Abstract
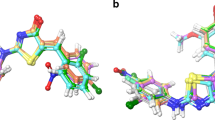
A series of new 5-aryliden-2-imino-4-thiazolidinones (5a–e and 6a–e) were synthesized via a three-step reaction and characterized by physicochemical and spectral data. The uniqueness of the derivatives lies in the fact that none of them had an acidic group, like conventional NSAIDS, but exhibited significant in vivo activity in acute inflammation models. In particular, 5-(3-chlorobenzyliden)-2-(pyridin-2-yl-imino)-4-thiazolidinone(5a) and 5-(3-chlorobenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6a) showed remarkable paw oedema inhibition (67.76 and 74.47 % oedema inhibition, respectively, after 3 h) comparable to that of Ibuprofen (74.56 % oedema inhibition, after 3 h) at half of the dose of the standard drug. Also, compounds 5a (72.86 %) and 6a (80.20 %) were found to possess significant inhibition of albumin denaturation when screened for in vitro anti-inflammatory activity. In addition, these compounds were docked into the known active site of COX-2 protein using Glide XP and QPLD algorithms, and the binding-free energy was calculated using Prime MM/GBSA simulation methods. The combined use of molecular docking and MM/GBSA methods gave a good correlation between the predicted binding-free energy and experimentally determined biological activities. It was also evident from the docking results that 2-methylisoxazolylimino or 2-(pyridin-2-yl-imino substitution and 3-chloro moiety on 5-benzylidin nucleus of these 4-thiazolidinone derivatives can easily occupy the COX-2 binding pocket, considered as the critical interaction for COX-2 inhibition. Moreover, pharmacokinetic properties of all the synthesized compounds were predicted, with good results. Further, the synthesized derivatives showed neither acute toxicity nor symptoms of gastric ulceration, at extended doses, owing to the absence of an acidic group.











Similar content being viewed by others
References
Agrawal OP, Sonar PK, Saraf SK (2013) 4-Thiazolidinone and 1-thia-3,4,9-triaza fluorene conjugates: synthesis, characterization and antimicrobial screening. Med Chem Res 22:1972–1978
Aulton ME (2002) Pharmaceutics: the science of dosage form design, 2nd edn. Churchill Livingstone, Edinburg
Balsamo A, Coletta I, Guglielmotti A, Landolfi C, Mancini F, Martinelli A, Milanese C, Minutolo F, Nencetti S, Orlandini E, Pinza M, Rapposelli S, Rossello A (2003) Synthesis of heteroaromatic analogues of (2-aryl-1-cyclopentenyl-1-alkylidene)-(arylmethyloxy)amine COX-2 inhibitors: effects on the inhibitory activity of the replacement of the cyclopentene central core with pyrazole, thiophene or isoxazole ring. Eur J Med Chem 38:157–168
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita MG (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Chawla P, Singh R, Saraf SK (2011a) Effect of chloro and fluoro groups on the antimicrobial activity of 2,5-disubstituted 4-thiazolidinones: a comparative study. Med Chem Res 21:3263–3271
Chawla P, Singh R, Saraf SK (2011b) Syntheses and evaluation of 2,5-disubstituted 4-thiazolidinone analogues as antimicrobial agents. Med Chem Res 21:2064–2071
Chen H, Bai J, Jiao L, Guo Z, Yin Q, Li X (2009) Design, microwave-assisted synthesis and HIV-RT inhibitory activity of 2-(2,6-dihalophenyl)-3-(4,6-dimethyl-5-(un)substituted-pyrimidin-2-yl)thiazolidin-4-ones. Bioorg Med Chem 17:3980–3986
de Leval X, Delarge J, Somers F, de Tullio P, Henrotin Y, Pirotte B, Dogne JM (2000) Recent advances in inducible cyclooxygenase (COX-2) inhibition. Curr Med Chem 7:1041–1062
Geronikaki A, Theofilidis G (1992) Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur J Med Chem 27:709–716
Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, Alam I, Saxena AK (2008) Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem 51:1601–1609
Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K (1996) A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem 271:15810–15814
Goel B, Ram T, Tyagi R, Bansal E, Kumar A, Mukherjee D, Sinha JN (1999) 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. Eur J Med Chem 34:265–269
Gouveia FL, de Oliveira RM, de Oliveira TB, da Silva IM, do Nascimento SC, de Sena KX, de Albuquerque JF (2009) Synthesis, antimicrobial and cytotoxic activities of some 5-arylidene-4-thioxo-thiazolidine-2-ones. Eur J Med Chem 44:2038–2043
Graul A, Martel AM, Castaner J (1997) Celecoxib: anti-inflammatory, cycloxygenase-2 inhibitor. Drugs Future 22:711–714
Harrold MW, Yee NS (2005) Principles of Pharmacodynamics and Medicinal Chemistry. In: Hansch C, Sammes PG, Taylor JB, Ramsden CA (eds) Comprehensive medicinal chemistry, 6th edn. Elsevier Publication, New Delhi (India), pp 270–271
Haviv F, DeNet RW, Michaels RJ, Ratajczyk JD, Carter GW, Young PR (1983) 2-[(Phenylthio)methyl]pyridine derivatives: new antiinflammatory agents. J Med Chem 26:218–222
Havrylyuk D, Mosula L, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R (2010) Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur J Med Chem 45:5012–5021
Hosni HM, Abdulla MM (2008) Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm 58:175–186
Kumar A, Bansal D, Bajaj K, Sharma S, Archana, Srivastava VK (2003) Synthesis of some newer derivatives of 2-amino benzoic acid as potent anti-inflammatory and analgesic agents. Bioorg Med Chem 11:5281–5291
Kumar A, Rajput CS, Bhati SK (2007) Synthesis of 3-[4’-(p-chlorophenyl)-thiazol-2’-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg Med Chem 15:3089–3096
Leval X, Julemont F, Delarge J, Pirotte B, Dogne JM (2002) New trends in dual 5-LOX/COX inhibition. Curr Med Chem 9:941–962
Lyne PD, Lamb ML, Saeh JC (2006) Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM–GBSA scoring. J Med Chem 49:4805–4808
Mizushima Y, Kobayashi M (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 20:169–173
Munoz C, Adasme F, Alzate-Morales JH, Vergara-Jaque A, Kniess T, Caballero J (2012) Study of differences in the VEGFR2 inhibitory activities between semaxanib and SU5205 using 3D-QSAR, docking, and molecular dynamics simulations. J Mol Graph Model 32:39–48
Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Sokovic M, Ciric A, Glamoclija J (2010) Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 18:426–432
Ottana R, Mazzon E, Dugo L, Monforte F, Maccari R, Sautebin L, De Luca G, Vigorita MG, Alcaro S, Ortuso F, Caputi AP, Cuzzocrea S (2002) Modeling and biological evaluation of 3,3’-(1,2-ethanediyl)bis[2-(4-methoxyphenyl)-thiazolidin-4-one], a new synthetic cyclooxygenase-2 inhibitor. Eur J Pharmacol 448:71–80
Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S, Vigorita MG (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13:4243–4252
Pavia DL, Lampman GM, Kriz GS (2007) Spectroscopy, 1st edn. Cenage Learning India Private Limited, Australia
Previtera T, Basile M, Vigorita MG, Fenech G, Occhiuto F, Circosta C, de Pasquale RC (1987) 3,3′-Di [1,3-thiazolidine-4-one] system. II. Anti-inflammatory and anti-histaminic properties in new substituted derivatives. Eur J Med Chem 22:67–74
Raghvan PV (2000) Expert consultant, CPCSEA, OECD, Guideline No. 420
Rawal RK, Tripathi R, Katti SB, Pannecouque C, De Clercq E (2007) Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg Med Chem 15:1725–1731
Sadashiva CT, Chandra JN, Kavitha CV, Thimmegowda A, Subhash MN, Rangappa KS (2009) Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Eur J Med Chem 44:4848–4854
Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT (1996) Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol 303:217–220
Sauzem PD, Machado P, Rubin MA, da S Sant’Anna G, Faber HB, de Souza AH, Mello CF, Beck P, Burrow RA, Bonacorso HG, Zanatta N, Martins MA (2008) Design and microwave-assisted synthesis of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles: novel agents with analgesic and anti-inflammatory properties. Eur J Med Chem 43:1237–1247
Smith QE (1960) Pharmacological screening tests progressive. In: Medicinal Chemistry, vol I. Butterworths, London
Sondhi SM, Jain S, Dinodia M, Kumar A (2008) Synthesis of some thiophene, imidazole and pyridine derivatives exhibiting good anti-inflammatory and analgesic activities. Med Chem 4:146–154
Thirumurugan P, Mahalaxmi S, Perumal P (2010) Synthesis and anti-inflammatory activity of 3-indolyl pyridine derivatives through one-pot multi component reaction. J Chem Sci 122:819–832
Tripathi AC, Gupta SJ, Fatima GN, Sonar PK, Verma A, Saraf SK (2014) 4-Thiazolidinones: The advances continue. Eur J Med Chem 72:52–77
The United State Pharmacopoeia. USP-NF-XXV (2002). United State Pharmacopoeial Convention Inc. Rockville, M.D
Vane J, Botting R (1987) Inflammation and the mechanism of action of anti-inflammatory drugs. Faseb J 1:89–96
Vazzana I, Terranova E, Mattioli F, Sparatore F (2004) Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents. ARKIVOC 5:364–374
Verma A, Saraf SK (2008) 4-thiazolidinone: a biologically active scaffold. Eur J Med Chem 43:897–905
Verma M, Sinha JN, Gujrati VR, Bhalla TN, Bhargava KP, Shanker K (1981) A new potent anti-inflammatory quinazolone. Pharmacol Res Commun 13:967–979
Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M (2008) 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: synthesis and structure-activity relationship. Bioorg Med Chem 16:3714–3724
Vigorita MG, Ottana R, Monforte F, Maccari R, Monforte MT, Trovato A, Taviano MF, Miceli N, De Luca G, Alcaro S, Ortuso F (2003) Chiral 3,3’-(1,2-ethanediyl)-bis[2-(3,4-dimethoxyphenyl)-4-thiazolidinones] with anti-inflammatory activity. Part 11: evaluation of COX-2 selectivity and modelling. Bioorg Med Chem 11:999–1006
Wang M, Liu Q, Yueyun ZLL, Zhou L, Sun M, Su H, Hua Y, Faming ZS (2008) Preparation of 5-membered heterocycles as regulators of glucagon-like peptide1 receptors (GLP1R). CN 101274918 A
Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JR, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, Kiefer JR, Carter J (2010) The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett 20:7159–7163
Zhang X, Li X, Li D, Qu G, Wang J, Loiseau PM, Fan X (2009) Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside-thiazolidinone hybrids and their antiparasitic activities. Bioorg Med Chem Lett 19:6280–6283
Zhou H, Wu S, Zhai S, Liu A, Sun Y, Li R, Zhang Y, Ekins S, Swaan PW, Fang B, Zhang B, Yan B (2008) Design, synthesis, cytoselective toxicity, structure-activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J Med Chem 51:1242–1251
Acknowledgments
This study was funded by the All India Council for Technical Education (AICTE), New Delhi, India, under the Research Promotion Scheme (RPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Tripathi, A.C., Tewari, A. et al. Ulcerogenicity devoid novel non-steroidal anti-inflammatory agents (NSAIDS): syntheses, computational studies, and activity of 5-aryliden-2-imino-4-thiazolidinones. Med Chem Res 24, 1927–1941 (2015). https://doi.org/10.1007/s00044-014-1270-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1270-z