Abstract
In intermittent streams, aquatic organisms use various strategies to face dry phases, but the relative contribution of these strategies to persist during dry phase remains unclear. Here, we investigated the in situ persistence of benthic invertebrates in the saturated hyporheic sediments and the “invertebrate seedbank” that persists in dry sediments across six Mediterranean intermittent streams. Taxonomic and functional responses within hyporheic and seedbank assemblages were compared with those in the benthic assemblages under connected flow conditions by combining field and mesocosms data. The dry phase duration in each stream was calculated to assess the responses of hyporheic and seedbank assemblages. Taxonomic composition and abundance-weighted traits related to resistance and resilience to face the dry phase in each assemblage type (benthic, hyporheic, seedbank) were determined. Taxonomic (richness and diversity) and functional (richness and dispersion) metrics were also calculated. We found that seedbank and hyporheic assemblages supported up to 16% and 40% of the benthic taxa, respectively. Only taxonomic and functional richness differed between assemblage types. Contrary to previous research, no clear relationship was established between diversity or the abundance of resistance traits and the duration of dry phase; however, a negative linear relationship was identified between the abundance of resilience traits and the duration of the dry phase. The increase in the frequency and duration of drying events due to climate change will reduce water availability in both saturated and unsaturated streambed sediments, compromising the persistence of aquatic biodiversity in intermittent streams.
Similar content being viewed by others
Introduction
Intermittent streams, characterized by shifts between wet and dry in-stream conditions, comprise more than 50% of global river networks (Messager et al. 2021). Flow interruption in these streams occurs gradually and varies in both time and space, modifying the lateral, longitudinal, and vertical dimensions of hydrological connectivity during different hydrological phases (Datry et al. 2014). Flow contraction can culminate in the complete loss of surface water during the dry phase. The dry phase affects ecosystem functions, such as organic matter processing and sediment transport, as well as riparian and aquatic biodiversity (Larned et al. 2010). Aquatic invertebrate communities in intermittent streams have various adaptations to face dry phases, including the use of remaining wet habitats as refuges, as hyporheic zone (HZ), or the capacity to persist within dry sediment, as a “seedbank” (Stubbington and Datry 2013). However, the contribution of both hyporheic refuge and invertebrate seedbank, as used by benthic communities to persist during dry phases, have not yet been compared (Hay et al. 2018; DelVecchia et al. 2022). In the context of global change, in which the spatial and temporal distribution of many intermittent streams is predicted to increase (Döll and Schmied 2012; Sauquet et al. 2021), understanding how invertebrates persist in situ during dry phases in wet and dry sediments, i.e., in the hyporheic zone and invertebrate seedbank, is necessary to inform actions that preserve their biodiversity (Datry et al. 2018).
Drying alters community composition, with reductions in taxonomic and functional diversity increasing with dry-phase severity (Arias‐Real et al. 2021; Crabot et al. 2021b). In natural intermittent streams, communities are adapted to drying, displaying different combinations of functional traits that promote resistance and/or resilience to face dry phases (Nimmo et al. 2015; Bogan et al. 2015). Resilience strategies enable communities to recover after flow resumption by aquatic or aerial dispersal from refuges including upstream, downstream, and/or nearby perennial reaches. Active dispersal can also enable escape of dry conditions to wet refuges, following environmental cues as flow recedes, potentially supporting subsequent community recovery (Drummond et al. 2015). In contrast, resistance strategies enable organisms to persist in situ during dry phases (Bogan et al. 2017). For instance, during dry phases, some invertebrates can use isolated pools in streambeds (Bonada et al. 2006) or move to the subsurface sediments of the HZ (Stubbington 2012; DelVecchia et al. 2022).
The capacity of the HZ to act as a refuge for aquatic invertebrates during dry phases varies spatially and temporally, influenced by factors such as sediment composition, moisture content, and water quality (Stubbington 2012). Should the dry phase persist, water levels within the HZ may also decline, affecting taxonomic composition and leading to the loss of desiccation-sensitive taxa (Stubbington et al. 2009). After the water table recedes through the subsurface, aquatic invertebrates can only persist in damp streambed sediments as invertebrate seedbanks, which include desiccation-tolerant life stages, such as dormant eggs or juveniles (Tronstad et al. 2005). Partially desiccation-tolerant taxa can also survive within the invertebrate seedbank, provided some sediment moisture remains (Loskotová et al. 2021). The invertebrate seedbank can support over 50% of benthic taxa, and it can make a particularly substantial contribution to community recovery in certain areas, such as isolated catchments or reaches without saturated HZ (Stubbington and Datry 2013). However, some taxa are eliminated as their taxon-specific desiccation-tolerance thresholds are surpassed (Stubbington et al. 2016). Hyporheic refuge and the invertebrate seedbank are well-documented individually, but how benthic invertebrates use these different in situ strategies to face dry phases remains unclear.
Our aim was to characterize the relative contributions of the hyporheic refuge and the invertebrate seedbank to supporting the persistence of benthic aquatic invertebrates in situ during dry phases in intermittent streams, by combining field data with mesocosm experiments. We predicted that (1) the taxonomic and functional diversity of hyporheic and seedbank assemblages will represent subsets of benthic communities, resulting in lower taxonomic and functional diversity; (2) the dry phase will result in a higher abundance of traits that confer resistance to these conditions in invertebrates from seedbank and the HZ, compared with those in benthic communities. This is because benthic taxa present in hyporheic refuge and invertebrate seedbank possess traits enabling them to tolerate dry phases. Specifically, the capacity to move into the HZ will be more abundant in taxa from HZ, whereas the ability to persist by using any desiccation-tolerant life stage will be more abundant among taxa from invertebrate seedbank; and (3) streams experiencing prolonged dry phases will show reduced taxonomic and functional diversity in hyporheic and seedbank assemblages, and an increased abundance of resistance traits, attributed to the decline in desiccation-sensitive taxa.
Methods
Study sites
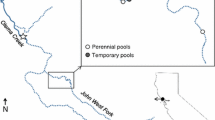
We sampled six reaches, one in each of six intermittent Mediterranean streams across Catalonia on the northeastern Iberian Peninsula (Fig. 1), during the summer of 2020. Selected sites had similar geology, stream order, and stream width. Specifically, stream orders spanned from three to four across an altitudinal range of 110–526 m a.s.l. The geology of streams was characterized by sandstone, granite, and marl formations, with the streambeds primarily composed of gravel and coarse sand. All streams had a natural flow intermittence, experiencing dry-phase durations between 16 and 69 days, starting between early and late summer. Throughout the study period, only two reaches (Tordera and Ebro) did not dry completely as they retained pools in the streambed (personal observation). Flow resumed almost simultaneously across all streams with the onset of autumn rains in mid-September 2020, following our dry phase sample collection (Table S1).
Field methods
Hydrological characterization
In June 2020, before the dry phase started, a temperature data logger (HOBO Pendant MX2201, Onset Corp, Bourne, MA, USA) was installed in the middle of each streambed, avoiding deep pools to obtain a more representative record of the flow disconnection in the stream. Hourly temperatures were recorded until the moment of sampling collection in early September 2020. We calculated the number of days without surface water (the dry-phase duration) for each stream using the daily temperature variation between the maximum and minimum streambed temperatures. This variation indicates water presence due to the higher specific heat capacity of water compared with air (Smith 1975). As a result, daily variability in temperatures is lower when water is present. Air temperature and rainfall data from proximate meteorological stations were utilized to elucidate any anomalies in daily temperature fluctuations, by comparing direct measurements of air temperature and precipitation patterns with the presence of flow. However, the presence of surface water can vary within a stream reach because drying occurs more quickly in riffles than in pools (Hwan and Carlson 2016).
Sample collection
To characterize invertebrate seedbanks, we excavated sediment samples from three points in each streambed at the end of the summer, just before the onset of autumn rainfalls, using a hand trowel. These points were selected for their lower slope, which favors the deposition of sediments. Larger surface clasts (> 5 cm) were intentionally avoided, and the depth of collection was restricted to a maximum of 15 cm. Each sampled area ranged from 0.5 to 2 m2. Approximately 8 L of dry sediment were collected at every reach, with an attempt to collect the same quantity from each area (~2.5–3 L). The sediments collected from each area were combined and transported to the laboratory.
We collected three replicate hyporheic water samples from a depth of 40 cm at 15–20 m intervals along the dry reach. Because hyporheic exchange flow can vary between riffles and pools (Käser et al. 2009), we exclusively sampled in riffle zones to minimize differences. To maximize richness estimates using a Bou–Rouch pump (Bou and Rouch 1967), we tried to pump 20 L of hyporheic water for each replicate. However, on several occasions, we were unable to find hyporheic water and had to reposition the pump multiple times to locate it where feasible. Consequently, we were able to extract a total of 60 L of hyporheic water from only one reach, while from three other reaches, we extracted between 35 and 50 L. The HZ in two reaches, Llobregat and Fluvià, was not sampled because they were dry at the sampled depth (Table S1). The pumped water was filtered through a 250 µm mesh to retain invertebrates (Sánchez-Morales et al. 2018).
Benthic invertebrates were collected at each reach just after the rainy season (February) to ensure that all streams were in the flowing phase and connected. We collected 5 min kick samples with a 250 μm net following a standardized multihabitat protocol that represented each habitat in proportion to its occurrence (Jáimez-Cuéllar et al. 2002).
Seedbank mesocosm setup
In the laboratory, we sieved 7.5 L of dry sediment from each reach through a 0.5 cm test sieve to remove large sediment grains. We then distributed it into three 8 L containers and covered each container with a 1 mm mesh to minimize external colonization and to retain emerging invertebrates. The containers were immediately flooded with 5 L of dechlorinated tap water, continuously aerated with air stones, and maintained at room temperature under a natural photoperiod of 13:11 h (light/dark). We sampled the water column using a 250 µm hand net for 30 s at 0, 8, 16, and 32 days to collect invertebrates. After 32 days, the experiment was stopped, and all invertebrates in the sediment were collected (Datry et al. 2012) by filtering the column water and screening the sediments from each mesocosm.
Invertebrate data
All invertebrates were identified to genus except for Diptera (which were identified to the subfamily or family level), Oligochaeta (identified to the subclass level), Nematoda (identified to the phylum level), and individuals that were too small for accurate identification. Micro-crustaceans were also observed, noting only their presence or absence at the class level (Ostracoda and Copepoda) and at the genus level (Daphnia), without quantifying their abundance (Table S2). We aggregated all replicate samples to calculate global community diversity indices and for data analysis due to the low number of individuals per sample and the high incidence of zeros in the replicate hyporheic samples and invertebrate seedbank samples. Relative abundances, rather than absolute abundances, were calculated because different methods were used to sample each assemblage type (benthic, hyporheic, seedbank). As a result, we obtained six samples for each type of assemblage, except for hyporheic assemblages.
Resistance and resilience traits
To assess the functional aspects of each prediction, we selected and classified trait categories that promote resistance and resilience during dry phases, considering both as distinct trait classes. We considered traits associated with resistance, including any desiccation-tolerant life stages (resistance forms’ trait), aerial respiration (spiracle or plastron), the capacity to move into the HZ, generalist feeding habits (herbivorous and detritivorous habits; Cummins 1973), and small body size, as resistance trait categories. We classified female wing size, large body size, aerial active dispersal, multivoltine capacity, short life cycle duration (≤ 1 year), and terrestrial adult life stage as resilience trait categories (Table S3). Trait data were compiled from Múrria et al. (2020) and Sarremejane et al. (2020). Values were assigned to represent the affinity of each taxon for each trait category, taking into account differences among taxa within the same genus (referred to as potential trait adaptability) using a fuzzy code approach (Chevenet et al. 1994). All category scores were standardized to proportions by setting the category sum to 1. Our final trait set had a total of 27 categories belonging to 11 traits.
For each assemblage, we calculated an abundance-weighted trait matrix by multiplying the taxa–trait matrix by the relative abundance taxa matrix (Statzner et al. 2004). Furthermore, we calculated the average abundance-weighted values for each trait class (resistance or resilience) across each assemblage type.
Taxonomic and functional metrics
To represent invertebrate seedbank “importance” (sensu Stubbington and Datry 2013), we calculated the percentage of benthic taxa present in the HZ and the invertebrate seedbank for each stream. We also identified taxa that occurred exclusively in each assemblage type. Additionally, we calculated taxonomic richness (S) and the Shannon–Wiener index (H’) (Shannon 1948) for each assemblage.
To describe functional diversity, we built a functional space (FS) using the taxa–trait matrix that included all taxa from all assemblages (72 taxa) and all trait categories. The taxa–trait matrix was used to generate a dissimilarity matrix based on the Gower distances adapted to fuzzy coding traits (Pavoine et al. 2009). The FS was created using a principal coordinates analysis conducted with the dudi.pco function from the R package ade4 (Dray and Dufour 2007). We selected the five most informative axes based on their ability to represent the original trait-based dissimilarity among taxa (Maire et al. 2015). The 5-D FS explained 71.90% of the Gower dissimilarity matrix (with a mean squared deviation of 0.009). To identify the traits associated with each FS axis, we utilized Spearman correlation coefficients (Múrria et al. 2020). In addition, the FS was used to obtain two functional diversity metrics. The first metric, functional richness (FRic), was calculated as the minimum hypervolume of the FS occupied by invertebrate taxa in each assemblage (Villéger et al. 2008). Low values of FRic suggest that taxa capable of responding to environmental disturbance may be absent (Mason et al. 2005). The second metric, functional dispersion (FDis), represented the weighted mean distance of all invertebrate taxa to the weighted centroid of their assemblage within the FS (Laliberté and Legendre 2010), being equivalent to the multivariate dispersion (Anderson et al. 2006). FRic and FDis are complementary metrics; FRic captures the range of the FS covered by each assemblage, whereas FDis focuses on the variation in their trait abundances (Kuebbing et al. 2018). Both metrics were calculated using the R package FD (Laliberté and Legendre 2010; Laliberté et al. 2014).
Data analysis
To test differences between assemblage types in all metrics (taxonomic and functional metrics and the mean of abundance-weighted traits by trait class), we used the nonparametric Kruskal‒Wallis test (Ostertagová et al. 2014). Additionally, post hoc pairwise comparison tests were performed between assemblage types using a Wilcoxon rank sum test (Wilcoxon 1945) with a Bonferroni correction to a significance level at p < 0.05.
To assess the effect of dry-phase duration on the resilience and resistance of aquatic invertebrates in hyporheic and seedbank assemblages, we fitted linear and nonlinear regressions, utilizing nonlinear least squares for parameter estimation in the latter (Smyth 2002). We used only data from hyporheic and seedbank assemblages, with dry-phase duration as the explanatory variable, and the mean of abundance-weighted traits for each trait class, along with their different diversity metrics, as the different response variables. The effect of dry-phase duration on individual abundance-weighted trait categories was also tested using the same approach. For linear regressions (LM), residuals were visually examined for normality and homoscedasticity. All data were analyzed using R (R Core Team 2022).
Results
Taxonomic diversity
We identified a total of 24,487 individuals: belonging to 49 families and 41 genera, which included 23,970 from 34 taxa in the benthic assemblages, 415 from 24 taxa in the hyporheic assemblages, and 102 from 21 taxa in the invertebrate seedbank (Table S4). Four groups each accounted for more than 5% individuals of the benthic assemblages: Diptera (51.46%), Mollusca (18.19%), Oligochaeta (12.77%), and Ephemeroptera (7.43%). Three groups each accounted > 5% individuals of the hyporheic assemblages: Diptera (62.41%), Oligochaeta (22.41%), and Ephemeroptera (7.47%). Finally, five groups each accounted > 5% individuals of the seedbank assemblages: Diptera (41.18%), Oligochaeta (21.57%), Coleoptera (13.73%), Ephemeroptera (8.82%), and Hirudinea (5.88%). Diptera was the only order present in all seedbanks. Nematomorpha, Coleoptera, and Ephemeroptera were present in all hyporheic assemblages.
Seedbank and hyporheic invertebrates represented 4.5–16% and 8.7–40% of benthic taxa, respectively (Table S5). Four taxa were exclusively found in invertebrate seedbanks (Nebrioporus sp., Eriopterini, Sciomyzidae, Erpobdellidae), with each taxon limited to a single stream. Similarly, four taxa were exclusive to the HZ (Ecdyonurus sp., Forcipomyiinae, Glossosomatidae, Polycentropodidae), each restricted to a single stream. Thirty-three taxa were present only in benthic assemblages (e.g., Micronecta sp. and Sericostoma sp.), but only the family Lymnaeidae was encountered in all benthic assemblages (Table S6).
Taxonomic richness was highest for benthic assemblages (S = 22 ± 2 taxa per sample), moderate for hyporheic assemblages (11 ± 2 taxa), and lowest for seedbank assemblages (6 ± 1 taxa) (Kruskal–Wallis: χ2 = 13.353; d.f. = 2, p = 0.0013; associated Wilcoxon paired test: 0.01 < all Bonferroni p-adjusted < 0.05; Fig. 2a). The Shannon‒Wiener index was comparable in all assemblages, showing values between 1.50 ± 0.12 and 1.86 ± 0.31 (Kruskal–Wallis: χ2 = 5.5772; d.f. = 2, p = 0.0615; Fig. 2b).
Taxonomic (a, b) and functional (c, d) metric values for benthic, hyporheic and seedbank assemblages: a taxonomic richness (S), b Shannon‒Wiener diversity (H’), c functional richness (FRic), and d functional dispersion (FDis). Solid lines represent the median, while dashed lines represent the mean. Different letters indicate statistically significant differences among assemblage types
Functional diversity
The first two axes of the FS jointly explained 43.77% of the original trait variation, with axes 1 and 2 explaining 23.79% and 19.98%, respectively (Fig. 3a). Axis 1 was highly correlated (> 0.50, Spearman correlation) with feeding habit, body size, voltinism, and female wing length, aligning with the resistance trait categories at one extreme (generalist feeding habits and small body size) and the resilience trait categories at the other (large body size and high female wing length). Axis 2 was highly correlated (> 0.50, Spearman correlation) with adult life stage, life cycle duration, and female wing length, primarily corresponding to the resilience trait categories at one of its extremes (terrestrial adult stage and a life cycle duration of 1 year or less; Table S8). Orders plotted in three main clusters: Diptera, Ephemeroptera, Plecoptera, and Trichoptera in the upper left-hand quadrant; Odonata and Megaloptera with positive axis 1 values; and all eight other orders with negative axis 2 values (Fig. 3a).
Functional space (FS) plot on two axes: a FS with taxa represented by points colored according to taxonomic group. The dotted line denotes the boundary of the total FS created by all taxa. Categories of selected traits that are correlated with each axis are located at the corresponding axis extreme. b FS occupied by each assemblage, with small points representing individual taxa; red crosses indicating the assemblage centroids; and black crosses marking the centroid of the all-taxa ordination. Biv Bivalvia, Col Coleoptera, Cru Crustacea, Dip Diptera, Eph Ephemeroptera, Hem Hemiptera, Hir Hirudinea, Meg Megaloptera, Moll Mollusca, Nem Nematomorpha, Oli Oligochaeta, Odo Odonata, Ple Plecoptera, Tri Trichoptera
Benthic assemblages occupied more functional space than their respective hyporheic and seedbank assemblages (Fig. 3b). Two hyporheic assemblages occupied > 50% of the total FS, while the other two hyporheic assemblages occupied less space. All seedbank assemblages occupied < 50% of the FS, and the two smallest FS corresponded to the streams without HZ (Fig. 3b). Functional richness was highest for benthic assemblages (0.58 ± 0.11), and lowest for hyporheic (0.06 ± 0.01) and seedbank (0.05 ± 0.01) assemblages (Kruskal–Wallis: χ2 = 11.765; d.f. = 2, p = 0.0028; associated Wilcoxon paired test: Bonferroni p-adjustedall benthic = 0.014; Fig. 2c). Functional dispersion was not significantly different among assemblage types, showing values between 0.27 ± 0.05 and 0.32 ± 0.03 (Kruskal–Wallis: χ2 = 4.0772; d.f. = 2, p = 0.1302; Fig. 2d).
Resistance and resilience traits
The mean of abundance-weighted resistance trait categories was lowest for hyporheic assemblages (0.25 ± 0.20), and highest for benthic assemblages (0.47 ± 0.05) and seedbank assemblages (0.35 ± 0.11) (Kruskal–Wallis: χ2 = 11.486; d.f. = 2, p = 0.0032; associated Wilcoxon paired test: Bonferroni p-adjustedall hyporheic = 0.015; Fig. 4a). Generalist feeding habits were the predominant resistance trait category across all assemblage types, with weighted abundances ranging from 0.51 ± 0.14 to 0.82 ± 0.13. This was closely followed by the small body size, with weighted abundances between 0.35 ± 0.31 and 0.58 ± 0.17. The capacity to move into the HZ demonstrated moderate weighted abundances in both benthic (0.28 ± 0.05) and hyporheic (0.19 ± 0.06) assemblages, whereas the abundance of a resistance forms’ trait was more pronounced in dry sediment (0.47 ± 0.15) (Table S9). Two trait categories varied among the assemblage types: resistance forms’ trait, which was lowest for HZ (Kruskal–Wallis: χ2 = 9.7996; d.f. = 2, p = 0.0074; associated Wilcoxon paired test: 0.02 < Bonferroni p-adjustedhyporheic < 0.04), and aerial respiration, which was highest for the seedbank (Kruskal–Wallis: χ2 = 13.066; d.f. = 2, p = 0.0015; associated Wilcoxon paired test: 0.006 < Bonferroni p-adjustedall seedbank < 0.02).
Abundance-weighted traits for benthic, hyporheic, and seedbank assemblages: a mean of abundance-weighted trait by trait class; b resistance categories, and c resilience category. Solid lines indicate the median, and dashed lines represent the mean. Traits and trait categories are described in Table S2. Different letters indicate statistically significant differences
The mean of abundance-weighted resilience trait categories was statistically comparable in all assemblages, showing values between 0.25 ± 0.21 and 0.41 ± 0.04 (Kruskal–Wallis: χ2 = 1.8382; d.f. = 2, p = 0.3989; Fig. 4a). A short life cycle duration (≤ 1 year) was the predominant resilience trait category for all assemblage types, with weighted abundances ranging from 0.45 ± 0.40.01 to 0.72 ± 0.11. In benthic and hyporheic assemblages, this was followed by the terrestrial adult stage (0.65 ± 0.20 and 0.40 ± 0.38, respectively) and multivoltinism (0.47 ± 0.13 and 0.32 ± 0.28, respectively). In contrast, in seedbank assemblages, large body size and terrestrial adult stage were the subsequent most abundant trait categories (0.50 ± 0.14 and 0.42 ± 0.30, respectively). Notably, large body size varied among the assemblage types (Kruskal–Wallis: χ2 = 6.8843; d.f. = 2, p = 0.032; associated Wilcoxon paired test: Bonferroni p-adjustedseedbank–hyporheic = 0.039; Fig. 4c).
Effects of dry-phase duration on taxonomic and functional assemblages
Only the mean of abundance-weighted resilience traits differed in response to dry-phase duration in hyporheic and seedbank assemblages following a linear regression (LM: F1,8 = 17.75; d.f. = 8, p = 0.0029). Notably, as the dry-phase duration increased, the abundance of these traits decreased (Fig. 5a). Specifically, both the terrestrial adult stage (LM: F1,8 = 34.9; d.f. = 8, p = 0.0003) and short life cycle duration (LM: F1,8 = 14.51; d.f. = 8, p = 0.0052) also followed a negative linear relationship with the dry-phase duration (Fig. 5b). However, taxonomic and functional metrics, along with the mean of abundance-weighted resistance traits, did not conform to any discernible model (Fig. S1).
Linear relationship between dry-phase duration and (a) the mean of abundance-weighted resilience traits and (b) the terrestrial adult stage and short life cycle duration, both categories of resilience traits. Lilac points represent hyporheic assemblages, whereas orange points represent seedbank assemblages. Benthic assemblages were excluded
Discussion
This study is the first to compare the contribution of seedbank and hyporheic refuge used by benthic aquatic invertebrates to face dry phases in situ in intermittent streams. Our results showed that persistence in the seedbank and the HZ are strategies employed by a subset of benthic invertebrate taxa during dry phases in Mediterranean streams. Taxonomic and functional richness were higher in benthic assemblages than in those in the HZ and in invertebrate seedbanks, confirming our initial hypothesis that both hyporheic and seedbank assemblages are subsets of benthic assemblages. Contrary to our second hypothesis, no significant differences in the abundance-weighted resistance traits were observed between benthic and seedbank assemblages; consistently, hyporheic assemblages showed the lowest abundances. Finally, while no clear relationship was established between diversity or the abundance of resistance traits, and the duration of dry phases, as was expected, a linear negative relationship was identified between the abundance of resilience traits and the duration of the dry phase.
Contribution of hyporheic and seedbank invertebrate assemblages supporting benthic communities
Consistent with our first hypothesis, nearly all taxa and traits observed in the HZ and invertebrate seedbanks were also identified in benthic communities, which showed the highest taxonomic and functional richness. These findings suggest that hyporheic and seedbank assemblages represent subsets of the benthic communities (Stubbington 2012; Stubbington and Datry 2013). The taxonomic overlap among all assemblage types, ranging between 5% and 40%, demonstrated that some benthic taxa might persist in the HZ and/or the seedbank. Concretely, the percentage of benthic taxa in the HZ (8.7–40%) corresponded to some previous studies. For instance, Boulton (1992) found that 35% and 69% of benthic taxa were present in the HZ of temperate and arid intermittent streams, respectively. However, the taxonomic richness in these sites was limited to 8 and 10 taxa, similar to the taxonomic richness found in the HZ of our study (between 8 and 13 taxa). In contrast, Datry (2012) reported a 75% overlap of benthic taxa in HZ in a temperate stream but noted a 10% decrease in similarity between benthic and hyporheic assemblages for every 10 days of prolonged dry-phase duration. On the other hand, the percentage of benthic taxa found in the invertebrate seedbanks in our study streams (between 4.5% and 16%) was lower than in previous studies. For example, Datry et al. (2012) found two to three times more benthic taxa in the invertebrate seedbank of a temperate intermittent stream under similar dry-phase durations. However, these percentages varied from 33% to 5%, in arid and Mediterranean streams, respectively, with low taxonomic richness (9 and 3, respectively), similar to our findings (Stanley et al. 1994; Chester and Robson 2011).
Oligochaeta and Diptera were abundant in the studies previously mentioned (Boulton 1992; Chester and Robson 2011; Datry 2012; Datry et al. 2012). In our streams, both Oligochaeta and multiple Diptera families were also well represented in all assemblage types. Both groups include taxa with desiccation-tolerant life stages and the capacity to move into the HZ, with certain Oligochaeta species also having aerial respiration (Tachet et al. 2010). Moreover, these groups include semi-aquatic species, explaining their potential to be among the few taxa that persist in invertebrate seedbanks during extended dry phases (Tronstad et al. 2005; Chester and Robson 2011; Datry et al. 2012; Stubbington et al. 2016). Some Ephemeroptera found in the HZ, such as Caenis sp., are characterized as a burrower and can actively move into the HZ (Tachet et al. 2010; Robertson 2010). However, the early instars of Ecdyonurus sp. and Baetis sp. are transported passively into the HZ by down-welling water, resulting in their temporary inhabitation of these subsurface sediments (Boulton 2000; Tachet et al. 2010). On the other hand, Ephemeroptera found in seedbanks, including Procloeon sp. and Caenis sp., have summer egg diapause (Clifford 1982; Brittain 1990). Coleoptera taxa identified in the HZ can move into this refuge, whereas none of those recorded in the seedbank (e.g., Nebrioporus sp.) are recognized as having desiccation-tolerant life stages (Tachet et al. 2010). The survival of these Coleoptera in the dry streambed can be attributed to their aerial respiration and certain level of desiccation tolerance, conferred by their impermeable protective cuticle (Holdgate 1956; Pallarés et al. 2017).
We identified seven benthic taxa in the HZ that were not present in the invertebrate seedbanks, such as Hydropsyche sp., which does not show desiccation-tolerant life stages (Tachet et al. 2010). Conversely, six benthic taxa were found in the invertebrate seedbanks but not in the HZ, such as Procloeon sp., which enters summer diapause and is multivoltine, adaptations for avoiding aquatic environments during the dry phase (Bonada et al. 2007a, b). Furthermore, four taxa exclusively in the HZ, with three represented by one to two individuals, and Ecdyonurus sp. by ten individuals in a single sample. Conversely, four taxa were only found in the invertebrate seedbank, each represented by four to six individuals per sample. Given the very low densities of these taxa in both the HZ and in invertebrate seedbanks, we suggest that they may also have been present in the benthic sediments but were missed by kick sampling. This method captures only a proportion of the taxa present (e.g., 62% and 78% of families and 50% and 68% of species in 3 and 6 min sampling, respectively; Furse et al. 1981).
Differences in trait responses across assemblage types
Contrary to our second prediction, the hyporheic assemblages showed the lowest abundance-weighted resistance traits, in part because small body size, generalist feeding habits, and the capacity to move into the HZ were common resistance traits in benthic and seedbank, as well as hyporheic assemblages. These traits are either directly or indirectly associated with the ability to move into and persist within the HZ. For instance, body size affects an organism’s ability to penetrate sediment due to the dimensions of interstitial pathways (Stubbington 2012), while generalist feeding habits are advantageous given the limited abundance and diversity of food resources in the HZ (Burrell and Ledger 2003). In addition, a few hyporheic taxa (e.g., Glossostomidae and Forcipomyinae), although not burrowers, show crawler behavior and small sizes, enabling them to seek refuge in the HZ following the water table receding through the subsurface sediments in dry phases (Vander Vorste et al. 2016; Maazouzi et al. 2017). This ability is influenced by interactions between taxon characteristics (i.e., their traits) and environmental factors, including sediment characteristics, such as grain size, and local hydrological conditions, such as the direction and strength of hyporheic exchange (Stubbington 2012; Dole-Olivier et al. 2022).
Seedbank and benthic assemblages had similar abundances of resistance traits, with the third most abundant trait in seedbank assemblages being the presence of resistance forms. In contrast, aerial respiration showed low-weighted abundances (< 0.4) across all assemblages, and had its highest abundance in seedbank assemblages. Although aerial respiration becomes particularly advantageous when water flow decreases and water quality deteriorates, especially when dissolved oxygen concentrations decline (Bonada et al. 2007b; Mellado-Díaz et al. 2007), it is not necessarily associated with the persistence in HZ or in invertebrate seedbanks, even though if some taxa have both trait classes. For instance, within the Mollusca, most freshwater pulmonates can tolerate hypoxic conditions, as may occur in the HZ during dry phases, and also tolerate exposure to air. They can also extend this tolerance by entering a dormant, aestivating state (Poznańska et al. 2015).
On the other hand, we did not find differences in abundance-weighted resilience traits between assemblage types. The only exception was the large body size trait category, which showed the lowest abundances in hyporheic assemblages, suggesting that maximum body size may limit the use of HZ (Mathers et al. 2019). The similar trait composition across assemblage types and the taxonomic and functional overlapping (see above), reflect the dominance of traits that enable aquatic invertebrates to tolerate dry phases in intermittent natural streams of Mediterranean basin (Bonada et al. 2007a).
Dry-phase duration and resistance strategies
Contrary to our third hypothesis, taxonomic and functional diversity, as well as resistance traits, did not show a clear relationship with the duration of dry phase in hyporheic refuges and seedbanks. Despite the limited number of samples and total individuals, we identified a negative correlation between abundance-weighted resilience traits and the duration of dry phase. As dry-phase duration increases, environmental conditions become harsher, filtering out the less desiccation-tolerant taxa (Datry 2012; Datry et al. 2012), which may also possess resilience traits. In addition, we found that the resilience trait categories of terrestrial adult stage and short life cycle duration (≤ 1 year) also showed a negative linear relationship with dry phase duration. The decline of these traits may indicate avoidance behavior in response to harsher conditions during more severe dry phases (Strachan and Chester 2015). However, we must be cautious with these relationships given the low number of samples, which prevents drawing definitive conclusions.
On the other hand, two of the study reaches with the longest dry phases (Llobregat and Fluvià) lacked water in the HZ, preventing invertebrates from finding refuge in the shallow hyporheic sediments that we sampled, or forcing invertebrate to migrate into deeper sediments find refuge (Clinton et al. 1996). The effectiveness of most resistance strategies decreases with an increase in dry-phase harshness, influenced by the presence of water in the HZ or isolated pools, or moisture in the streambed sediment (Bogan et al. 2017). With extended dry phases and water loss, only few invertebrates are adapted to such harsh conditions (Fritz and Dodds 2004). For instance, many invertebrate seedbank taxa, which have various desiccation-tolerant life stages (e.g., eggs, juveniles), can persist during moderate-intensity dry phases, but as intensity increases, only taxa with cocoons and housing against desiccation can survive (Crabot et al. 2021a). Consequently, extended, severe dry phases can significantly alter aquatic invertebrate community composition (Doretto et al. 2018; Chanut et al. 2022).
Conclusions and prospects for future research
Further research is required to extend our understanding of the invertebrate traits associated with resistance and to evaluate the impact of dry phases on the effectiveness of resistance strategies (Stubbington and Datry 2013; DelVecchia et al. 2022). Our results showed that the taxa within hyporheic refuges and invertebrate seedbanks constituted up to 40% and 16%, respectively, of benthic invertebrate taxa. Additionally, a similar abundance of traits to face with the dry phase was observed across all assemblage types, suggesting a biota adapted to seasonal drying events in Mediterranean streams. Ongoing climate change, as well as human activities including water abstraction, are intensifying the frequency and duration of stream drying (Datry et al. 2018). This escalation may compromise the availability of water necessary to sustain the HZ and to maintain adequate moisture levels to support life in streambed sediments. In light of these challenges, freshwater management strategies should aim to ensure a sufficient supply of water to preserve aquatic biodiversity and the integrity of their recovery mechanisms during dry phases in naturally intermittent streams.
Data availability
The invertebrate data can be found in the Supplementary Information (Table S4). Two trait databases are also available: one (Sarremejane et al. 2020) in the data repository Figshare (https://doi.org/https://doi.org/10.6084/m9.figshare.c.5000633), and the other (Múrria et al. 2020) in the Supplementary Information of the paper.
Change history
15 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00027-024-01050-5
References
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Arias-Real R, Gutiérrez-Cánovas C, Menéndez M et al (2021) Diversity mediates the responses of invertebrate density to duration and frequency of rivers’ annual drying regime. Oikos 130:2148–2160. https://doi.org/10.1111/oik.08718
Bogan MT, Boersma KS, Lytle DA (2015) Resistance and resilience of invertebrate communities to seasonal and supraseasonal drought in arid-land headwater streams. Freshw Biol 60:2547–2558. https://doi.org/10.1111/fwb.12522
Bogan MT, Chester ET, Datry T et al (2017) Resistance, resilience, and community recovery in intermittent rivers and ephemeral streams. Intermittent rivers and ephemeral streams. Elsevier, pp 349–376
Bonada N, Rieradevall M, Prat N, Resh VH (2006) Benthic macroinvertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. J North Am Benthol Soc 25:32–43. https://doi.org/10.1899/0887-3593(2006)25[32:BMAAMC]2.0.CO;2
Bonada N, Dolédec S, Statzner B (2007a) Taxonomic and biological trait differences of stream macroinvertebrate communities between mediterranean and temperate regions: implications for future climatic scenarios. Glob Change Biol 13:1658–1671. https://doi.org/10.1111/j.1365-2486.2007.01375.x
Bonada N, Rieradevall M, Prat N (2007b) Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 589:91–106. https://doi.org/10.1007/s10750-007-0723-5
Bou C, Rouch R (1967) Un nouveau champ de recherches sur la faune aquatique souterraine. CR Acad Sci 265:369–370
Boulton AJ, Stanley EH, Fisher SG, Lake PS (1992) Over-summering strategies of macroinvertebrates in intermittent streams in Australia and Arizona. In: Robarts RD, Bothwell ML (eds) Aquatic ecosystems in semi-arid regions: implications for Resource Management, National Hydrological Research Institute Symposium Series 7. Environment Canada, Saskatoon, Canada, pp 227–237
Boulton A (2000) The Subsurface Macrofauna. In: Jones JB, Mulholland PJ (eds) Streams and Ground Waters. Academic Press, pp 337–361
Brittain JE (1990) Life history strategies in Ephemeroptera and Plecoptera. In: Campbell IC (ed) Mayflies and Stoneflies: Life Histories and Biology. Springer, Netherlands, Dordrecht, pp 1–12
Burrell GP, Ledger ME (2003) Growth of a stream-dwelling caddisfly (Olinga feredayi:Conoesucidae) on surface and hyporheic food resources. J North Am Benthol Soc 22:92–104. https://doi.org/10.2307/1467980
Chanut PCM, Drost A, Siebers AR et al (2022) Flow intermittency affects structural and functional properties of macroinvertebrate communities in alpine streams. Freshw Biol 68:212–228. https://doi.org/10.1111/fwb.14018
Chester ET, Robson BJ (2011) Drought refuges, spatial scale and recolonisation by invertebrates in non-perennial streams: drought refuges and recolonisation in streams. Freshw Biol 56:2094–2104. https://doi.org/10.1111/j.1365-2427.2011.02644.x
Chevenet F, Dolédec S, Chessel D (1994) A fuzzy coding approach for the analysis of long-term ecological data. Freshw Biol 31:295–309. https://doi.org/10.1111/j.1365-2427.1994.tb01742.x
Clifford HF (1982) Life cycles of mayflies (Ephemeroptera), with special reference to voltinism. Quaest Entomol 18:15–90
Clinton SM, Grimm NB, Fisher SG (1996) Response of a hyporheic invertebrate assemblage to drying disturbance in a desert stream. J North Am Benthol Soc 15:700–712. https://doi.org/10.2307/1467817
Crabot J, Mondy CP, Usseglio-Polatera P et al (2021a) A global perspective on the functional responses of stream communities to flow intermittence. Ecography 44:1511–1523. https://doi.org/10.1111/ecog.05697
Crabot J, Polášek M, Launay B et al (2021b) Drying in newly intermittent rivers leads to higher variability of invertebrate communities. Freshw Biol 66:730–744. https://doi.org/10.1111/fwb.13673
Cummins KW (1973) Trophic relations of aquatic insects. Annu Rev Entomol 18:183–206. https://doi.org/10.1146/annurev.en.18.010173.001151
Datry T (2012) Benthic and hyporheic invertebrate assemblages along a flow intermittence gradient: effects of duration of dry events: river drying and temporary river invertebrates. Freshw Biol 57:563–574. https://doi.org/10.1111/j.1365-2427.2011.02725.x
Datry T, Corti R, Philippe M (2012) Spatial and temporal aquatic-terrestrial transitions in the temporary Albarine River, France: responses of invertebrates to experimental rewetting: invertebrates in dry river sediments. Freshw Biol 57:716–727. https://doi.org/10.1111/j.1365-2427.2012.02737.x
Datry T, Larned ST, Tockner K (2014) Intermittent rivers: a challenge for freshwater ecology. Bioscience 64:229–235. https://doi.org/10.1093/biosci/bit027
Datry T, Boulton AJ, Bonada N et al (2018) Flow intermittence and ecosystem services in rivers of the Anthropocene. J Appl Ecol 55:353–364. https://doi.org/10.1111/1365-2664.12941
DelVecchia AG, Shanafield M, Zimmer MA et al (2022) Reconceptualizing the hyporheic zone for nonperennial rivers and streams. Freshw Sci 41:167–182. https://doi.org/10.1086/720071
Dole-Olivier M-J, Creuzé des Châtelliers M, Galassi DMP, et al (2022) Drivers of functional diversity in the hyporheic zone of a large river. Sci Total Environ 843:156985. https://doi.org/10.1016/j.scitotenv.2022.156985
Döll P, Schmied HM (2012) How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A Global-Scale Analysis Environ Res Lett 7:014037. https://doi.org/10.1088/1748-9326/7/1/014037
Doretto A, Piano E, Falasco E et al (2018) Investigating the role of refuges and drift on the resilience of macroinvertebrate communities to drying conditions: an experiment in artificial streams: resilience mechanisms of macroinvertebrates to droughts. River Res Appl 34:777–785. https://doi.org/10.1002/rra.3294
Dray S, Dufour A-B (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20. https://doi.org/10.18637/jss.v022.i04
Drummond LR, McIntosh AR, Larned ST (2015) Invertebrate community dynamics and insect emergence in response to pool drying in a temporary river. Freshw Biol 60:1596–1612. https://doi.org/10.1111/fwb.12591
Fritz KM, Dodds WK (2004) Resistance and resilience of macroinvertebrate assemblages to drying and flood in a tallgrass prairie stream system. Hydrobiologia 527:99–112. https://doi.org/10.1023/B:HYDR.0000043188.53497.9b
Furse MT, Wright JF, Armitage PD, Moss D (1981) An appraisal of pond-net samples for biological monitoring of lotic macro-invertebrates. Water Res 15:679–689. https://doi.org/10.1016/0043-1354(81)90160-3
Hay SE, Jenkins KM, Kingsford RT (2018) Diverse invertebrate fauna using dry sediment as a refuge in semi-arid and temperate Australian rivers. Hydrobiologia 806:95–109. https://doi.org/10.1007/s10750-017-3343-8
Holdgate MW (1956) Transpiration through the cuticles of some aquatic insects. J Exp Biol 33:107–118. https://doi.org/10.1242/jeb.33.1.107
Hwan JL, Carlson SM (2016) Fragmentation of an intermittent stream during seasonal drought: intra-annual and interannual patterns and biological consequences. River Res Appl 32:856–870. https://doi.org/10.1002/rra.2907
Jáimez-Cuéllar P, Vivas S, Bonada N et al (2002) Protocolo GUADALMED (PRECE). Limnetica 21:187–204. https://doi.org/10.23818/limn.21.25
Käser DH, Binley A, Heathwaite AL, Krause S (2009) Spatio-temporal variations of hyporheic flow in a riffle-step-pool sequence. Hydrol Process 23:2138–2149. https://doi.org/10.1002/hyp.7317
Kuebbing SE, Maynard DS, Bradford MA (2018) Linking functional diversity and ecosystem processes: a framework for using functional diversity metrics to predict the ecosystem impact of functionally unique species. J Ecol 106:687–698. https://doi.org/10.1111/1365-2745.12835
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Laliberté E, Legendre P, Shipley B (2014) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12.1
Larned ST, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary-river ecology. Freshw Biol 55:717–738. https://doi.org/10.1111/j.1365-2427.2009.02322.x
Loskotová B, Straka M, Polášek M et al (2021) Macroinvertebrate seedbank survival in pristine and nutrient-enriched intermittent streams and its contribution to flow phase communities. Hydrobiologia 848:1911–1923. https://doi.org/10.1007/s10750-021-04566-1
Maazouzi C, Galassi D, Claret C et al (2017) Do benthic invertebrates use hyporheic refuges during streambed drying? A manipulative field experiment in nested hyporheic flowpaths. Ecohydrology 10:e1865. https://doi.org/10.1002/eco.1865
Maire E, Grenouillet G, Brosse S, Villéger S (2015) How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob Ecol Biogeogr 24:728–740. https://doi.org/10.1111/geb.12299
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111:112–118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Mathers KL, Hill MJ, Wood CD, Wood PJ (2019) The role of fine sediment characteristics and body size on the vertical movement of a freshwater amphipod. Freshw Biol 64:152–163. https://doi.org/10.1111/fwb.13202
Mellado-Díaz A, Alonso MLS, Gutiérrez MRV-A (2007) Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshw Biol. https://doi.org/10.1111/j.1365-2427.2007.01854.x
Messager ML, Lehner B, Cockburn C et al (2021) Global prevalence of non-perennial rivers and streams. Nature 594:391–397. https://doi.org/10.1038/s41586-021-03565-5
Múrria C, Iturrarte G, Gutiérrez-Cánovas C (2020) A trait space at an overarching scale yields more conclusive macroecological patterns of functional diversity. Glob Ecol Biogeogr 29:1729–1742. https://doi.org/10.1111/geb.13146
Nimmo DG, Mac Nally R, Cunningham SC et al (2015) Vive la résistance: reviving resistance for 21st century conservation. Trends Ecol Evol 30:516–523. https://doi.org/10.1016/j.tree.2015.07.008
Ostertagová E, Ostertag O, Kováč J (2014) Methodology and application of the Kruskal-Wallis test. Appl Mech Mater 611:115–120. https://doi.org/10.4028/www.scientific.net/AMM.611.115
Pallarés S, Botella-Cruz M, Arribas P et al (2017) Aquatic insects in a multistress environment: cross-tolerance to salinity and desiccation. J Exp Biol Jeb. https://doi.org/10.1242/jeb.152108
Pavoine S, Vallet J, Dufour A-B et al (2009) On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118:391–402. https://doi.org/10.1111/j.1600-0706.2008.16668.x
Poznańska M, Goleniewska D, Gulanicz T et al (2015) Effect of substratum drying on the survival and migrations of a freshwater pulmonate snail Planorbarius corneus (Linnaeus, 1758). Hydrobiologia 747:177–188. https://doi.org/10.1007/s10750-014-2130-z
R Core Team (2022) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Robertson ALW (2010) Ecology of the hyporheic zone: origins, current knowledge and future directions. Fundam Appl Limnol 176:279–289. https://doi.org/10.1127/1863-9135/2010/0176-0279
Sánchez-Morales M, Sabater F, Muñoz I (2018) Effects of urban wastewater on hyporheic habitat and invertebrates in Mediterranean streams. Sci Total Environ 642:937–945. https://doi.org/10.1016/j.scitotenv.2018.06.132
Sarremejane R, Cid N, Stubbington R et al (2020) DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Sci Data 7:386. https://doi.org/10.1038/s41597-020-00732-7
Sauquet E, Beaufort A, Sarremejane R, Thirel G (2021) Predicting flow intermittence in France under climate change. Hydrol Sci J 66:2046–2059. https://doi.org/10.1080/02626667.2021.1963444
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Smith K (1975) Water temperature variations within a major river system. Hydrol Res 6:155–169. https://doi.org/10.2166/nh.1975.0011
Smyth GK (2002) Nonlinear regression. In: El-Shaarawi AH, Piegorsch WW (eds) Encyclopedia of environmetrics. Wiley, Chichester, pp 1405–1411
Stanley EH, Buschman DL, Boulton AJ et al (1994) Invertebrate resistance and resilience to intermittency in a desert stream. Am Midl Nat 131:288. https://doi.org/10.2307/2426255
Statzner B, Dolédec S, Hugueny B (2004) Biological trait composition of European stream invertebrate communities: assessing the effects of various trait filter types. Ecography 27:470–488. https://doi.org/10.1111/j.0906-7590.2004.03836.x
Strachan SR, Chester ET (2015) Robson, BJ Freshwater invertebrate life history strategies for surviving desiccation. Spr Sci Rev 3:57–75. https://doi.org/10.1007/s40362-015-0031-9
Stubbington R (2012) The hyporheic zone as an invertebrate refuge: a review of variability in space, time, taxa and behaviour. Mar Freshw Res 63:293. https://doi.org/10.1071/MF11196
Stubbington R, Datry T (2013) The macroinvertebrate seedbank promotes community persistence in temporary rivers across climate zones. Freshw Biol 58:1202–1220. https://doi.org/10.1111/fwb.12121
Stubbington R, Wood PJ, Boulton AJ (2009) Low flow controls on benthic and hyporheic macroinvertebrate assemblages during supra-seasonal drought. Hydrol Process 23:2252–2263. https://doi.org/10.1002/hyp.7290
Stubbington R, Gunn J, Little S et al (2016) Macroinvertebrate seedbank composition in relation to antecedent duration of drying and multiple wet-dry cycles in a temporary stream. Freshw Biol 61:1293–1307. https://doi.org/10.1111/fwb.12770
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douce: systématique, biologie, écologie, ([Nouvelle édition revue et augmentée]). CNRS Éditions, Paris
Tronstad LM, Tronstad BP, Benke AC (2005) Invertebrate seedbanks: rehydration of soil from an unregulated river floodplain in the south-eastern U.S. Freshw Biol 50:646–655. https://doi.org/10.1111/j.1365-2427.2005.01351.x
Vander Vorste R, Malard F, Datry T (2016) Is drift the primary process promoting the resilience of river invertebrate communities? A manipulative field experiment in an intermittent alluvial river. Freshw Biol 61:1276–1292. https://doi.org/10.1111/fwb.12658
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Wilcoxon F (1945) Individual Comparisons by Ranking Methods. Biom Bull 1:80–83. https://doi.org/10.2307/3001968
Acknowledgements
We express our gratitude to X. Herbera for his assistance during the field campaigns and for identifying the invertebrates. We thank the Fluvial Systems Research Group from ICRA (Institut Català de Recerca de l’Aigua, Girona) for their valuable support with the water chemical analyses. We also acknowledge the contributions of the reviewers from Aquatic Science, particularly R. Stubbington, whose substantial input greatly enhanced this article. This work was funded through projects CGL2017-88640-C2-2-R and PID2020-115708RB-C21 funded by MCIN/AEI/https://doi.org/10.13039/501100011033. A.V. received a pre-doctoral grant PRE2018-084817 (FPI). R.A.-R. received a “Margarita Salas” postdoctoral grant from the Spanish Ministry of Universities and the Next Generation EU—Recovery, Transformation and Resilience Plan.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
AV. Main author. She contributed substantially to the study’s conception, data acquisition (field and experimental designs, field samplings, experiment execution, sample processing), data analysis, writing the original draft of the manuscript, edited, approved, and submitted it. RAR. She contributed substantially to the data acquisition (field sampling), reviewing data analysis, reviewing the manuscript, and approved it. MM. She contributed substantially to the study’s conception, supervision, data acquisition (field and experimental designs, field samplings), reviewing data analysis, reviewing the manuscript and approved it. IM. She contributed substantially to the study’s conception, data acquisition (field and experimental designs, field samplings), reviewing data analysis, drafting and reviewing the manuscript, and approved it. Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
This work was funded through projects CGL2017-88640-C2-2-R and PID2020-115708RB-C21 funded by MCIN/AEI/https://doi.org/10.13039/501100011033. A.V. received a predoctoral grant PRE2018-084817 (FPI). R.A.-R. received a “Margarita Salas” postdoctoral grant from the Spanish Ministry of Universities and the Next Generation EU—Recovery, Transformation and Resilience Plan. All authors agree with the content of the manuscript, approve its submission for publication, and declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to all authors given and family name are interchanged.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viza, A., Arias-Real, R., Menéndez, M. et al. The role of seedbanks and hyporheic refuges in supporting benthic invertebrate community resistance and resilience to dry phases. Aquat Sci 86, 16 (2024). https://doi.org/10.1007/s00027-023-01034-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01034-x