Abstract
In inland aquatic ecosystems, drying and salinity can co-occur as natural stressors, affecting aquatic invertebrate communities. Despite recent appreciation of the importance of temporary waterbodies for terrestrial invertebrates, knowledge about the effects of drying on dynamics of aquatic and terrestrial invertebrate communities is scarce, especially in saline ecosystems. This study analyzed structural and compositional responses of both communities to the coupled effects of drying and salinity in two streams and two shallow lakes in Spain, during three hydrological phases: wet, contraction, and dry. In the two studied saline streams, the contraction phase presented the highest aquatic and terrestrial abundance and richness, and the main compositional changes were mainly due, to an increase in aquatic lentic taxa (e.g., Coleoptera), and Araneae and Formicidae as terrestrial taxa. In shallow lakes, which presented highly variable salinity conditions, the highest abundance and diversity values were found at the wet phase for aquatic invertebrates and at the dry phase for terrestrial invertebrates. Compositional invertebrate community changes were due to a decrease in Rotifera and Anostraca (aquatic taxa) in the contraction phase for aquatic communities, and to an increase of Araneae, Coleoptera, and Formicidae (terrestrial taxa) at the dry phase for the terrestrial. Our study evidences the significant effect of drying on both aquatic and terrestrial invertebrates communities in natural inland saline waters and the need to integrate aquatic and terrestrial perspectives to study temporary inland waters.
Similar content being viewed by others
Introduction
Natural inland saline ecosystems (salinity ≥ 3 g/L−1) are common worldwide, especially in semi-arid and arid zones (Hart et al. 1991; Alcorlo et al. 1996; Gómez et al. 2005; Álvarez et al. 2006). Water salinity in these ecosystems is mainly explained by their catchment lithology, often characterized by the presence of gypsum and halite-rich evaporite rocks (Gómez et al. 2005; Millán et al. 2011). Salinity acts as a natural stressor, which may reduce aquatic invertebrate diversity and promotes changes in community composition along increasing salinity gradients (de Necker et al. 2021) in both lotic (Nielsen et al. 2003; Arribas et al. 2009; Suárez et al. 2017) and lentic ecosystems (Karagianni et al. 2018; Zsuga et al. 2021). This is due primarily to salinity increasing osmotic pressure, which affects the development, physiology, and behavior of aquatic organisms with even lethal effects if their tolerance limits are exceeded (Carter et al. 2020). As a result, taxa capable of inhabiting inland water saline ecosystems are frequently rare or endemic (Alcorlo 1999; Alcorlo et al. 2001; Millán et al. 2011; Karagianni et al. 2018; Zsuga et al. 2021), which contributes to the increase of the ecological value of these aquatic ecosystems (Arribas et al. 2009; Vidal-Abarca et al. 2013; Karagianni et al. 2018).
In inland waters of semi-arid and arid areas, water salinity often co-occurs with drying, as these regions are characterized by wide interannual and seasonal hydrological variability with recurrent flood and drought events (Millán et al. 2011; Suárez et al. 2017). Similar to water salinity, drying acts as a natural stressor shaping the structure and composition of aquatic invertebrate communities (e.g., Sánchez-Montoya et al. 2007, 2018; Vidal-Abarca et al. 2013; Gutiérrez-Cánovas et al. 2015; Schriever et al. 2015). Drying turns both lotic and lentic ecosystems into a changing mosaic of aquatic and terrestrial habitats due to the alternation between wet (presence of surface water) and dry phases (absence of surface water) detached by a contraction phase (surface water retraction) (Alcorlo and Baltanás 1999; Boix et al. 2004; Mormul et al. 2015; Costigan et al. 2016; Datry et al. 2016). In the Mediterranean Basin, the contraction phase typically begins from late spring to early summer, followed by a long dry phase that can extend beyond the summer (Sánchez-Montoya et al. 2018). In late autumn or early winter, the wet phase is reestablished subject to some unpredictability depending of the climatic conditions (Comín et al. 1992; Vidal-Abarca et al. 2000; Boix et al. 2004; Gómez et al. 2005; Sánchez-Montoya et al. 2018). In both lotic and lentic ecosystems, the contraction phase contracts aquatic habitats by reducing the water surface and the water column depth, and consequently expands terrestrial habitats by increasing the area of exposed shore bars and dry beds (Boulton et al. 2003, 2017; García-Roger et al. 2013; Golec-Fialek et al. 2021). Under these variable hydrological conditions, aquatic invertebrates that inhabit temporary aquatic ecosystems are specialists with resistance forms (e.g., eggs, cocoons, diapauses) or resilience strategies (e.g., aerial dispersal mode, movement to the hyporheic zone) to withstand drying (Bonada et al. 2007; Díaz et al. 2008; Vander Vorste et al. 2016; Bogan et al. 2017; Sánchez-Montoya et al. 2018; Golec-Fialek et al. 2021).
In lotic ecosystems, drying usually decreases aquatic invertebrates abundance and richness, particularly in streams with low microhabitat diversity and riffles domain (Waterkeyn et al. 2008; Datry 2012; Datry et al. 2014; Leigh and Datry 2016). In addition, community composition may change due to the replacement of riffle taxa [Ephemeroptera, Plecoptera, and Trichoptera (EPT)] by pool taxa [Odonata, Coleoptera, and Heteroptera (OCH)] as a result of the lowering riffle/pool habitat proportion (Sánchez-Montoya et al. 2018; Bogan et al. 2019; Bonada et al. 2020; Miliša et al. 2022), even when the hydrological connectivity between aquatic habitats remains (Bonada et al. 2006). In lentic ecosystems, as drying progresses, a decrease in abundance and richness of aquatic invertebrates has been reported, particularly in shallow lakes (e.g., Brucet et al. 2006; Chaparro et al. 2011). However, no clear response in aquatic invertebrate composition has been identified in these types of aquatic ecosystems, probably due to the highly unpredictable hydrological variation that characterize temporary shallow lakes especially in arid and semi-arid areas (Comín et al. 1992; Chaparro et al. 2011).
In addition, fewer studies have analyzed the coupled effect of drying and salinity in inland waters (but see Suárez et al. 2017; Botwe et al. 2018; Karagianni et al. 2018), compared with each natural stressor separately. Aquatic invertebrate communities in saline temporary inland waters have to cope with both adverse effects, where water salinity may act as a stronger environmental stressor than drying, at least for lotic ecosystems (Velasco et al. 2006, 2019; Suárez et al. 2017; Cañedo-Argüelles et al. 2019). These two natural stressors constrain aquatic invertebrate communities, resulting in a net antagonist interaction in which the net effect of the two stressors may not be as strong as the sum of their independent effects (Jackson et al. 2016; Suárez et al. 2017). Negative effects can be especially important during drying when salinity, temperature, turbidity, and pH may increase and dissolved oxygen may decrease, shaping aquatic invertebrate communities in both lotic and lentic ecosystems (Southwood 1988; McCulloch et al. 2008; Mosley 2015; Suárez et al. 2017; Gómez et al. 2017; Karagianni et al. 2018; de Necker et al. 2021).
During drying, in parallel to the reduction of aquatic habitats for aquatic invertebrates, the expansion of terrestrial habitats in the form of exposed shore bars and dry beds have positive implications for terrestrial invertebrate communities (Sánchez-Montoya et al. 2016, 2020a,b; Steward et al. 2017, 2022; Vidal-Abarca et al. 2020). Recent studies have described not only the dynamics of terrestrial invertebrate communities that colonize these new terrestrial habitats (e.g., Sánchez-Montoya et al. 2016, 2020a, b; Steward et al. 2017, 2022), but also the contribution of these communities to overall biodiversity in temporary inland ecosystems (e.g., Bunting et al. 2021; Bruno et al. 2022). Particularly for lotic ecosystems, dry riverbeds have been identified as valuable terrestrial habitats, harboring abundant and diverse communities of ground-dwelling arthropods (Steward et al. 2011, 2012; Corti and Datry 2016; Sánchez-Montoya et al. 2016, 2020a, b). Dry riverbeds provide favorable conditions to terrestrial invertebrates such as low occupancy, few competing resources, food availability, and mating sites, especially during the onset of drying (e.g., Sánchez-Montoya et al. 2016, 2020a, b; Steward et al. 2022). Ants, spiders, beetles, mites, and springtails have been identified as the main taxa comprising these communities, which colonize dry beds from fringing terrestrial habitats (riparian and uplands) and may differ from them in composition terms (Steward et al. 2011; Sánchez-Montoya et al. 2016, 2020a,b). For lentic ecosystems, little attention has been paid to the terrestrial invertebrates that colonize dry lakebeds during drying (i.e., playas) to date, although some studies support the idea of dry lakebeds as valuable habitats for ground-dwelling arthropods (Thièry 1987; Rueda and Montes 1987, 1988; García 1991; Batzer and Wu 2020).
Despite the growing knowledge about drying responses of aquatic invertebrates during the last decade, few studies have integrated the dry phase of temporary water bodies reflecting contributions from both terrestrial and aquatic invertebrate communities that would provide a more comprehensive and inclusive view of waterbodies biodiversity and their responses to drying, especially in saline ecosystems. This is a major shortcoming if we consider that saline temporary inland ecosystems represent 25% of lotic ecosystems worldwide (Meybeck 1995), and their spatial extend is predicted to further increase in the near future because of ongoing climate change, land-use alteration, and human exploitation of water resources (Döll and Schmied 2012; Pachauri 2014; Woolway et al. 2022).
In this study, we examined the coupled effect of water salinity and drying on the structure and composition of both aquatic and terrestrial invertebrate communities during three contrasting hydrological phases (wet, contraction, and dry) in lotic and lentic naturally saline ecosystems. Based on potential changes in physical and chemical conditions (i.e., increasing in salinity and terrestrial habitats), we hypothesized that both drying and coupled salinity increase would drive substantial changes in the structure and composition of aquatic communities. Similarly, the terrestrial habitat availability, which increases over drying, would affect the structure and composition of terrestrial invertebrate communities. For aquatic invertebrates, we predicted structural changes with the decreasing of abundance and taxonomic richness in streams and lakes along drying and, consequently, higher diversity in the wet phase compared with the contraction phase. We also predicted that the composition of aquatic communities would differ between the wet and contraction phases, with an increase in the proportion of lentic to lotic taxa in the case of lotic ecosystems. For terrestrial invertebrates, we predicted higher abundance and richness in streams and lakes in the contraction and dry phases than in the wet phase, and significant changes in their composition among the three hydrological study phases.
Methods
Study area and sampling sites
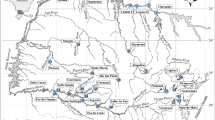
This study was carried out in lotic and lentic inland saline temporary ecosystems: two streams (Parra and Serranos, the Segura River Basin) and two shallow lakes (Muerte and Piñol, the Monegros district, Zaragoza) in SE and NE Spain, respectively (Table 1 and Fig. 1). According to the Köppen–Geiger climate classification (Rubel and Kottek 2010), Segura River Basin exhibits a warm semi-arid climate (BSh) and Monegros district a cold semi-arid climate (BSk). The studied streams and shallow lakes are located in calcareous sedimentary basins with the presence of evaporite rocks, which explain their high salinity waters (Table 1). According to saline type classifications, Parra and Serranos streams are classified as hyposaline during both the wet and contraction phases (3–20 g/L) (Arribas et al. 2009; Millán et al. 2011), while Muerte and Piñol lakes cover a range from hyposaline in the wet phase (3–20 g/L) to hypersaline in the contraction phase (> 50 g/L) (Hammer 1986). The dominant benthic vegetation in streams is characterized by periphyton in both Parra and Serranos and, Chara sp. and Chlorophyceae only in Serranos. In shallow lakes, the dominant benthic vegetation is microbial mat in Muerte, while in Piñol, benthic vegetation is absence. Riparian vegetation in streams is characterized by the presence of halophytes, shrubs, and by salt marsh plants (xerophytes and halophytes) in shallow lakes (Table 1).
We sampled the four study sites in three contrasting hydrological phases (wet, contraction, and dry) In both lotic and lentic ecosystems, the wet phase was considered when the discharge (streams), wetted bed area, and water column depth (streams and shallow lakes) were maximum, assuming high interannual variation among cycles. The contraction phase was defined as the period when the aquatic habitat drastically reduced in size at least at 50% in both ecosystems, which implied highly decreasing in discharge (streams), water surface area, and water depth (streams and lakes) compared with the previous wet phase. Finally, the dry phase was considered when surface water was totally absent in both ecosystem types. In general, in both studied streams the wet phase has a duration of 3–4 months, followed by a 2–3 month contraction phase and finally an extensive dry phase of around 6–7 months (e.g., Alcorlo 1999; Sánchez-Montoya et al. 2018). In streams, the wet phase was sampled in February (W1), the contraction phase from mid-June to early July (C1), and the dry phase from July to September (D1: 1 month after dry up) in 2020 (Fig. S1). Shallow lakes, which are subject to higher levels of hydrological variation than streams, were sampled more frequently, but combining years 2020 and 2021 due to the failure to sample the contraction phase in 2020 as a result of the COVID-19 pandemic lockdown. Consequently, the wet phase was sampled twice, once in January of 2020 (W1) and once in early March in 2021 (W1 and W2, respectively) The contraction phase was sampled only once, in mid-March of 2021 (C2). The dry phase was sampled three times, in June (D1: 1 day after dry up) and July (D2: 1 month after dry up) of 2020, and in July of 2021 (D3: 3 months after dry up) (Table 1 and Figure S1).
Environmental characterization
Water surface area (lakes), water column depth (streams and lakes), and water velocity (streams) were recorded along the aquatic reaches during the wet and contraction phases. Surface water flow was estimated in streams as the product of the average water velocity (current meter MiniAir2; Schiltknecht Co., Zurich, Switzerland) and cross-sectional area (width and depth of the channel) at each sampling site. Surface water in shallow lakes was estimated through aerial images on the same day of sampling with EO Browser (Sinergise Ltd). At three points of each sampling site and hydrological phase, water conductivity (mS/cm), water salinity (g/L), dissolved oxygen (mg/L), oxygen saturation (%), pH, and water temperature (°C) were measured using handheld probes (Intellical HQ40D, Hach Lange, USA).
Aquatic and terrestrial invertebrates sampling
Different types of sampling transects were established depending on the lotic and lentic nature of ecosystems, and the type of community sampled. In streams, the aquatic samples were collected along a longitudinal 100 m aquatic transect in the wet and contraction phases. Ten aquatic invertebrate samples were collected in each stream along transects by using hand net in pools and a surber sampler in riffles (both 20 cm × 20 cm, mesh size 250 µm), which were distributed based on the relative proportion of pools and riffles along the study aquatic reaches. In shallow lakes, five aquatic invertebrate samples were taken by using a hand net (25 cm diameter, mesh size 53 µm) distributed regularly along an aquatic transect of 50 m radial reach in the wet and contraction phases.
In streams and shallow lakes, the terrestrial samples were collected along a 100 m terrestrial transect parallel to the streams and lakes shoreline in the wet and contraction phases, following Sánchez-Montoya et al. (2016, 2020b). In the dry phase, the center of the dry riverbeds and dry lakebeds were considered as the terrestrial transects. In streams and shallow lakes, ten terrestrial invertebrate samples were obtained by using pitfall traps, installed 10 m apart along the terrestrial transect in the three hydrological phases. Pitfall traps consisted of plastic containers filled with water (width: 13 cm; length: 19.5 cm; depth: 7 cm) inserted into the sediment for 24 h. Salt was added as a preservative, and detergent to break surface tension and to avoid captured terrestrial arthropods escaping. Both the aquatic and terrestrial invertebrate communities were preserved with ethanol (70%) and were identified and counted by a stereo microscope. Aquatic invertebrates from streams were identified at the genus level, except for Diptera at the family level. Aquatic taxa from shallow lakes were identified at the species level, except for Nematoda. Terrestrial invertebrates in the four study sites were identified at the species level for Araneae, Formicidae, and Coleoptera, and at morphospecies for the rest of taxa (in accordance with Steward et al. 2011). Aerial terrestrial taxa (Diptera, Lepidoptera, and Hymenoptera) were not included in the study because pitfall traps are unsuitable for sampling these taxa (Corti et al. 2013; Sánchez-Montoya et al. 2016, 2020a, b).
Data analysis
To test the effect of drying on environmental conditions, values of water physical and chemical parameters for each site and hydrological phase were log-transformed through log (x + 1) and their normality and homogeneity of variance were analysed by Shapiro–Wilk and Barlett tests, respectively. We tested for differences between hydrological phases (wet and contraction) at each site, by running one-way analyses of variance (ANOVA) over the log-transformed values of each physical and chemical parameter.
For aquatic and terrestrial communities, to ensure the relative completeness of the sampling, we first estimated sampled coverage (SC) (Chao and Jost 2012) for both communities in each phase. Secondly, for testing differences in aquatic and terrestrial communities over drying, we considered the total abundance and taxonomic richness separately in each study site and hydrological phase. Abundance and taxonomic richness data were log-transformed through log (x + 1) and their normality and homogeneity of variance were analysed by Shapiro–Wilk and Barlett tests, respectively. Then, in streams we tested for differences between hydrological phases at each site by running ANOVA parametric analysis over the log-transformed abundance and richness data. In shallow lakes, because they did not comply with the assumptions of ANOVA, we used a Kruskal–Wallis nonparametric analysis. When necessary, we performed post hoc tests [Tukey’s Honest Significant Difference (HSD) test or the nonparametric Wilcoxon test] to identify pair-wise comparisons among phases.
Secondly, a Bray–Curtis dissimilarity matrix was constructed from the logarithmically transformed abundance data though log (x + 1). Taxa that represented less than 0.1% of the total abundance were considered rare and were removed before analyses to avoid statistical artifacts (McCune and Grace 2002). To evaluate the spatial arrangement of the dataset according to the phase for both streams and shallow lakes, we performed a nonmetric multidimensional scaling analysis (NMDS), and to examine differences in both aquatic and terrestrial composition for each stream and shallow lake among phases, we used the permutational multivariate analysis of variance (PERMANOVA); both analyses operated on the Bray–Curtis dissimilarity matrix.
Finally, we performed a similarity percentage analysis (SIMPER) for each community and site separately, to calculate dissimilarity among phases and the contribution of individual taxa to these dissimilarities. For richness, NMDS and PERMANOVA were used using the highest taxonomic resolution available, whereas for the SIMPER, the invertebrate family level was used. Specifically for NMDS, to proceed with the analysis, samples with zero individuals were previously eliminated, and then 2D stress values were calculated.
All the analyses were performed with R software (R Core Team 2020). We used the aov, kruskal.test, TukeyHSD, and pairwise.wilcox.test functions from the basic R package. For the rest of the analyses, we used the packages vegan (Oksanen et al. 2013) and pairwise.adonis (Martinez 2020).
Results
Environmental conditions in streams and shallow lakes during drying
In the studied two streams from the wet to the contraction phase, in parallel to the reduction of more than 50% of the discharge, channel width, and water depth, we detected a significant variation in the physical and chemical water features (Table 1 and Table S1). Whereas water salinity, oxygen saturation, pH, and temperature significantly increased, dissolved oxygen tended to decrease. Both streams displayed an increasing proportion of connected pools and a decreasing proportion of riffles in the contraction phase compared with the wet phase (Fig. S1). In the two studied shallow lakes, a 50% reduction in the surface area was observed in the contraction phases compared with the previous wet phases, as well as reductions of between 20% and 60% in the water column depth (Table 1). In the contraction phase, the water surface was fragmented into different sized disconnected aquatic patches in both studied lakes. Considering the same hydrological cycle, physical and chemical data obtained from these different aquatic patches were significantly different from those obtained during the wet phase in both shallow lakes (Table 1 and Table S1). In some cases, the observed tendency was a decrease in dissolved oxygen and oxygen saturation, a temperature increase, and pH stabilization (Table S2). Water salinity only increased in Piñol and, in contrast, it remained stable in Muerte.
Aquatic and terrestrial invertebrate community structure
In the two streams, we collected a total of 4325 aquatic invertebrates over the two sampling occasions (W1 and C1), represented mainly by Diptera (83.9%), Ephemeroptera (5.6%), Coleoptera (4.8%), Odonata (3.6%), and Heteroptera (1.5%) (Table S3). The remaining invertebrates (0.5%) belonged to Oligochaeta, Trichoptera, and Mollusca. In the two shallow lakes, we collected a total of 64,121 aquatic invertebrates over the three sampling occasions (W1, W2, and, C2), represented mostly by Ostracoda (67.9%) and Nematoda (24.7%). Rotifera (4.9%), Anostraca (2.1%), Diptera (0.2%), and Copepoda (0.1%) were minority taxa. The sample coverage obtained was ≥ 90% in each sampling phase for both streams and shallow lakes (Table S4). In both streams, the total abundance and taxonomic richness of aquatic invertebrates were significantly higher in the contraction than in the wet phase (Table S5 and Fig. 2). The opposite pattern was observed in the two studied shallow lakes for the two structural metrics between W2 and C2, except for invertebrate abundance at Piñol (Tables S6 and S7 and Fig. 2).
Abundance and taxonomic richness mean values (± SE) of aquatic (left) and terrestrial (right) invertebrate communities calculated for each hydrological phase in each studied streams (up) and shallow lakes (down). Different letters within each panel indicate statistically significant differences based on Tukey’s HSD tests
Concerning terrestrial invertebrates, in the two studied streams we collected 841 individuals along the three sampling occasions (W1, C1, and D1). This community was composed mainly of Heteroptera (46.7%), Formicidae (21.1%), Collembola (14.9%), Araneae (8.8%), Coleoptera (4.5%), and, Orthoptera (3.2%). The remaining invertebrates (0.5%) belonged to Acari, Dermaptera, and Opiliones (Table S3). In both shallow lakes, we collected a total of 778 individuals from the six sampling occasions (W1, W2, C2, D1, D2, and D3). Terrestrial communities were comprised mainly of Formicidae (40.2%), Collembola (30.4%), Araneae (9.9%), and Coleoptera (15.6%). The remaining 3.7% was represented by Acari, Psocodea, Dermaptera, and Orthoptera. The sample coverage obtained was, in general, ≥ 90% in each sampling phase for both streams and shallow lakes, with the exceptions of D1 in Serranos, W1 in Muerte, and C2 in Piñol (Table S4). The total abundance and richness of terrestrial invertebrates differed significantly among phases in both ecosystem types (Tables S5 and S6 and Fig. 2). In streams, abundances were significantly higher in the contraction than in the wet and dry phases, and richness was significantly lower in wet than in the other two phases (Table S8 and Fig. 2). In shallow lakes, the contraction phase exhibited the lowest abundance of terrestrial invertebrates (Table S9 and Fig. 2), which differed mainly from the dry phases in both studied lakes.
Aquatic and terrestrial invertebrate community composition
In the two streams, the composition of aquatic invertebrates significantly differed between the wet and contraction phases (Table 2 and Fig. 3). In both streams, Diptera were mainly responsible for compositional differences (Table S10). Lower abundances of Chironomidae in Parra and Culicidae in Serranos in the contraction phase compared with the wet phase were found, but the opposite pattern was detected for Simuliidae in Parra and Ceratopogonidae in Serranos. In addition, we recorded in both streams higher abundances of Coleoptera (Dytiscidae, Hydrophilidae, and Hydraenidae in Parra; Hydrophilidae in Serranos) and Heteroptera (Corixidae and Notonectidae in Parra; Corixidae and Nepidae in Serranos) in the contraction phase than in the wet phase (Table S10). Finally, higher abundance of Ephemeroptera (Baetidae and Caenidae), Trichoptera (Sericostomidae), and Odonata (Libellulidae) at Parra were recorded in the contraction phase than in the wet phase (Table S10).
Similarly, in both shallow lakes, the composition of aquatic invertebrates significantly differed between the wet and contraction phases (Table 2 and Table S11, and Fig. 3). Rotifera (Hexarthridae), Anostraca (Branchinectidae), Ostracoda (Cyprididae), and Copepoda (Diaptomidae) were the most important taxa that accounted for the compositional differences in both lakes, and Nematoda only at Muerte. In both lakes, higher abundances were detected in the wet phase than in the contraction phase for all recorded taxa, except for Ostracoda at Piñol (Table S10).
Regarding terrestrial invertebrates in the studied streams, their composition was significantly distinct among the three hydrological phases, except for the dry and contraction phases at Serranos (Table 2 and Table S12, and Fig. 3). In both streams, higher dissimilarities were found between wet and dry phases, followed by wet and contraction, and contraction and dry phases (Table S13). At Parra, dissimilarities were due to Formicidae, which presented significantly lower abundances in the wet phase than in the other two phases. Also, Araneae (Lycosidae and Linyphiidae) presented significantly lower abundances in the wet than in the dry phase, and Collembola and Coleoptera (Carabidae and Coccinelidae) showed the opposite pattern. Finally, Heteroptera presented higher abundances in the contraction phase than in the other two phases and Araneae (Salticidae) had higher abundances in the contraction phase than in the dry phase. At Serrano, dissimilarities were due to Araneae (Salticidae, Titanoecidae, and Zodariidae), which presented significantly lower abundances in the wet than in the dry phase, and Coleoptera (Carabidae and Staphylinidae) with lower abundances in the wet phase than in the contraction phase. The opposite pattern was recorded for Collembola, with significantly higher abundance in the wet phase than in the other two phases, similar to Parra. Orthoptera (Gryllidae) also presented significantly higher abundances in the dry phase than in the other two phases (Table S13).
In the studied shallow lakes, the composition of terrestrial invertebrates changed also significantly among the three hydrological phases (Table 2 and Table S12, and Fig. 3). In both lakes, in general, higher dissimilarities were detected between the dry and contraction phases, followed by wet and dry, and wet and contraction phases (Table S13). In Muerte, community differences were due to the significantly higher abundances of Formicidae in the dry phases (D1 and D3 with respect to the wet phases, and in D1 and D2 with respect to the contraction phase) compared with the other phases. In addition, Araneae (Gnaphosidae in D1 and Dyctinidae in D3) and Coleoptera (Carabidae in D1 and D2) presented significantly higher abundances in the dry phase than in the wet and contraction phases. Also, Collembola presented significantly higher abundances in the dry phase D1 with respect to the wet phases and, finally, Araneae (Gnaphosidae) and Coleoptera (Chrysomelidae, Elateridae and, Staphylinidae) presented significantly higher abundances in the wet phases than the contraction phase. In Piñol, the abundance of Araneae (Dyctinidae Lycosidae, and Theridiidae) was in general significantly higher in the dry phases than in the wet and contraction phases. The abundances of Collembola in D1, Formicidae in D2 and D3, and Coleoptera (Anthicidae) in D3 were significantly higher than in the wet phases. Finally, abundances of Coleoptera (Anthicidae, Carabidae, and Staphylinidae) was significantly higher in the wet phases than in the contraction phases (Table S13).
Discussion
Only recently, the dry phase of inland water ecosystems and their associated dry beds have been identified as key moments and habitats, respectively, for a diverse and distinct terrestrial invertebrate community, primarily in rivers and streams (e.g., Sánchez-Montoya et al. 2016, 2020a, b). Concomitantly, less attention has been paid to other key aspects such as (i) the importance of the dry phase in saline ecosystems; (ii) the temporal dynamics of terrestrial invertebrates in wet, contraction, and dry phases, especially in saline inland waters; and, finally, (iii) parallels and contrasts of aquatic and terrestrial invertebrate response patterns during drying in saline both lotic and lentic ecosystems.
In this study, we analyzed changes in the structure and composition patterns of both aquatic and terrestrial invertebrate communities in response to both coupled stressors, water salinity, and drying, in lotic and lentic naturally saline ecosystems. With this purpose, we studied in detail the communities response along the three hydrological phases: wet, contraction, and dry. This allowed identifying (i) different hydrological phases characterized by different abundances and richness values, (ii) the level of consistency in structural and compositional responses to drying across aquatic and terrestrial communities in saline ecosystems, and (iii) which terrestrial and aquatic invertebrate taxa are responsible for compositional changes along different hydrological phases.
Aquatic invertebrate communities and their structural and compositional responses to drying in saline ecosystems
According to our hypothesis, drying promoted changes in the structure and composition of aquatic communities not only in saline streams but also in saline shallow lakes. This finding expands previous knowledge about the effect of drying in freshwater ecosystems to saline ecosystems (Bonada et al. 2006; García-Roger et al. 2011).
In streams, many studies have described how drying reduces abundance and taxonomic richness of aquatic communities (e.g., Boulton 2003; García-Roger et al. 2013; Datry 2012; Datry et al. 2017; Stubbington et al. 2017), in conjunction with changes in their composition by taxa replacement (e.g., Pace et al. 2013; Stubbington et al. 2017). In our studied streams, drying also changed the aquatic invertebrate structure but, conversely to our predictions, the highest abundances and richness occurred in the contraction phase, unlike the conceptual model described by Boulton (2003). That model proposed a sharp taxa richness decline as flow discharge or water level decreases. In our study, we detected the increasing proportion of connected pools and associated OCH taxa in the contraction phase and the persistence of runs with the significant increase of Ephemeroptera abundance. All these taxa were mainly responsible for changes in community composition from the wet to the contraction phase. This finding may be the consequence of not only the persistence of hydrological connectivity among aquatic habitats in the contraction phase, but also the slight increase in water salinity during drying as result of the maintained flow (e.g., Gómez et al. 2017; Bonada et al. 2020). Under these hydrological conditions, aquatic invertebrate communities would have avoided the niche filtering imposed for isolated pool habitats in harsher drying scenarios (Bonada et al. 2006), allowing the coexistence of both lotic and lentic taxa (Buffagni et al. 2020), as well as the adverse effect of highly increasing salinity expected in disconnected pools (e.g., Vidal-Abarca et al. 2013). In addition, it must be considered that the reduction of the surface flow during the contraction phase generally promotes an increase in organic matter deposited in pools as detritus that can be utilized as food resources for aquatic communities. This may explain the increase in detritivore abundance (Acuña et al. 2005). In general, our findings support the idea that connected pools promote high density and diversity of aquatic invertebrates compared with disconnected pools, acting as biodiversity reservoirs for entire drainage networks (Bonada et al. 2006; Bogan et al. 2017; Hill and Milner 2018).
Conversely to the studied streams, in shallow lakes we observed significantly lower diversity in the contraction phase than in the previous wet phase, according to our prediction. The detected significant drop in abundance (only in Muerte) and richness (in both Muerte and Piñol) of aquatic invertebrates may respond to the aquatic habitat constraints imposed by the contraction phase (e.g., Dodson 1992; O’Brien et al. 2004; Quintana et al. 2006). However, observed abundance patterns cannot be explained by severe physical and chemical conditions imposed by contraction phase in both study lakes, as previously detected in other lakes (Ginatullina et al. 2017; Senner et al. 2018; de Necker et al. 2021). In our study, in both lakes, the water surface of the previous wet phase (within the same hydrological cycle) was fragmented into different sized isolated aquatic patches in the contraction phase, but only resulted in slight changes in water physical and chemical conditions. We assume that this fact was because the aquatic patches were not isolated enough to promote drastic physical and chemical changes. Nevertheless, as an exception, water salinity increased in Piñol (1.8-fold). This fact, together with the aquatic habitat constraints, explains the lower richness and higher abundance during the contraction phase in Piñol, where the aquatic community shifted to have only salinity tolerant species (Ostracoda), which represented the total abundance. In addition, for dissolved oxygen, despite lower values at the contraction phase for both lakes , those values were always above the oxygen requirement of aquatic taxa in both lakes (Davis 1975; Karpowicz et al. 2020).
In both lakes, the main detected compositional changes were related to the loss of most taxa in the contraction phase compared with the wet phase, particularly crustaceans (Anostraca and Copepoda, among others) and rotifers. Similar changes have been previously described in shallow lakes (Brucet et al. 2005, 2006). In our study, the community presented in the contraction phase at Muerte, characterized by stable salinity conditions, was dominated mainly by nematodes, which are described as taxa that withstand and tolerate harsh environmental conditions due to resilience mechanisms, which allow a rapid and effective response to environmental disturbances such as hydrological changes (Gascón et al 2006). At Piñol, Ostracoda Candelacypris aragonica was the dominant taxa in the contraction phase characterized by a significant increase in water salinity concentration. This is a consequence of the ability of Ostracoda to survive in isolated shallow aquatic patches at high salinity concentrations (de Necker et al. 2021), especially for C. aragonica whose physiological optimum coincides with the high saline conditions of shallow lakes from Monegros district (Santamaría et al. 1992).
Terrestrial invertebrate communities and their structural and compositional responses to the salinity and drying
Terrestrial invertebrate communities have been recently described as an important biological component of the dry phase of inland waters, mainly in lotic ecosystems (rivers and streams), playing a key role in processing organic matter in temporary terrestrial habitats, such as exposed shore bars and dry beds habitats (e.g., Steward et al. 2017). Although there is evidence that dry beds of lentic ecosystems (shallow lakes) harbor a diverse terrestrial invertebrate community (e.g., Alcorlo 1999, 2004), the importance of this habitat for terrestrial invertebrate communities has been largely overlooked, in general in lentic aquatic ecosystems, and in particular in saline lakes.
According to our hypothesis, drying changed the structure and composition of terrestrial invertebrate communities in both the studied streams and shallow lakes, similar to that observed for aquatic communities. Our predictions were confirmed for both ecosystem types, since highest the abundance and richness values of terrestrial invertebrates were detected in the contraction and/or dry phases compared with the wet phase.
In the two studied streams, the highest abundance and richness values of terrestrial invertebrates were detected in the contraction phase compared with wet and even dry phases. This finding is in line with the habitat continuum model proposed recently by Steward et al. (2022), which described how richness and abundance of terrestrial invertebrates increase as surface water disappears (i.e., contraction phase and at the onset of the dry phase), but decrease over the dry phase. In the onset of the dry phase, dry riverbeds may represent an opportunity for terrestrial invertebrates, providing them a newly unexploited habitat with competitor–predator free spaces, low occupancy, and suitable mating sites and food resources from stranded aquatic organisms (e.g., fish, insects, algae; Sánchez-Montoya et al. 2016, 2020a,b; Steward et al. 2017, 2022). This temporary food subsidy may benefit opportunistic predators, scavengers, and detritivores such as Coleoptera, Heteroptera, Araneae (especially Lycosidae), Formicidae, Collembola, and Orthoptera, among others (Sánchez-Montoya et al. 2016, 2020a,b; Steward et al. 2022). However, the fact that soil moisture diminishes as drying progresses, together with the loss of aquatic food resources, may explain the detected reduction of the diversity and abundances in dry beds. In these conditions, only persisting specialist taxa remained (Steward et al. 2022).
In our study, drying also changed the terrestrial community composition in the two studied streams from the wet to the contraction and dry phases. Similarly to previous studies (Pflug and Wolters 2001; Lessel et al. 2011; Xu et al. 2012; McCluney and Sabo 2012), drying led to a drastic reduction in Collembola in the contraction and dry phases, probably due to the marked dependence of this taxa on soil moisture (Marx et al. 2012). Collembola are very sensitive to soil environmental changes (Flórián et al. 2019), and may be directly influenced by the drying effect on their physiology, behavior, and reproduction, or indirectly on resources availability (reducing mainly fungi and bacteria, which depend directly on soil moisture) (Xu et al. 2012). Conversely, the transition from the wet to the contraction and dry phases was characterized by the increased abundance of mainly Araneae and Formicidae in the two studied streams. Some Formicidae and Araneae (e.g., Lycosidae) taxa were frequently found along exposed terrestrial bars and dry riverbeds during drying, according to previous studies (Steward et al. 2011; Sánchez-Montoya et al. 2016, 2020a,b). These two groups of taxa may benefit from the accumulation of stranded aquatic organisms (e.g., algae and aquatic invertebrates) as a temporary food subsidy during drying. This may be the case of ants that prey on aquatic biota in exposed bars and riverbeds (Steward et al. 2017) and some spiders that prey on both emerging aquatic insects in the contraction phase (e.g., Paetzold et al. 2005). Therefore, our results not only confirm those previous findings reported in freshwater streams about the drying effect on terrestrial invertebrate communities, but also extend the knowledge to saline streams. In addition, it must be considered that dry riverbeds can offer a wide diversity of microhabitats for the terrestrial invertebrates that inhabit the surface and interstitial spaces of dry beds (Steward et al. 2017, 2022). For instance, the highest abundance of Araneae and Formicidae on the dry beds at both Parra and Serranos were probably also related to spiders and ants preferring coarser substrates (rock–cobble) (Sánchez-Montoya et al. 2016; Steward et al. 2022), which characterized the two studied streams.
In the two shallow lakes, as predicted, we observed the highest values of terrestrial invertebrate abundances and richness in dry phases. As far as we know, our study describes, for the first time, the dynamic of terrestrial invertebrate communities in the dry lakebeds, although the occasional presence of similar terrestrial taxa has been previously observed in similar lakes (e.g., Rueda and Montes 1987; García 1991; Alcorlo 1999). Thus, current findings demonstrate that dry lakebeds may also act as key habitats for terrestrial invertebrates, similarly to dry riverbeds. Conversely to what was proposed by the habitat continuum model for temporary rivers (Steward et al. 2022), the later dry phases in shallow lakes also showed similar abundance and richness for terrestrial invertebrates compared with the onset of drying. These results evidence that the application of this model in lentic ecosystems must be proven. In compositional terms, in the current study, the dry phase exhibited the highest abundance of Formicidae and Araneae, similar to the pattern found in streams. In addition, the dry phase of both shallow lakes showed the highest abundance of Coleoptera. Some families of predatory beetles, such as ground beetles (Carabidae) and rove beetles (Staphylinidae), which were present in the studied shallow lakes, are common inhabitants of dry beds, similarly to spiders and ants (Sánchez-Montoya et al. 2016; Steward et al. 2017). In addition, we observed high abundances of larvae and adults of tiger beetles (Cicindelidae and Carabidae) in the burrows constructed in the studied sandy dry lakebeds, in accordance with their habitat preferences (Schultz 1989; Rodríguez-Flores et al. 2016; Brust et al. 2005). This finding indicates the potential key role of sandy dry lakebeds for some particular terrestrial invertebrates as favorable habitats. Finally, and conversely to the studied streams, the lowest values of these metrics were recorded for the contraction phase. In general, these findings highlight that comparative studies across regions are required to further support the present results evidencing potential complex array of interactions between regional and local conditions predicting responses of aquatic and terrestrial invertebrate assemblages over hydrological phases, especially in areas subject to high level of hydrological variations.
Conclusions
Our findings showed the significant effect of drying on both aquatic and terrestrial invertebrate communities in saline streams and shallow lakes. Aquatic habitat constraints, and its resulting effect in water salinity, seems to be a determinant factor shaping aquatic communities along drying. Also, drying and the consequent expansion of terrestrial habitat, represented an opportunity to increase terrestrial invertebrate diversity in saline streams and shallow lakes.
In the studied streams, the stable physical and chemical conditions in the contraction phase (characterized by hydrological connectivity and steady salinity) favored high abundance and taxonomic richness for aquatic invertebrates. However, in the shallow lakes, not only the aquatic habitat constraints but also water salinity produced a shift in community composition, decreasing the abundance and diversity of the aquatic community in the contraction phase in some occasions. In parallel, the expansion of terrestrial habitats in both streams and shallow lakes during the progress of drying increased the diversity of terrestrial invertebrates. This was especially important for predators and scavengers such as Araneae, Formicidae, and Coleoptera, which reached the highest values in the intermediate phase of drying in streams (contraction phase) and spread during the dry phase of saline shallow lakes.
This study supports the need to integrate both aquatic and terrestrial communities into analyses of biodiversity variability of temporary saline aquatic ecosystems. Considering that drying and salinity are increasing globally as a result of climate change and human activities (Jeppensen et al. 2015; Estévez et al. 2019; Woolway et al. 2022), the role of the dry phase and its contribution to inland water biodiversity should be central for the conservation of not only freshwater but also saline aquatic ecosystems.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
22 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00027-024-01081-y
References
Acuña V, Muñoz I, Giorgi A, Omella M, Sabater F, Sabater S (2005) Drought and postdrought recovery cycles in an intermittent Mediterranean stream: structural and functional aspects. J N Am Benthol Soc 24:919–933. https://doi.org/10.1899/04-078.1
Alcorlo P (1999) Redes Tróficas en Lagunas Salinas Temporales de la comarca de Los Monegros (Zaragoza). PhD Thesis, Universidad Autónoma de Madrid.
Alcorlo P (2004) Las redes tróficas en las lagunas salinas temporales de Los Monegros (Zaragoza, España). Ecosistemas Revista Científica y Técnica De Ecología y Medio Ambiente. https://doi.org/10.7818/re.2014.13-2.00
Alcorlo P, Baltanás A (1999) Limnología de las lagunas salinas de Los Monegros y caracterización de sus comunidades animales. Boletín De La s.e.a 24:113–120
Alcorlo P, Baltanás A, Montes C (1996) Is it possible to predict the salinity of Iberian salt lakes from their conductivity? Hydrobiologia 330:137–142. https://doi.org/10.1007/BF00020002
Alcorlo P, Baltanás A, Montes C (2001) Food-web structure in two shallow salt lakes in Los Monegros (NE Spain): energetic vs dynamic constraints. Hydrobiologia 466:307–316. https://doi.org/10.1023/A:1014594408119
Álvarez S, Díaz P, López-Archilla AI, Guerrero MC (2006) Phytoplankton composition and dynamics in three shallow temporary salt lakes (Monegros, Spain). J Arid Environ 65:553–571. https://doi.org/10.1016/j.jaridenv.2005.09.001
Arribas P, Gutiérrez-Cánovas C, Abellán P, Sánchez-Fernández D, Picazo F, Velasco J, Millán A (2009) Tipificación de los ríos salinos ibéricos. Ecosistemas 18(3):1–13
Batzer DP, Wu H (2020) Ecology of terrestrial arthropods in freshwater wetlands. Annu Rev Entomol 65:101–119. https://doi.org/10.1146/annurev-ento-011019-024902
Bogan M, Chester ET, Datry T, Murphy AL, Robson BJ, Ruhi A, Stubbington R, Whitney JE (2017) Resistance, resilience, and community recovery in intermittent rivers and ephemeral streams. Chapter 4.8. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 349–376. https://doi.org/10.1016/B978-0-12-803835-2.00013-9
Bogan M, Leidy RA, Neuhaus L, Hernandez CJ, Carlson SM (2019) Biodiversity value of remnant pools in an intermittent stream during the great California drought. Aquat Conserv Mar Freshwat Ecosyst 29:976–989. https://doi.org/10.1002/aqc.3109
Boix D, Sala J, Quintana XD, Moreno-Amich R (2004) Succession of the animal community in a Mediterranean temporary pond. J N Am Benthol Soc 23:29–49. https://doi.org/10.1899/0887-3593(2004)023%3c0029:SOTACI%3e2.0.CO;2
Bonada N, Rieradevall M, Prat N, Resh VH (2006) Benthic macroinvertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. J N Am Benthol Soc 25:32–43. https://doi.org/10.1899/0887-3593(2006)25[32:BMAAMC]2.0.CO;2
Bonada N, Rieradevall M, Rieradevall M (2007) Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 589:91–106. https://doi.org/10.1007/S10750-007-0723-5
Bonada N, Cañedo-Argüelles M, Gallart F, von Schiller D, Fortuño P, Latron J, Llorens P, Múrria C, Soria M, Vinyoles D, Cid N (2020) Conservation and management of isolated pools in temporary rivers. Water (switzerland) 12(10):1–24. https://doi.org/10.3390/w12102870
Botwe PK, Carver S, Magierowski R, McEvoy P, Goonan P, Madden C, Barmuta LA (2018) Effects of salinity and flow interactions on macroinvertebrate traits in temporary streams. Ecol Ind 89:74–83. https://doi.org/10.1016/j.ecolind.2018.01.036
Boulton AJ (2003) Parallels and contrasts in the effects of drought on stream macroinvertebrate assemblages. Freshw Biol 48:1173–1185. https://doi.org/10.1046/J.1365-2427.2003.01084.X
Boulton AJ, Rolls RJ, Jaeger KL, Datry T (2017) Hydrological connectivity in intermittent rivers and ephemeral streams. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 79–108. https://doi.org/10.1016/B978-0-12-803835-2.00004-8
Brucet S, Boix D, Moreno-Amich R, Quintana XD (2005) Zooplankton structure and dynamics in permanent and temporary Mediterranean salt marshes: taxon-based and size-based approaches. Arch Hydrobiol 162:535–555. https://doi.org/10.1127/0003-9136/2005/0162-0535
Brucet S, Boix D, López-Flores R, Badosa A, Quintana XD (2006) Size and species diversity of zooplankton communities in fluctuating Mediterranean salt marshes. Estuar Coast Shelf Sci 67:424–432. https://doi.org/10.1016/j.ecss.2005.11.016
Bruno D, Hermoso V, Sánchez-Montoya MM, Belmar O, Gutiérrez-Cánovas C, Cañedo-Argüelles M (2022) Ecological relevance of non-perennial rivers for the conservation of terrestrial and aquatic communities. Conservation Biology 6:e13982
Brust ML, Hoback WW, Knisley CB (2005) Biology, habitat preference, and larval description of Cicindela cursitans Leconte (Coleoptera: Carabidae: Cicindelinae). Bulletin 59:379–390. https://doi.org/10.1649/798.1
Buffagni A, Erba S, Cazzola M, Barca E, Belfiore C (2020) The ratio of lentic to lotic habitat features strongly affects macroinvertebrate metrics used in southern Europe for ecological status classification. Ecol Indic. https://doi.org/10.1016/J.ECOLIND.2020.106563
Bunting G, England J, Gething K, Sykes T, Webb J, Stubbington R (2021) Aquatic and terrestrial invertebrate community responses to drying in chalk streams. Water Environ J 35:229–241. https://doi.org/10.1111/wej.12621
Cañedo-Argüelles M, Kefford B, Schäfer R (2019) Salt in freshwaters: causes, effects and prospects - introduction to the theme issue. Philos Trans R Soc B. https://doi.org/10.1098/rstb.2018.0002
Carter MJ, Flores M, Ramos-Jiliberto R (2020) Geographical origin determines responses to salinity of Mediterranean caddisflies. PLoS ONE. https://doi.org/10.1371/journal.pone.0220275
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Chaparro G, Marinone MC, Lombardo RJ, Schiaffino MR, de Souza Guimarães A, O’Farrell I (2011) Zooplankton succession during extraordinary drought–flood cycles: a case study in a South American floodplain lake. Limnologica 41:371–381. https://doi.org/10.1016/j.limno.2011.04.003
Comín FA, Rodó X, Comín P (1992) Lake Gallocanta (Aragón, NE Spain): a paradigm of fluctuations at different scales of time. Limnetica 8:79–86
Corti R, Datry T (2016) Terrestrial and aquatic invertebrates in the riverbed of an intermittent river: parallels and contrasts in community organisation. Freshw Biol 61:1308–1320. https://doi.org/10.1111/fwb.12692
Corti R, Larned ST, Datry T (2013) A comparison of pitfall-trap and quadrat methods for sampling ground dwelling invertebrates in dry riverbeds. Hydrobiologia 717:13–26. https://doi.org/10.1007/s10750-013-1563-0
Costigan KH, Jaeger KL, Goss CW, Fritz KM, Goebel PC (2016) Understanding controls on flow permanence in intermittent rivers to aid ecological research: integrating meteorology, geology and land cover. Ecohydrology 9:1141–1153. https://doi.org/10.1002/eco.1712
Datry T (2012) Benthic and hyporheic invertebrate assemblages along a flow intermittence gradient: effects of duration of dry events. Freshw Biol 57:563–574
Datry T, Larned ST, Fritz KM, Bogan MT, Wood PJ, Meyer EI, Santos AN (2014) Broad-scale patterns of invertebrate richness and community composition in temporary rivers: effects of flow intermittence. Ecography 37:94–104
Datry T, Pella H, Leigh C, Bonada N, Hugueny B (2016) A landscape approach to advance intermittent river ecology. Freshw Biol 61:1200–1213. https://doi.org/10.1111/fwb.12645
Datry T, Corti R, Heino J, Hugueny B, Rolls RJ, Ruhí A (2017) Habitat fragmentation and metapopulation, metacommunity, and metaecosystem dynamics in intermittent rivers and ephemeral streams. Chapter 4.9. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 377–403. https://doi.org/10.1016/B978-0-12-803835-2.00014-0
Davis JC (1975) Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J Fish Res Board Can 32(12):2295–2332. https://doi.org/10.1139/f75-268
de Necker L, Brendonck L, van Vuren J, Wepener V, Smit NJ (2021) Aquatic invertebrate community resilience and recovery in response to a supra-seasonal drought in an ecologically important naturally Saline Lake. Water 13:948. https://doi.org/10.3390/w13070948
Díaz AM, Suárez ML, Vidal-Abarca MR (2008) Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshw Biol 53:1–21. https://doi.org/10.1111/J.1365-2427.2007.01854.X
Dodson S (1992) Predicting crustacean zooplankton species richness. Limnol Oceanogr 37:848–856. https://doi.org/10.4319/LO.1992.37.4.0848
Döll P, Schmied HM (2012) How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ Res Lett. https://doi.org/10.1088/1748-9326/7/1/014037
Estévez E, Rodríguez-Castillo T, González-Ferreras AM, Cañedo-Argüelles M, Barquín J (2019) Drivers of spatio-temporal patterns of salinity in Spanish rivers: a nationwide assessment. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2018.0022
Flórián N, Ladányi M, Ittzés A, Kröel-Dulay G, Ónodi G, Mucsi M, Szili-Kovács T, Gergócs V, Dányi L, Dombos M (2019) Effects of single and repeated drought on soil microarthropods in a semi-arid ecosystem depend more on timing and duration than drought severity. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0219975
García C (1991) Estudio de un medio acuático fluctuante: la laguna atalasohalina de Fuente de Piedra (Málaga). PhD Thesis, University of Malaga
García-Roger EM, Sánchez-Montoya MM, Gómez R et al (2011) Do seasonal changes in habitat features influence aquatic macroinvertebrate assemblages in perennial versus temporary Mediterranean streams? Aquat Sci 73:567–579. https://doi.org/10.1007/s00027-011-0218-3
García-Roger EM, Sánchez-Montoya MM, Cid N, Erba S, Karaouzas I, Verkaik I, Rieradevall M, Gómez R, Súarez ML, Vidal-Abarca MR, Demartini D, Buffagni A, Skoulikidis N, Bonada N, Prat N (2013) Spatial scale effects on taxonomic and biological trait diversity of aquatic macroinvertebrates in Mediterranean streams. Fundam Appl Limnol 183(2):89–105. https://doi.org/10.1127/1863-9135/2013/0429
Gascón S, Boix D, Sala X, Quintana XD (2006) Nematode assemblages and their responses to disturbances: A case study from the Empordà wetlands (northeastern Iberian Peninsula). J N Am Benthol Soc 25:643–655. https://doi.org/10.1899/0887-3593(2006)25[643:NAATRT]2.0.CO;2
Ginatullina E, Atwell L, Saito L (2017) Resilience and resistance of zooplankton communities to drought-induced salinity in freshwater and saline lakes of Central Asia. J Arid Environ 144:1–11. https://doi.org/10.1016/J.JARIDENV.2017.04.010
Golec-Fialek C, Lansac-Tôha FM, Bonecker CC (2021) Response of the zooplankton community to extreme hydrological variations in a temporary lake in a neotropical floodplain system. Limnologica. https://doi.org/10.1016/J.LIMNO.2020.125834
Gómez R, Hurtado I, Suárez ML, Vidal-Abarca MR (2005) Ramblas in south-east Spain: threatened and valuable ecosystems. Aquat Conserv Mar Freshw Ecosyst 15:387–402. https://doi.org/10.1002/aqc.680
Gómez R, Arce MI, Baldwin DS, Dahm CN (2017) Water physicochemistry in intermittent rivers and ephemeral streams. Chapter 3.1. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 109–134
Gutiérrez-Cánovas C, Sánchez-Fernandez D, Velasco J, Millán A, Bonada N (2015) Similarity in the difference: Changes in community functional features along natural and anthropogenic stress gradients. Ecology 96:2458–2466. https://doi.org/10.1890/14-1447.1
Hammer UT (1986) Saline lake ecosystems of the world 59. Springer Science & Business Media
Hart BT, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1991) A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210:105–144. https://doi.org/10.1007/BF00014327
Hill MJ, Milner VS (2018) Ponding in intermittent streams: A refuge for lotic taxa and a habitat for newly colonising taxa? Sci Total Environ 628–629:1308–1316. https://doi.org/10.1016/j.scitotenv.2018.02.162
Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT (2016) Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Change Biol 22:180–189. https://doi.org/10.1111/GCB.13028
Jeppensen E, Brucet S, Naselli-Flores L et al (2015) Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750:201–227. https://doi.org/10.1007/s10750-014-2169-x
Karagianni A, Stamou G, Katsiapi M, Polykarpou P, Dörflinger G, Michaloudi E (2018) Zooplankton communities in Mediterranean temporary lakes: THE case of saline lakes in Cyprus. Ann Limnol. https://doi.org/10.1051/limn/2018007
Karpowicz M, Ejsmont-Karabin J, Kozłowska J, Feniova I, Dzialowski AR (2020) Zooplankton community responses to oxygen stress. Water 12:706. https://doi.org/10.3390/W12030706
Leigh C, Datry T (2016) Drying as primary hydrological determinant of biodiversity in river systems: a broadscale analysis. Ecography 39:1–13
Lessel T, Marx MT, Eisenbeis G (2011) Effects of ecological flooding on the temporal and spatial dynamics of carabid beetles (Coleoptera, Carabidae) and springtails (Collembola) in a polder habitat. ZooKeys 100:421–446. https://doi.org/10.3897/ZOOKEYS.100.1538
Martinez P (2020) pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.4
Marx MT, Guhmann P, Decker P (2012) Adaptations and predispositions of different middle European arthropod taxa (Collembola, Araneae, Chilopoda, Diplopoda) to flooding and drought conditions. Animals 2:564–590. https://doi.org/10.3390/ANI2040564
McCluney KE, Sabo JL (2012) River drying lowers the diversity and alters the composition of an assemblage of desert riparian arthropods. Freshw Biol 57:91–103. https://doi.org/10.1111/J.1365-2427.2011.02698.X
McCulloch GP, Irvine K, Eckardt FD, Bryant R (2008) Hydrochemical fluctuations and crustacean community composition in an ephemeral saline lake (Sua Pan, Makgadikgadi Botswana). Hydrobiologia 596:31–46. https://doi.org/10.1007/s10750-007-9055-8
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Meybeck M (1995) Global distribution of lakes. In: D. M, Lerman Abraham GJR, Imboden (ed) Physics and chemistry of lakes. Springer Berlin Heidelberg, p 1. https://doi.org/10.1007/978-3-642-85132-2_1
Miliša M, Stubbington R, Datry T, Cid N, Bonada N, Šumanović M, Milošević D (2022) Taxon-specific sensitivities to flow intermittence reveal macroinvertebrates as potential bioindicators of intermittent rivers and streams. Sci Total Environ 804:150022. https://doi.org/10.1016/j.scitotenv.2021.150022
Millán A, Velasco J, Gutiérrez-Cánovas C, Arribas P, Picazo F, Sánchez-Fernández D, Abellán P (2011) Mediterranean saline streams in southeast Spain: what do we know? J Arid Environ 75:1352–1359. https://doi.org/10.1016/j.jaridenv.2010.12.010
Mormul RP, de Esteves AF, Farjalla VF, Bozelli RL (2015) Space and seasonality effects on the aquatic macrophyte community of temporary Neotropical upland lakes. Aquat Bot. https://doi.org/10.1016/j.aquabot.2015.06.007
Mosley LM (2015) Drought impacts on the water quality of freshwater systems; review and integration. Earth Sci Rev 140:203–214. https://doi.org/10.1016/J.EARSCIREV.2014.11.010
Nielsen DL, Brock MA, Rees GN, Baldwin DS (2003) Effects of increasing salinity on freshwater ecosystems in Australia. Aust J Bot 51:655. https://doi.org/10.1071/BT02115
O’Brien WJ, Barfield M, Bettez ND, Gettel GM, Hershey AE, McDonald ME, Miller MC, Mooers H, Pastor J, Richards C, Schuldt J (2004) Physical, chemical, and biotic effects on arctic zooplankton communities and diversity. Limnol Oceanogr 49:1250–1261. https://doi.org/10.4319/LO.2004.49.4_PART_2.1250
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, Ohara R, Wagner H (2013) Vegan: Community ecology package (R package version 2).
Pace G, Bonada N, Prat N (2013) Long-term effects of climatic-hydrological drivers on macroinvertebrate richness and composition in two Mediterranean streams. Freshw Biol 58:1313–1328. https://doi.org/10.1111/fwb.12129
Pachauri RK (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. In IPCC.
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic terrestrial linkages along a Braided-River: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759. https://doi.org/10.1007/s10021-005-0004-y
Pflug A, Wolters V (2001) Influence of drought and litter age on Collembola communities. Eur J Soil Biol 37:305–308. https://doi.org/10.1016/S1164-5563(01)01101-3
Quintana XD, Boix D, Badosa A, Brucet S, Compte J, Gascon S, López-Flores R, Sala X, Moreno-Amich R (2006) Community structure in mediterranean shallow lentic ecosystems: size-based vs. taxon-based approaches. Limnetica 25:303–320. https://doi.org/10.23818/LIMN.25.21
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing
Rodríguez-Flores PC, Gutiérrez-Rodríguez J, Aguirre-Ruiz EF, García-París M (2016) Salt lakes of La Mancha (Central Spain): A hot spot for tiger beetle (Carabidae, Cicindelinae) species diversity. ZooKeys 2016:63. https://doi.org/10.3897/ZOOKEYS.561.6042
Rubel F, Kottek M (2010) Observed and projected climate shifts 1901–2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol Z 19(2):135–141
Rueda F, Montes C (1988) Los Carábidos (Col. Carabidae) de las orillas de las lagunas salinas de la Península Ibérica. Aspectos faunísticos. Actas III Congreso Ibérico De Entomología 651–662.
Rueda F, Montes C (1987) Riparian carabids of saline aquatic ecosystems. Acta Phytopath Entom Hung 22:247–263
Sánchez-Montoya MM, Puntí T, Suárez ML, Vidal-Abarca MR, Rieradevall M, Poquet JM, Zamora-Munoz C, Robles S, Álvarez M, Alba-Tercedor J, Toro M, Pujante A, Munné A, Prat N (2007) Concordance between ecotypes and macroinvertebrate assemblages in Mediterranean streams. Freshw Biol 52:2240–2255
Sánchez-Montoya MM, von Schiller D, Ruhí A, Pechar GS, Proia L, Miñano J, Vidal-Abarca MR, Súarez ML, Tockner K (2016) Responses of ground-dwelling arthropods to surface flow drying in channels and adjacent habitats along Mediterranean streams. Ecohydrology 9:1376–1387. https://doi.org/10.1002/eco.1733
Sánchez-Montoya MM, von Schiller D, Barberá GG, Díaz AM, Arce MI, del Campo R, Tockner K (2018) Understanding the effects of predictability, duration, and spatial pattern of drying on benthic invertebrate assemblages in two contrasting intermittent streams. PLoS ONE. https://doi.org/10.1371/journal.pone.0193933
Sánchez-Montoya MM, Guerrero-Brotons M, Miñano J, Gómez R (2020a) Effects of debris piles and pools along dry riverbeds on nutrients, microbial activity, and ground-dwelling arthropods: a Namibian ephemeral river case. J Arid Environ. https://doi.org/10.1016/j.jaridenv.2019.104082
Sánchez-Montoya MM, Tockner K, von Schiller D, Miñano J, Catarineu C, Lencina JL, Barberá GG, Ruhi A (2020b) Dynamics of ground-dwelling arthropod metacommunities in intermittent streams: The key role of dry riverbeds. Biol Conserv. https://doi.org/10.1016/j.biocon.2019.108328
Santamaría L, Balsa J, Bidondo B, Baltanás A, Montes C (1992) Salinity tolerance of three ostracode species (Crustacea:Ostracoda) of Iberian saline lakes. Hydrobiologia 246:89–98. https://doi.org/10.1007/BF00014696
Schriever TA, Bogan MT, Boersma KS, Cañedo-Argüelles M, Jaeger KL, Olden JD, Lytle DA (2015) Hydrology shapes taxonomic and functional structure of desert stream invertebrate communities. Freshw Sci 34:399–409. https://doi.org/10.1086/680518
Schultz TD (1989) Habitat Preferences and Seasonal Abundances of Eight Sympatric Species of Tiger Beetle, Genus Cicindela (Coleoptera: Cicindelidae), in Bastrop State Park, Texas. Source Southwestern Nat 34:468–477
Senner NR, Moore JN, Seager ST, Dougill S, Kreuz K, Senner SE (2018) A salt lake under stress: relationships among birds, water levels, and invertebrates at a Great Basin saline lake. Biol Cons 220:320–329. https://doi.org/10.1016/j.biocon.2018.02.003
Southwood TRE (1988) Tactics, strategies and templets. Oikos 52:3–18. https://doi.org/10.2307/3565974
Steward AL, Marshall JC, Sheldon F, Harch B, Choy S, Bunn SE, Tockner K (2011) Terrestrial invertebrates of dry river beds are not simply subsets of riparian assemblages. Aquat Sci 73:551–566. https://doi.org/10.1007/s00027-011-0217-4
Steward AL, von Schiller D, Tockner K, Marshall JC, Bunn SE (2012) When the river runs dry: human and ecological values of dry riverbeds. Front Ecol Environ 10:202–209. https://doi.org/10.1890/110136
Steward AL, Langhans SD, Corti R, Datry T (2017) The biota of intermittent rivers and ephemeral streams: terrestrial AND semiaquatic invertebrates. Chapter 4.4. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 245–266. https://doi.org/10.1016/B978-0-12-803835-2.00008-5
Steward AL, Datry T, Langhans SD (2022) The terrestrial and semi-aquatic invertebrates of intermittent rivers and ephemeral streams. Biol Rev 97:1408–1425. https://doi.org/10.1111/brv.12848
Stubbington R, Bogan MT, Bonada N, Boulton AJ, Datry T, Leigh C, Vander Vorste R (2017) The biota of intermittent rivers and ephemeral streams: aquatic invertebrates. Chapter 4.3. In: Boulton A, Datry T, Bonada N (eds) Intermittent rivers and ephemeral streams: ecology and management. Academic Press, Burlington, pp 217–237. https://doi.org/10.1016/B978-0-12-803835-2.00007-3
Suárez ML, Sánchez-Montoya MM, Gómez R, Arce MI, del Campo R, Vidal-Abarca MR (2017) Functional response of aquatic invertebrate communities along two natural stress gradients (water salinity and flow intermittence) in Mediterranean streams. Aquat Sci 79:1–12. https://doi.org/10.1007/s00027-016-0475-2
Thièry A (1987) Les Crustaces Branchiopodes Anostraca, Notostraca Et Conchostraca Des Milieux Limniques Temporaires (Dayas) Au Maroc. Taxonomie, Biogeographie, Ecologie. Doctoral dissertation, Aix-Marseille 3), Faculte des Sciences et techniques de St Jerôme, Universite de Droit d'Economie et des Sciences d'Aix-Marseille. pp 406
Vander Vorste R, Corti R, Sagouis A, Datry T (2016) Invertebrate communities in gravel-bed, braided rivers are highly resilient to flow intermittence. Freshw Sci 35:164–177. https://doi.org/10.1086/683274
Velasco J, Millán A, Hernández J, Gutiérrez-Cánovas C, Abellán P, Sánchez-Fernández D, Ruiz M (2006) Response of biotic communities to salinity changes in a Mediterranean hypersaline stream. Aquat Biosyst 2:12. https://doi.org/10.1186/1746-1448-2-12
Velasco J, Gutiérrez-Cánovas C, Botella-Cruz M, Sánchez-Fernández D, Arribas P, Carbonell JA, Millán A, Pallarés S (2019) Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos Trans R Soc B. https://doi.org/10.1098/rstb.2018.0011
Vidal-Abarca MR, Suárez ML, Moreno J, Sánchez I (2000) Hidroquímica de un río de características semiáridas (Río Chicamo;Murcia). Análisis Espacio-Temporal Limnética 18:57–73
Vidal-Abarca MR, Sánchez-Montoya MM, Guerrero C, Gómez R, Arc MI, García-García V, Suárez ML (2013) Effects of intermittent stream flow on macroinvertebrate community composition and biological traits in a naturally saline Mediterranean stream. J Arid Environ. https://doi.org/10.1016/j.jaridenv.2013.09.008
Vidal-Abarca MR, Gómez R, Sánchez-Montoya MM, Arce MI, Nicolás N, Suárez ML (2020) Defining dry rivers as the most extreme type of non-perennial fluvial ecosystems. Sustainability 12:7202. https://doi.org/10.3390/SU12177202
Waterkeyn A, Grillas P, Vanshoenwinkel B, Brendonck L (2008) Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients. Freshw Biol 53:1808–1822. https://doi.org/10.1111/j.1365-2427.2008.02005.x
Woolway RI, Sharma S, Smol JP (2022) Lakes in hot water: the impacts of a changing climate on aquatic ecosystems. Bioscience 72(11):1050–1061. https://doi.org/10.1093/biosci/biac052
Xu GL, Kuster TM, Günthardt-Goerg MS, Dobbertin M, Li MH (2012) Seasonal exposure to drought and air warming affects soil Collembola and Mites. PLoS ONE. https://doi.org/10.1371/journal.pone.0043102
Zsuga K, Inelova Z, Boros E (2021) Zooplankton community structure in shallow saline steppe inland waters. Water 13:1164. https://doi.org/10.3390/w13091164
Acknowledgements
This study was financially supported by the Spanish Ministry of Science, Innovation and Universities (project ref: RTI2018-097950-B-C22; PID2021-126143OB-C22). We thank the sampling permission from Dirección General de Medio Natural de la Consejería de Agua, Agricultura, Ganadería, Pesca y Medio Ambiente de la Región de Murcia (AUF/2020/0003) and Instituto Aragonés de Gestión Ambiental (500201/21B/2019/03522 and 500201/19/2021/04021). We thank Eva Pérez and Andrea Montesinos for their help in sampling campaigns and Helen Warburton for the English revision.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Z.F., R.G., P.A., and MM.SM. carried out the conceptualization, conducting the research, data analysis and data interpretation. Z.F., R.G., P.A., J.M., and MM.SM. carried out developing methods. Z.F., R.G., P.A., J.B., and MM.SM. wrote the main manuscript text and carried out the figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest and funding
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to error in the author names.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freixinos, Z., Gómez, R., Alcorlo, P. et al. Disentangling responses of aquatic and terrestrial invertebrates to drying in saline streams and shallow lakes. Aquat Sci 86, 57 (2024). https://doi.org/10.1007/s00027-024-01072-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01072-z