Abstract
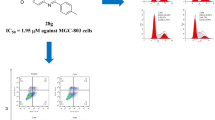
A series of 1,3-diphenylurea quinoxaline derivatives were synthesized and characterized by 1H, 13C NMR, and HR-ESI-MS analyses. In vitro cytotoxicity of the synthesized compounds was evaluated by MTT assay against MGC-803, H460, T-24, HeLa, HepG2, and SMMC-7721 human cancer cell lines. The results showed that most of the compounds exhibited effective cytotoxicity to the tested cancer cell lines. The mechanism of action of the two best active quinoxaline derivatives, compounds 2d and 2g was also investigated. They may be potent anticancer agents.










Similar content being viewed by others
References
Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. https://doi.org/10.1016/j.canlet.2017.04.024.
Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39:22 https://doi.org/10.1186/s40880-019-0368-6.
Tariq S, Somakala K, Amir M. Quinoxaline: An insight into the recent pharmacological advances. Eur J Med Chem. 2018;143:542–57. https://doi.org/10.1016/j.ejmech.2017.11.064.
Qi J, Dong H, Huang J, Zhang S, Niu L, Zhang Y, et al. Synthesis and biological evaluation of N-substituted 3-oxo-1,2,3,4-tetrahydro-quinoxaline-6-carboxylic acid derivatives as tubulin polymerization inhibitors. Eur J Med Chem. 2018;143:8–20. https://doi.org/10.1016/j.ejmech.2017.08.018.
Acharya BR, Chatterjee A, Ganguli A, Bhattacharya S, Chakrabarti G. Thymoquinone inhibits microtubule polymerization by tubulin binding and causes mitotic arrest following apoptosis in A549 cells. Biochimie. 2014;97:78–91. https://doi.org/10.1016/j.biochi.2013.09.025.
Kamal A, Reddy NV, Nayak VL, Reddy VS, Prasad B, Nimbarte VD, et al. Synthesis and biological evaluation of benzo[b]furans as inhibitors of tubulin polymerization and inducers of apoptosis. ChemMedChem. 2014;9:117–28. https://doi.org/10.1002/cmdc.201300366.
Perez-Perez MJ, Priego EM, Bueno O, Martins MS, Canela MD, Liekens S. Blocking blood flow to solid tumors by destabilizing tubulin: an approach to targeting tumor growth. J Med Chem. 2016;59:8685–711. https://doi.org/10.1021/acs.jmedchem.6b00463.
Tantak MP, Klingler L, Arun V, Kumar A, Sadana R, Kumar D. Design and synthesis of bis(indolyl)ketohydrazide-hydrazones: identification of potent and selective novel tubulin inhibitors. Eur J Med Chem. 2017;136:184–94. https://doi.org/10.1016/j.ejmech.2017.04.078.
Horio T, Murata T. The role of dynamic instability in microtubule organization. Front Plant Sci. 2014;5:511 https://doi.org/10.3389/fpls.2014.00511.
Prota AE, Bargsten K, Zurwerra D, Field JJ, Diaz JF, Altmann KH, et al. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587–90. https://doi.org/10.1126/science.1230582.
Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med. Res Rev. 2011;31:443–81. https://doi.org/10.1002/med.20242.
Montana M, Correard F, Khoumeri O, Esteve MA, Terme T, Vanelle P. Synthesis of new quinoxalines containing an oxirane ring by the TDAE strategy and in vitro evaluation in neuroblastoma cell lines. Molecules. 2014;19:14987–98. https://doi.org/10.3390/molecules190914987.
Lan J, Huang L, Lou H, Chen C, Liu T, Hu S, et al. Design and synthesis of novel C14-urea-tetrandrine derivatives with potent anti-cancer activity. Eur J Med Chem. 2018;143:1968–80. https://doi.org/10.1016/j.ejmech.2017.11.007.
Sun S, He Z, Huang M, Wang N, He Z, Kong X, et al. Design and discovery of thioether and nicotinamide containing sorafenib analogues as multikinase inhibitors targeting B-Raf, B-RafV600E and VEGFR-2. Bioorg Med Chem. 2018;26:2381–91. https://doi.org/10.1016/j.bmc.2018.03.039.
Zhan WH, Li YY, Huang WP, Zhao YJ, Yao ZG, Yu SY, et al. Design, synthesis and antitumor activities of novel bis-aryl ureas derivatives as Raf kinase inhibitors. Bioorg Med Chem. 2012;20:4323–9. https://doi.org/10.1016/j.bmc.2012.05.051.
Xu Z, Yang F, Wei D, Liu B, Chen C, Bao Y, et al. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36:1965–77. https://doi.org/10.1038/onc.2016.356.
Li M, Su Y, Zhang F, Chen K, Xu X, Xu L, et al. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and antimiRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomaterialia. 2018;75:413–26. https://doi.org/10.1016/j.actbio.2018.05.049.
Busschaert N, Kirby IL, Young S, Coles SJ, Horton PN, Light ME, et al. Squaramides as potent transmembrane anion transporters. Angew Chem Int Ed Engl. 2012;51:4426–30. https://doi.org/10.1002/anie.201200729.
Chen JN, Wang XF, Li T, Wu DW, Fu XB, Zhang GJ, et al. Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem. 2016;107:12–25. https://doi.org/10.1016/j.ejmech.2015.10.045.
Rodriguez R, Miller KM, Forment JV, Bradshaw CR, Nikan M, Britton S, et al. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat Chem Biol. 2012;8:301–10. https://doi.org/10.1038/nchembio.780.
Chtchigrovsky M, Eloy L, Jullien H, Saker L, Segal-Bendirdjian E, Poupon J, et al. Antitumor trans-N-heterocyclic carbene-amine-Pt(II) complexes: synthesis of dinuclear species and exploratory investigations of DNA binding and cytotoxicity mechanisms. J Med Chem. 2013;56:2074–86. https://doi.org/10.1021/jm301780s.
Wei JH, Chen ZF, Qin JL, Liu YC, Li ZQ, Khan TM, et al. Water-soluble oxoglaucine-Y(III), Dy(III) complexes: in vitro and in vivo anticancer activities by triggering DNA damage, leading to S phase arrest and apoptosis. Dalton Trans. 2015;44:11408–19. https://doi.org/10.1039/c5dt00926j.
Qin QP, Qin JL, Meng T, Lin WH, Zhang CH, Wei ZZ, et al. High in vivo antitumor activity of cobalt oxoisoaporphine complexes by targeting G-quadruplex DNA, telomerase and disrupting mitochondrial functions. Eur J Med Chem. 2016;124:380–92. https://doi.org/10.1016/j.ejmech.2016.08.063.
Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. https://doi.org/10.1038/nature03097.
Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–86. https://doi.org/10.1038/sj.onc.1207233.
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. https://doi.org/10.1038/nrc1317.
Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790–803. https://doi.org/10.1038/nrd3253.
Zeng L, Chen Y, Liu J, Huang H, Guan R, Ji L, et al. Ruthenium(II) complexes with 2-phenylimidazo[4,5-f][1,10]phenanthroline derivatives that strongly combat cisplatin-resistant tumor cells. Sci Rep. 2016;6:19449. https://doi.org/10.1038/srep19449.
Chen ZF, Qin QP, Qin JL, Liu YC, Huang KB, Li YL, et al. Stabilization of G-quadruplex DNA, inhibition of telomerase activity, and tumor cell apoptosis by organoplatinum(II) complexes with oxoisoaporphine. J Med Chem. 2015;58:2159–79. https://doi.org/10.1021/jm5012484.
Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808–12.
Bhattacharjee RN, Park KS, Kumagai Y, Okada K, Yamamoto M, Uematsu S, et al. VP1686, a Vibrio type III secretion protein, induces toll-like receptor-independent apoptosis in macrophage through NF-kappaB inhibition. J Biol Chem. 2006;281:36897–904. https://doi.org/10.1074/jbc.M605493200.
Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32. https://doi.org/10.1158/1078-0432.CCR-08-0144.
Qin JL, Qin QP, Wei ZZ, Yu YC, Meng T, Wu CX, et al. Stabilization of c-myc G-Quadruplex DNA, inhibition of telomerase activity, disruption of mitochondrial functions and tumor cell apoptosis by platinum(II) complex with 9-amino-oxoisoaporphine. Eur J Med Chem. 2016;124:417–27. https://doi.org/10.1016/j.ejmech.2016.08.054.
Gou Y, Wang J, Chen S, Zhang Z, Zhang Y, Zhang W, et al. α-N-heterocyclic thiosemicarbazone Fe(III) complex: Characterization of its antitumor activity and identification of anticancer mechanism. Eur J Med Chem. 2016;123:354–64. https://doi.org/10.1016/j.ejmech.2016.07.041.
Noh J, Kwon B, Han E, Park M, Yang W, Cho W, et al. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun. 2015;6:6907 https://doi.org/10.1038/ncomms7907.
Hong Y, Sengupta S, Hur W, Sim T. Identification of novel ROS inducers: quinone derivatives tethered to long hydrocarbon chains. J Med Chem. 2015;58:3739–50. https://doi.org/10.1021/jm501846y.
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–5. https://doi.org/10.1073/pnas.88.9.3671.
Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. https://doi.org/10.1021/ar700135m.
Fromenty B, Pessayre D. Impaired mitochondrial function in microvesicular steatosis effects of drugs, ethanol, hormones and cytokines. J Hepatol. 1997;26:43–53. https://doi.org/10.1016/s0168-8278(97)80496-5.
Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33.
Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. https://doi.org/10.1007/978-1-4020-6554-5_2.
Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. https://doi.org/10.1016/s0962-8924(98)01273-2.
Carvallo-Chaigneau F, Trejo-Solis C, Gomez-Ruiz C, Rodriguez-Aguilera E, Macias-Rosales L, Cortes-Barberena E, et al. Casiopeina III-ia induces apoptosis in HCT-15 cells in vitro through caspase-dependent mechanisms and has antitumor effect in vivo. Biometals. 2008;21:17–28. https://doi.org/10.1007/s10534-007-9089-4.
Acknowledgements
We gratefully acknowledge the financial support from the Science and Technology Project of Guangxi (No. AB18221005), Science and Technology Major Project of Guangxi (No. AA17204058-21), Guangxi Key Laboratory of Special Non-wood Forest Cultivation & Utilization (No.18-A-04-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, GZ., Ouyang, XL., Mo, ZY. et al. Synthesis and biological evaluation of novel 1,3-diphenylurea quinoxaline derivatives as potent anticancer agents. Med Chem Res 30, 1496–1511 (2021). https://doi.org/10.1007/s00044-021-02745-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02745-2