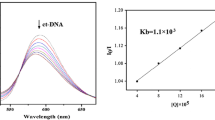
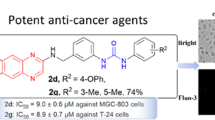
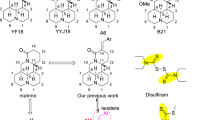
Abstract
Novel ursolic acid derivatives were synthesized, and their structures were confirmed by MS, IR, 1H NMR and 13C NMR spectral analysis. In vitro antitumor activities of these compounds against MGC-803 (gastric cancer cell) and Bcap-37 (breast cancer cell) human cancer cell lines were evaluated by MTT assay. The pharmacological screening results revealed that many derivatives exhibited moderate to high activities against the tested cell lines, and that most demonstrated more potent inhibitory activities than that of ursolic acid. Preliminarily mechanism study of representative compound 3h were carried out by acridine orange/ethidium bromide staining, Hoechst 33258 staining, terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling assay, and flow cytometry which indicated that compound 3h can induce cell apoptosis of MGC-803 cells, and the apoptosis ratio reached 34.59 % after 36 h treatment at 10 μM.






Similar content being viewed by others
Abbreviations
- ADM:
-
Adriamycin
- AO/EB:
-
Acridine orange/ethidium bromide
- 13C NMR:
-
13C Nuclear magnetic resonance
- DMF:
-
N,N-dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- EDCI:
-
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- HCPT:
-
10-Hydroxyl camptothecine
- HOBt:
-
1-Hydroxybenzotriazole
- 1H NMR:
-
Proton nuclear magnetic resonance
- IR:
-
Infra-red
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TUNEL:
-
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
- UA:
-
Ursolic acid
References
Achiwa Y, Hasegawa K, Udagawa Y (2005) Molecular mechanism of ursolic acid induced apoptosis in poorly differentiated endometrial cancer HEC108 cells. Oncol Rep 14:507–512
Babalola IT, Shode FO (2013) Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J Pharmacogn Phytochem 2:214–222
Chadalapaka GJI, Mclees AA (2008) Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett 18:2633–2639
Checker R, Sandur SK, Sharma D, Patwardhan RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB, Sainis KB (2012) Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS One 7:e31318
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Farha AK, Geetha BS, Mangalam SN, Dhanya SR, Latha PG, Remani P (2013) Apoptosis mediated cytotoxicity induced by isodeoxyelephantopin on nasopharyngeal carcinoma cells. Asian J Pharm Clin Res 6:51–56
Furtado RA, Rodrigues ÉP, Araújo FRR, Oliveira WL, Furtado MA, Castro MB, Cunha WR, Tavares DC (2008) Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon. Toxicol Pathol 36:576–580
Gao N, Cheng S, Budhraja A, Gao Z, Chen J, Liu EH, Huang C, Chen D, Yang Z, Liu Q, Li P, Shi X, Zhang Z (2012) Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br J Pharmacol 165:1813–1826
Guo L, Wu JZ, Han T, Cao T (2008) Chemical composition, antifugal and antitumor properties of ether extracts of Scapania verrucosa Heeg. and its endophytic fungus Chaetomium fusiforme. Molecules 13:2114–2125
Han B, Peng Z (2014) Anti-HIV triterpenoid components. J Chem Pharm Res 6:438–443
Hanai A, Yang WL, Ravikumar TS (2001) Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome C release. Int J Cancer 93:526–533
Harmand PO, Duval R, Liagre B, Jayat-Vignoles C, Beneytout JL, Delage C, Simon A (2003) Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int J Oncol 23:105–112
Honda T, Finlay HJ, Gribble GW (1997) New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 7:1623–1628
Honda T, Gribble GW, Suh N (2000a) Novel synthetic oleanane and ursane triterpenoids with various enone functionalities in ring a as inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:1866–1877
Honda T, Rounds BAV, Bore L, Finaly HJ, Facaloro FG (2000b) Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:4233–4246
Honda T, Rounds BV, Bore L (1999) Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 9:3429–3434
Hsu YL, Kuo PL, Lin CC (2004) Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci 75:2303–2316
Hua SX, Huang RZ, Ye MY, Pan YM, Yao GY, Zhang Y, Wang HS (2015) Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur J Med Chem 95:435–452
Ikeda Y, Murakami A, Ohigashi H (2008) Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res 52:26–42
Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A (2009) Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules 14:2016–2031
Kashiwada Y, Nagao T, Hashimoto A, Ikeshiro Y, Okabe H, Cosentino LM, Lee KH (2000) Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J Nat Prod 63:1619–1622
Kashiwada Y, Wang HK, Nagao T, Kitanaka S, Yasuda I, Fujioka T, Yamagishi T, Cosentino LM, Kozuka M, Okabe H, Ikeshiro Y, Hu CQ, Yeh E, Lee KH (1998) Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod 61:1090–1095
Liobikas J, Majiene D, Trumbeckaite S, Kursvietiene L, Masteikova R, Kopustinskiene DM, Savickas A, Bernatoniene J (2011) Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J Nat Prod 74:1640–1644
Liu MC, Yang SJ, Jin LH, Hu DY, Wu ZB, Yang S (2012) Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 17:6156–6169
Liu MC, Yang SJ, Jin LH, Hu DY, Xue W, Song BA, Yang S (2012) Synthesis and cytotoxicity of novel ursolic acid derivatives containing an acyl piperazine moiety. Eur J Med Chem 58:128–135
Ma CM, Cai SQ, Cui JR, Wang RQ, Tu PF, Masao H, Mohsen D (2005) The cytotoxic activity of ursolic acid derivatives. Eur J Med Chem 40:582–589
Meng Y, Song Y, Yan Z, Xia Y (2010) Synthesis and in vitro cytotoxicity of novel ursolic acid derivatives. Molecules 15:4033–4040
Meng YQ, Liu D, Cai LL (2009) The synthesis of ursolic acid derivatives with cytotoxic activity and the investigation of their preliminary mechanism of action. Bioorg Med Chem 17:848–854
Mitsuda S, Yokomichi T, Yokoigawa J, Kataoka T (2014) Ursolic acid, a natural pentacyclic triterpenoid, inhibits intracellular trafficking of proteins and induces accumulation of intercellular adhesion molecule-1 linked to high-mannose-type glycans in the endoplasmic reticulum. FEBS Open Bio 4:229–239
do Nascimento PG, Lemos TL, Bizerra AM, Arriaga ÂM, Ferreira DA, Santiago GM, Braz-Filho R, Costa JG (2014) Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 19:1317–1327
Ovesná Z, Vachálková A, Horváthová K, Tóthová D (2004) Pentacyclic triterpenoic acids: new chemoprotective compounds. Neoplasma 51:327–333
Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A (2013) Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol 85:1579–1587
Shao JW, Dai YC, Xue JP, Wang JC, Lin FP, Guo YH (2011) In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur J Med Chem 46:2652–2661
Somova LO, Nadar A, Rammanan P, Shode FO (2003) Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10:115–121
Weng H, Tan ZJ, Hu YP, Shu YJ, Bao RF, Jiang L, Wu XS, Li ML, Ding Q, Wang XA, Xiang SS, Li HF, Cao Y, Tao F, Liu YB (2014) Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int 14:96
Xu X, Gao X, Jin L, Bhadury PS, Yuan K, Hu D, Song B, Yang S (2011) Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Div 6:14
Yoo KH, Park JH, Cui EJ, Kim KI, Kim JY, Kim J, Hong SG, Baek NI, Chung IS (2012) 3-O-acetyloleanolic acid induces apoptosis in human colon carcinoma HCT-116 cells. Phytother Res 26:1541–1546
Zhu RX, Zhao L, Zhang YK, Luo P, Liu SH, Wang JG (2015) Sequential treatment with ursolic acid chlorophenyl triazole followed by 5-fluorouracil shows synergistic activity in small cell lung cancer cells. Bangladesh J Pharmacol 10:197–204
Acknowledgments
The authors wish to thank the Key Technologies R&D Program (2014BAD23B01), National Nature Science Foundation of China (21372052, 21462012), Research Project of Chinese Ministry of Education (213033A), Guizhou Province S&T Program ([2012]6012, [2015]35) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Ming-Chuan Liu and Sheng-Jie Yang contributed equally to this work.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, MC., Yang, SJ., Jin, LH. et al. Synthesis and evaluation as potential antitumor agents of novel ursolic acid derivatives. Med Chem Res 25, 2267–2279 (2016). https://doi.org/10.1007/s00044-016-1680-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1680-1