mitchell D, Buglyó P, Thompson KH, Yuen VG, McNeill JH, Orvig C (2003) Vanadyl-thiazolidinedione combination agents for diabetes therapy. Bioconjug Chem 14:212–21
Wild S1, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053
Yanagisawa H, Takamura M, Yamada E, Fujita S, Hagisawa Y (2000) Novel oximes having 5-benzyl-2,4-thiazolidinedione as antihyperglycemic agents: synthesis and structure-activity relationship. Bioorg Med Chem Lett 10:373–375
Acknowledgments
We would like to thank Indian Institute of Technology (BHU) for providing financial assistance through Sprouting grant for faculty (SGF) and Institute research project (IRP) scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shrivastava, S.K., Batham, A., Sinha, S.K. et al. Design, synthesis and evaluation of novel thiazolidinedione derivatives as anti-hyperglycemic and anti-hyperlipidemic agents. Med Chem Res 25, 2258–2266 (2016). https://doi.org/10.1007/s00044-016-1675-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1675-y