Abstract
Ischemia-reperfusion injury (IRI) is a major event in renal transplantation, leading to adverse outcomes. Bone marrow mesenchymal stem cells (BMSCs) are novel promising therapeutics for repairing kidney injuries. The therapeutic efficacy of BMSCs with ISL1 overexpression in renal IRI and its underlying mechanism need to be investigated. The unilateral renal IRI rat model was established to mimic clinical acute kidney injury. Rats were injected with PBS, BMSCs-Scrambled or BMSCs-ISL1 via the tail vein at the timepoint of reperfusion, and then sacrificed after 24 h of reperfusion. The administration of BMSCs-ISL1 significantly improved renal function, inhibited tubular cells apoptosis, inflammation, oxidative stress in rats. In vitro, HKC cells subjected to H2O2 stimulation were pretreated with the conditioned medium (CM) of BMSCs-Scrambled or BMSCs-ISL1. The pretreatment of ISL1-CM attenuated apoptosis and oxidative stress induced by H2O2 in HKC cells. Our proteomic data suggested that haptoglobin (Hp) was one of the secretory proteins in ISL1-CM. Subsequent experiments confirmed that Hp was the important paracrine factor from BMSCs-ISL1 that exerted anti-apoptotic and antioxidant functions. Mechanistically, Hp played a cytoprotective role via the inhibition of ERK signaling pathway, which could be abrogated by Ro 67-7476, the ERK phosphorylation agonist. The results suggested that paracrine action may be the main mechanism for BMSCs-ISL1 to exert protective effects. As an important anti-apoptotic and antioxidant factor in ISL1-CM, Hp may serve as a new therapeutic agent for treating IRI, providing new insights for overcoming the long-term adverse effects of stem cell therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is an encountered syndrome that leading to rapidly decreased kidney function, characterized by increasing incidence, considerable mortality, high costs and lack of effective treatment [1]. The pathogenesis of AKI involves multiple stressors including hypoxia, inflammation and oxidant injury [2]. Besides causing acid-base or electrolyte disturbances, volume overload, and immune dysfunction, AKI is negatively associated with long-term survival [3, 4]. As the most common cause of AKI, ischemia-reperfusion injury (IRI) could lead to renal tubular epithelial cells and endothelial cells injury, activation of immune response, oxidative damage, and interstitial fibrosis [5, 6]. As an inevitable event in the deceased donor transplantation, IRI contributes to delayed graft function (DGF), acute and chronic organ rejection, and graft failure [7, 8]. Effective therapies are in urgent need to limit IRI and improve graft function.
Mesenchymal stem cells (MSCs) are a subset of multipotent cells, capable of differentiating into cells of multiple lineages, such as osteoblasts, adipocytes, myoblasts, and others [9, 10]. MSCs are known for their hypoimmunogenicity, availability from different tissues, and immunomodulatory capabilities [11, 12]. Numerous studies demonstrated that MSCs could ameliorate kidney injury by supporting renal tubular epithelial cell survival, inhibiting apoptosis, suppressing immune response, and stimulation of angiogenesis [13, 14]. The principal mechanism that MSCs exert the therapeutic efficacy is their paracrine action, through the release of various mediators, including pro-survival and immunosuppressive molecules, growth factors, exosomes, cytokines, and various metabolites [14, 15]. The tumorigenesis and immunogenicity are some of the safety and efficacy concerns of MSCs therapies in clinical use [16, 17]. Extracellular vesicles derived from MSCs could be utilized as cell-free therapeutics, which have shown great immunomodulatory and regenerative functions, thus providing new perspectives for MSCs therapy [18, 19]. In addition, conditioned medium (CM) derived from MSCs have displayed therapeutic efficacy against IRI by inhibition of inflammatory and apoptosis [20,21,22,23]. Pretreatment with hypoxia, certain chemical agents or gene modification could enhance the paracrine effects of MSCs to attenuate IRI, which may suggest a better approach to improve the therapeutic benefits of MSCs [24, 25].
Insulin gene enhancer binding protein 1 (ISL1), a LIM homeodomain transcription factor, has been reported to play vital roles in cardiogenesis, neuronal development, cancer progression, and the maturation of islet cells [26,27,28]. Our previous work demonstrated that ISL1-overexpressing bone marrow mesenchymal stem cells (BMSCs) reduce the apoptosis of grafted islets through the paracrine function [29]. It has been reported that ISL1 overexpression in human MSCs promoted cell survival and improved cardiac function in the model of myocardial infarction [30]. In addition, exosomes derived from ISL1-MSCs were proved its cytoprotective and pro-angiogenesis abilities in ischemic heart disease [31]. However, whether ISL1 overexpression in BMSCs could lead to alleviation of renal IRI and the improvement of renal function has not been investigated.
In this study, we demonstrated the therapeutic effects of ISL1-overexpressing BMSCs (BMSCs-ISL1) in attenuating renal injury and protecting renal function in a rat IRI model. Furthermore, we found that the conditioned medium (CM) of ISL1-CM displayed anti-apoptotic and antioxidant properties in vitro. Our proteomic analyses revealed that secretory proteins haptoglobin (Hp) may be beneficial for human kidney proximal tubular cells (HKC) against H2O2-induced injury. Moreover, our results indicated that Hp exerted its cytoprotective function by inhibiting extracellular-signal relate kinase (ERK) signaling pathway. Therefore, our study provides a new strategy for boosting the benefits of cell therapy based on MSCs and explores potential protective mechanisms of ISL1-CM.
Materials and methods
Isolation and culture of BMSCs
BMSCs were isolated by the whole bone marrow adherent method from Sprague-Dawley rats (male, 60–80 g) as previously described [32]. Briefly, the rats were sacrificed by injecting overdose pentobarbital, and then soaked in 75% ethanol alcohol for 15 min. BMSCs were isolated from the femur and tibia, then suspended with 5 ml of the BMSCs complete medium (Cyagen, Guangzhou, China) and seeded in T25 culture flasks (Corning, NY, USA) at 37 °C in 5% CO2 and 95% humidity. After 48 h, the culture medium was replaced. The adherent cells were passaged when 80% confluent. BMSCs from passage 3–5 were used in the experiments. The animals were obtained from the experimental animal center of Xi’an Jiaotong University, Xi’an, China. The protocol was approved by the Xi’an Jiaotong University Committee on Animal Care regulations.
The construction of BMSCs-ISL1 or BMSCs-scrambled
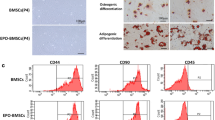
The detailed description of the construction of BMSCs-ISL1 or BMSCs-Scrambled has been reported in our prior study [29]. In brief, Adenovirus expressing ISL1 and an empty adenoviral vector were constructed from Hanbio Technology Ltd (Shanghai, China). BMSCs were transfected with adenoviruses harboring EGFP or ISL1 according to the manufacturer’s instructions. The titers of adenoviruses used in this study were 1.8 × 1010 PFU/ml. The culture medium was replaced 24 h after infection. 72 h after the transduction, the cells were selected by 2.5 µg/ml puromycin (Sigma-Aldrich, St. Louis, USA). The green fluorescence could be detected by a fluorescence microscope (Zeiss, Jena, Germany). BMSCs-ISL1 or BMSCs-Scrambled could differentiate into adipocytes and osteoblasts, identified by Oil Red O staining (Cyagen, Guangzhou, China) and Alizarin Red S staining (Cyagen, Guangzhou, China), respectively. BMSCs-ISL1 and BMSCs-Scrambled were identified by staining with allophycocyanin (APC)-conjugated anti-CD29, phycoerythrin (PE)- conjugated anti-CD90, APC-conjugated anti-CD45 and PE-conjugated anti-CD11b/c monoclonal antibodies (eBioscience, CA, USA). Isotype IgG antibodies (eBioscience, CA, USA) were employed as negative controls. Flow cytometry analyses were performed using a FACS Calibur flow cytometer (BD Biosciences, New Jersey, USA). The data were analyzed using FlowJo software (version 10; Treestar, OR, USA).
Preparation of conditioned medium (CM) of BMSCs-ISL1 or BMSCs-Scrambled
Upon 80% confluence of BMSCs-ISL1 or BMSCs-Scrambled, the culture medium was removed and then the cells were washed with phosphate-buffered saline (PBS; Servicebio) twice before treated with Iscove’s Modified Dulbecco Medium (IMDM; Procell, Wuhan, China) for 24 h at 37 °C in a humidified atmosphere with 5% CO2. The conditioned medium (CM) was then collected, centrifuged at 3000 rpm for 10 min at 4 °C, filtered with 0.22‐µm sterile filters (Millipore, MA, USA) and stored at − 80 °C for further use. Both CM of BMSCs-ISL1 (ISL1-CM) and BMSCs-Scrambled (Scrambled-CM) were diluted with the culture medium of HKC cells at ratio of 1:9 before use.
Renal IRI model and in vivo treatment
Male Sprague-Dawley rats (140–160 g) were obtained from the experimental animal center of Xi’an Jiaotong University, Xi’an, China and raised in a temperature-controlled and pathogen-free environment with a 12 h light/dark cycle. All animal experiments conducted in this study were in compliance with the regulations by the Xi’an Jiaotong University Committee on Animal Care. Rat IRI model was then established. Briefly, rats were food-deprived for 12 h before the surgery and anesthetized with intraperitoneal injection of pentobarbital (50 mg/kg). The right unilateral nephrectomy was then performed. The left renal pedicle was exposed by lumbodorsal incision and then clamped with a nontraumatic vascular clamp for 60 min. In the Sham group, the right unilateral nephrectomy was performed and the left renal pedicle was exposed but without clamped. During the procedure, the body temperature of rats was maintained at 37 °C using a rectal probe and heat pad. The nontraumatic vascular clamp was removed to restore blood flow, and the kidney was inspected to confirm reperfusion. At the timepoint of reperfusion, rats were administered intravenously via the tail vein with PBS (200 µl), BMSCs-Scrambled (2 × 105 cells in 200 µl PBS) or BMSCs-ISL1 (2 × 105 cells in 200 µl PBS). All rats were euthanized after 24 h of reperfusion, and the blood samples and kidneys were collected for further investigation. Serum levels of creatinine (SCr) and blood urea nitrogen (BUN) were determined by commercial kit reagents (Jiancheng Bioengineering Institute, Nanjing, China).
Cell culture and treatment
Human kidney proximal tubular cells (HKC) were purchased from the Chinese Academy of Medical Sciences cell bank. The cells were cultured in Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (Ham) (DMEM/F12 (1:1), Gibco, USA) containing 10% fetal bovine serum (Gibco), penicillin (100 U/mL, Gibco), and streptomycin (0.1 g/mL, Gibco) at 37 °C in a humidified atmosphere with 5% CO2. HKC cells were treated with IMDM, Scrambled-CM or ISL1-CM for 24 h and then stimulated with 250 µM H2O2 for 4 h. In the subsequent experiments, haptoglobin (MCE, Shanghai, China) was used to treat HKC cells. ERK phosphorylation agonist (S)-2-(4-fluorophenyl)-1-(toluene-4-sulfonyl)-pyrrolidine (Ro 67-7476) was purchased from TargetMol (Massachusetts, USA).
Cell viability assay and cell death analysis
HKC cells (5,000 cells/well) were seeded into 96-well plates and incubated overnight. HKC cells were then treated with CM or Hp at different concentrations for 24 h before being exposed to H2O2. The viability of cells was determined through Cell Counting Kit‐8 (CCK-8) assay (Beyotime Biotechnology, China) by following the manufacturer’s instruction. Optical density values were measured at 450 nm on a microplate reader (BioTek, CA, USA). Cell viability was calculated as relative values compared to the control cells.
The apoptosis of HKC cells was measured using an Annexin V-APC and 7-AAD kit (Multisciences, Beijing, China) and detected using flow cytometry, according to manufacturer’s instructions. The percentages of dead cells were analyzed and quantified with FlowJo software (version 10; Treestar, OR, USA).
Histology analysis and immunohistochemical staining
Kidney tissues were fixed with 4% paraformaldehyde solution, followed by paraffin embedding and slicing (4 μm). The sliced sections were deparaffinized and stained with Periodic Acid-Schiff (PAS) staining. The tubular injury was scored as follows: 0, no damage; 1, < 25%; 2, 25 ~ 50%; 3, 50 ~ 75%; 4, > 75%. The tubular injury score was calculated as the average score from 10 random high-power fields (× 20 objective). The tubular injury was evaluated in a blinded manner. For immunohistochemistry staining, renal sections were incubated in 3% H2O2 for 10 min, blocked with 5% bovine serum albumin for 1 h at 37 °C and then incubated with primary antibodies against CD68 (GB113109, Servicebio, 1:100) overnight at 4 °C. Then the sections were washed and incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody for 1 h at room temperature. DAB staining (G1212, Servicebio, China) was performed and the nuclei were counterstained using hematoxylin (Servicebio, China). The sections were observed by an optical microscopy (NikonInstruments, Melville, NY) and 5 random fields (× 40 objective) were selected and measured.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
TUNEL staining was performed on the kidney sections to evaluate cell apoptosis, using one-step TUNEL assay kit (Beyotime Biotechnology, Jiangsu, China). The sections were observed and photographed under a fluorescence microscope (Zeiss, Jena, Germany). 5 fields of vision (× 40 objective) were randomly selected to count the number of TUNEL-positive cells.
Dihydroethidium (DHE) staining of kidney tissues
Cryosections from frozen kidney tissues (4 μm) were stained with DHE solution (5 µM) (S0063, Beyotime Biotechnology, China) for 60 min in the dark at 37 °C, then washed 3 times with PBS. The sections were counterstained with DAPI (Invitrogen) to label the nucleus. 5 fields of vision (× 20 objective) were randomly selected and then the fluorescence intensity of DHE was quantified by ImageJ software (version 1.53; National Institutes of Health).
Dichlorodihyfrofluorescein diacetate (DCFH-DA) staining of HKC cells
Reactive Oxygen Species Assay Kit (S0033S, Beyotime Biotechnology, China) was used to measure intracellular level ROS. In brief, HKC cells were incubated with fresh culture medium containing 10 µM DCFH-DA for 15 min, and then washed three times with PBS. The ROS levels were observed using a fluorescence microscope (Zeiss, Jena, Germany) and analyzed by ImageJ software (version 1.53; National Institutes of Health).
Measurement of oxidative stress
Kidney tissues (100 µg) were homogenized in saline into 10% homogenate. Cell lysates were extracted by radioimmunoprecipitation assay buffer. Superoxide dismutase (SOD) activities and malondialdehyde (MDA) levels were measured in kidney tissues and HKC cells to evaluate oxidative stress using commercial reagent kits (A001-1, Jiancheng Bioengineering Institute, Nanjing, China; G4300-96T, Servicebio, China). In addition, the concentrations of lactate dehydrogenase (LDH) in the serum of rats and supernatants of HKC cells were assessed by the LDH assay kit (S03034, Rayto Life Sciences Co., Ltd, Shenzhen, China).
Enzyme-linked immunosorbent assay (ELISA)
The levels of haptoglobin released in the conditioned medium were measured by the ELISA kits (Elabscience Biotech, Wuhan, China) in accordance with the manufacturer’s instructions.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from kidney tissues using TRIzol reagent (Thermo Fisher Scientific, USA). Total RNA (1 µg) was reverse-transcribed into cDNA using reverse transcription polymerase (Roche, Mannheim, Germany). RT-qPCR was performed using the ABI 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, USA) with quantitative SYBR Green PCR Master Mix (Qiagen, Hilden, Germany). The relative mRNA levels of target genes were calculated using the 2−ΔΔCt method. The expression of each gene was normalized to that of Gapdh. The primers are listed in Table S1.
Western blotting
Proteins were extracted from kidney tissues and HKC cells with RIPA lysis buffer (Beyotime Biotechnology, China) containing protease inhibitor and phosphatase inhibitor (Pierce, IL, USA). To detect haptoglobin in the conditioned medium, methanol–chloroform precipitation method was used [33]. Total protein (25 µg) was subjected to 10% SDS-PAGE gels and subsequently transferred onto polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin (BSA, Gibco, USA) for 1 h at room temperature, membranes were incubated with primary antibodies, including rabbit anti-ISL1 (1:5000, Novus Biologicals, Colorado, USA, #NBP2-14999), mouse anti-Haptoglobin (1:500, Santa Cruz Biotechnology, USA, #sc-376,893), mouse anti-Bcl-2 (1:1000, Cell Signaling Technology, USA, #15071S), rabbit anti-Bax (1:1000, Cell Signaling Technology, USA, #2772S), rabbit anti-ERK (1:1000, Cell Signaling Technology, USA, #4695S), rabbit anti-phospho-ERK (1:1000, Cell Signaling Technology, USA, #4370S), mouse anti-JNK (1:500, Santa Cruz Biotechnology, USA, #sc-137,019), mouse anti- phospho-JNK (1:500, Santa Cruz Biotechnology, USA, #sc-293,136) and mouse anti-GAPDH (1:5000, Proteintech, 60004-1-Ig) overnight at 4 °C. The membranes were washed with PBS containing Tween 20 and incubated with HRP-conjugated secondary antibodies (1: 5000, Abcam, Cambridge, UK) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence Western blotting detection kit (Tanon, Shanghai, China) and imaged by a chemiluminescence imaging system (Tanon 5200, Shanghai, China). Three independent biological replicates were performed. All protein expression was normalized to GAPDH. The intensity of each band was assessed using the ImageJ software.
Statistical analysis
The statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad Inc., CA, USA). All data are presented as the means ± standard deviation (SD). Comparisons between groups were tested using Student’s t test or one-way ANOVA with post hoc Tukey’s test where appropriate. A value of P < 0.05 was considered to indicate significance.
Results
BMSCs-ISL1 alleviates renal IRI in rats
After adenovirus transfection, BMSCs-ISL1 and BMSCs-Scrambled were obtained. BMSCs-ISL1 and BMSCs-Scrambled showed green fluorescence under a fluorescence microscope 72 h after transfection (Fig. S1a-b). Alizarin Red staining and Oil Red O staining demonstrated their capabilities of differentiating into osteoblasts and adipocytes (Fig. S1c-d). Flow cytometry was performed to detect the surface markers. Both groups of cells expressed phenotypic markers CD29 and CD90, while did not express CD45 or CD11b/c (Fig. S1e). The mRNA and protein expression levels of ISL1 were elevated in BMSCs-ISL1 (Fig. S1f-g). Above results indicated that BMSCs-ISL1 and BMSCs-Scrambled were successfully constructed and maintained the characteristics of BMSCs.
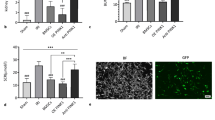
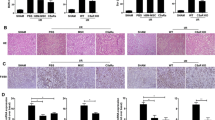
To investigate the protective efficacy of BMSCs-ISL1 in vivo, the rats were intravenously injected with PBS, BMSCs-Scrambled or BMSCs-ISL1 at the timepoint of reperfusion. After 24 h, the rats were sacrificed to obtain blood samples and kidney tissues (Fig. 1a). BMSCs-ISL1 treated rats showed improved renal function with markedly lower SCr and BUN levels (Fig. 1b-c). PAS staining showed that IRI caused cast formation, the loss of brush border and tubular necrosis, while BMSCs-ISL1 treatment significantly ameliorated renal damages (Fig. 1d-e). The infiltration of inflammatory cells was assessed by immunohistochemistry staining of CD68. The number of infiltrated macrophages markedly decreased in the BMSCs-ISL1-treated group (Fig. 1f-g). Moreover, the mRNA expression levels of kidney injury markers (Kim-1 and Ngal) and inflammatory factors (Nlrp3, Tnf-α, Il-6 and Il-1β) in the kidney tissues of BMSCs-ISL1 group were lower than those in the kidney tissues of BMSCs-Scrambled group (Fig. 1h-i). Collectively, these results indicated the potential therapeutic effects of BMSCs-ISL1 in protecting renal function and reducing the renal injuries.
BMSCs-ISL1 attenuate renal dysfunction and tubular injury induced by IRI in rats. a Schematic illustration of the animal model in this study. b-c The concentrations of SCr and BUN were measured in rats from different groups. The administration of BMSCs-ISL1 improved renal function significantly. (n = 5 biological replicates per group). d Representative images of PAS staining of the kidneys. Scale bar = 100 μm. e Tubular injury scores for histology grading in rats were analyzed. (n = 5 biological replicates per group). f Representative images of immunohistochemistry staining of CD68. g Quantification of CD68-positive cells. Five random fields were chosen from each section. Scale bar = 50 μm. (n = 5 biological replicates per group). h RT-qPCR analyses of Kim-1 and Ngal mRNA expression levels in renal tissues. (n = 3 biological replicates per group). i RT-qPCR analyses of renal inflammatory factors (Nlrp3, Tnf-α, Il-6 and Il-1β) mRNA expressions. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
BMSCs-ISL1 reduces apoptosis of renal tubular cells in the renal IRI model
Apoptosis is one of the mechanisms that contributing to cell death and organ damage in renal IRI [34]. TUNEL staining showed that IRI induced renal tubular cells apoptosis, however, the number of TUNEL-positive cells was lower in the kidneys of BMSCs-ISL1-treated rats (Fig. 2a-b). Besides, apoptotic markers were examined at both mRNA and protein levels. IRI increased Bax mRNA expression level while decreased Bcl-2 mRNA expression level. The administration of BMSCs-ISL1 could reverse the Bax and Bcl-2 mRNA expression levels (Fig. 2c). Similarly, Western blot results indicated that BMSCs-ISL1 upregulated Bcl-2/Bax protein ratio in the kidney tissues (Fig. 2d-e). Taken together, our results showed the anti-apoptotic effects of BMSCs-ISL1 in the rat renal IRI model.
BMSCs-ISL1 reduce IRI injury-induced apoptosis of renal tubular cells. a Representative images of TUNEL staining in kidneys of rats. Scale bar = 50 μm. b Quantification of TUNEL-positive cells. Five random fields were chosen from each section. (n = 5 biological replicates per group). c RT-qPCR analyses of Bcl-2 and Bax mRNA expression levels in renal tissues. (n = 3 biological replicates per group). d Western blot for Bcl-2 and Bax in the kidneys of rats. e Quantitative analysis of protein expression levels of Bcl-2 and Bax. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ****P < 0.0001. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
The conditioned medium (CM) of BMSCs-ISL1 ameliorates H2O2-stimulated renal tubular cells apoptosis
We collected the CM of BMSCs-Scrambled (Scrambled-CM) and BMSCs-ISL1 (ISL1-CM) to examine whether they could be protective in vitro. HKC cells were pretreated with CM for 24 h before being exposed to H2O2. Cell viability was determined by CCK-8 assay. We found a significantly elevated cell viability in the ISL1-CM group (Fig. 3a). Western blot results demonstrated that the stimulation of H2O2 increased the expression of Bax while decreased the expression of Bcl-2 in HKC cells. ISL1-CM significantly increased Bcl-2/Bax ratio compared to the Scrambled-CM (Fig. 3b-c). Subsequently, the results of flow cytometry with Annexin-V-APC/7AAD double staining demonstrated that the ratio of apoptotic cells in ISL1-CM-treated cells decreased dramatically in comparison with the Scrambled-CM-treated cells (20.20% ± 4.49% vs. 36.84% ± 7.51%) (Fig. 3d-e). Thus, our results corroborated that ISL1-CM could protect HKC cells from apoptosis in vitro.
ISL1-CM inhibits cell apoptosis induced by H2O2. a Cell viability of HKC cells was measured by CCK-8 assay. (n = 3 biological replicates per group). b-c Western blot analyses and quantification of Bcl-2 and Bax protein expression levels in HKC cells. (n = 3 biological replicates per group). d Flow cytometry with Annexin-V and 7AAD double staining was used to evaluate apoptosis in HKC cells. e Statistical analysis of the percentage of Annexin-V-positive cells. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
BMSCs-ISL1 inhibits oxidative stress against IRI in vivo and H2O2 stimulation in vitro
Oxidative stress plays an important role in renal IRI by activating several pathological processes [35]. To investigate whether BMSCs-ISL1 alleviated renal injury by inhibiting oxidative stress. DHE staining of kidney tissues were used to examine the level of reactive oxygen species (ROS) production. The results indicated that the administration of BMSCs-ISL1 led to a significant reduction of ROS (Fig. 4a-b). SOD and MDA levels in kidney tissues were then measured. IRI inhibited SOD activities and increased MDA levels significantly. The treatment of BMSCs-ISL1 was found to increase SOD activities and decrease MDA levels (Fig. 4c-d). Higher serum LDH levels were found after IRI, but BMSCs-ISL1-treated rats had lower serum LDH levels (Fig. 4e). DCFH-DA probe was used to assess the intracellular ROS levels. Fluorescence images showed that H2O2 increased the ROS production in HKC cells, while ISL1-CM markedly inhibited the ROS production (Fig. 4f-g). Likewise, reduced SOD activities and the accumulation of MDA in HKC cells after H2O2 stimulation were observed. The treatment of ISL1-CM mitigated oxidative stress by enhancing SOD activities and reducing MDA contents (Fig. 4h-i). Additionally, ISL1-CM significantly inhibited LDH release, suggesting the cell membrane was impaired less severely (Fig. 4j). Collectively, these results suggested that BMSCs-ISL1 protected renal IRI and H2O2-induced injury owing to its antioxidant properties.
BMSCs-ISL1 decrease oxidative damage in IRI-induced kidneys and H2O2-stimulated HKC cells. a Representative images for DHE staining in the kidneys. Scale bar = 100 μm. b Quantification of DHE staining of kidneys. Five random fields were chosen from each section. (n = 3 biological replicates per group). c-d SOD levels and MDA contents in the kidneys of each group. (n = 5 biological replicates per group). e Serum LDH levels were measured using the LDH assay kit. (n = 5 biological replicates per group). f Intracellular ROS levels were measured by DCFH-DA staining. Scale bar = 100 μm. g Quantification of the content of ROS in HKC cells. Five random fields were chosen to analyze the fluorescence intensity. (n = 3 biological replicates per group). h-i The levels of SOD and MDA in HKC cells. (n = 3 biological replicates per group). j The cellular damage was measured by the LDH release. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
Haptoglobin is one of the secretory proteins in ISL1-CM that protected HKC cells against H2O2-induced injury
Our previous study performed proteomics analysis of ISL1-CM and Scrambled-CM and found out 21 secretory proteins that expressed differentially (Table S2) [29]. We focused on haptoglobin (Hp) which showed the highest fold of upregulation in ISL1-CM compared to the Scrambled-CM (Fig. S2a). Enzyme-linked immunosorbent assay (ELISA) showed the markedly increase protein concentrations of Hp in ISL1-CM (Fig. S2b). Western blot showed that the content of Hp was higher in ISL1-CM than Scrambled-CM (Fig. S2c). The mRNA expression level was also upregulated in BMSCs-ISL1 (Fig. S2d). To evaluate the protective effect of Hp, we pretreated HKC cells with different concentrations of Hp for 24 h. The cell viability assessed by CCK-8 method displayed a significant increase at the concentration of 1 ng/ml with or without H2O2 stimulation. With the concentration increased to 2.5 ng/ml or 5 ng/ml, there was no significant elevation of cell viability compared to the low-dose pretreatment of Hp (Fig. 5a). Western blot results showed that Hp increased Bcl-2/Bax protein ratio when HKC cells were stimulated with H2O2, while there were no significant changes in the protein expression levels of Bcl-2 and Bax between control group and the control group with Hp pretreatment, suggesting that Hp only exerted protective effect in the presence of H2O2-induced injury (Fig. 5b-c; Fig. S3c, e). Flow cytometry showed that Hp resulted in the robust reduction in the apoptotic rate of HKC cells exposed to H2O2 (Fig. 5d, f).
Hp exerts cytoprotective effects against H2O2-induced injury. a Hp treatment elevated cell viability of HKC cells with or without H2O2 stimulation, assessed by CCK-8 assay. HKC and HKC with H2O2 stimulation were used as control groups. (n = 3 biological replicates per group). b-c Western blot showed that Hp increased Bcl-2/Bax protein ratio in HKC cells after H2O2 stimulation. Hp led to no significant change of Bcl-2/Bax protein ratio without H2O2 stimulation. (n = 3 biological replicates per group). d, f The percentage of apoptotic cells with or without H2O2 stimulation and Hp treatment was determined by flow cytometry. (n = 3 biological replicates per group). e Representative images of HKC cells stained with DCFH-DA. Hp inhibited H2O2-induced ROS production. Scale bar = 100 μm. g Quantification of DCFH-DA. Five random fields were chosen to analyze the fluorescence intensity. (n = 3 biological replicates per group). h-i The levels of SOD and MDA in H2O2-stimulated HKC cells after treatment with Hp. (n = 3 biological replicates per group). j The levels of LDH in supernatants were assessed. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
DCFH-DA staining showed Hp significantly reversed the excessive ROS production induced by H2O2 (Fig. 5e, g). Additionally, Hp increased SOD activities and decreased MDA contents in HKC cells (Fig. 5h-i). LDH release in the culture medium was markedly reduced in the Hp-treated group (Fig. 5j). Collectively, Hp may exert its cytoprotective role by reducing apoptosis and inhibiting oxidative stress.
Haptoglobin suppresses apoptosis and oxidative stress via ERK signaling pathway
ERK signaling pathway participating in apoptosis and ROS stress has been widely investigated [36, 37]. The results of Western blot showed that Hp inhibited the ERK phosphorylation induced by H2O2 but had no effect on the phosphorylation level of c-Jun N-terminal kinase (JNK) (Fig. 6a-b, Fig. S3a-b). Without the stimulation of H2O2, Hp treatment did not significantly change the phosphorylation of ERK (Fig. S3c-d). Subsequently, Ro 67-7476, the ERK phosphorylation agonist, was applied to confirm the correlation among Hp, ERK signaling pathway and the cytoprotective functions of Hp. The results of Western blot showed that Ro 67-7476 (1 µM for 5 min) caused the activation of p-ERK (Fig. S3f-g), which was consistent with other previous studies [38, 39]. H2O2 and Ro 67-7476 treatment led to markedly elevated p-ERK/ERK protein ratio compared with the H2O2 group, while Hp still inhibited the phosphorylation of ERK (Fig. 6c-d). Without the stimulation of H2O2, the treatment of Hp displayed not significant inhibition of the phosphorylation of ERK which was activated by Ro 67-7476 (Fig. S3c-d).
Hp limits H2O2-induced injury via ERK signaling pathway. a-b Western blot and quantitative analysis demonstrated Hp inhibited the phosphorylation of ERK induced by H2O2 stimulation. (n = 3 biological replicates per group). c-d Ro 67-7476 (1 µM for 5 min) activated the phosphorylation of ERK, while the phosphorylation of ERK was relieved in the Hp + Ro 67-7476 group compared to the Ro 67-7476 group. (n = 3 biological replicates per group). e-f Western blot showed that Ro 67-7476 incubation led to lower Bcl-2/Bax protein ratio with the presence of H2O2. Moreover, Ro 67-7476 + Hp group decreased the Bcl-2/Bax protein ratio compared to the Hp treatment group. (n = 3 biological replicates per group). g-h Ro 67-7476 + Hp treatment leaded to more apoptotic cells than the Hp treatment group, assessed by flow cytometry. (n = 3 biological replicates per group). i-j DCFH-DA staining images showed that Ro 67-7476 attenuated the antioxidant effect of Hp. (n = 3 biological replicates per group). k-l The SOD and MDA levels in HKC cells of different groups were measured. (n = 3 biological replicates per group). m LDH levels of each group in the cell supernatant. (n = 3 biological replicates per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant. Data are presented as mean ± SD. Statistically significant differences were determined by one-way ANOVA with Tukey’s post hoc comparison
Notably, Ro 67-7476 promoted the apoptosis of HKC cells that were exposed to H2O2, assessed by decreased Bcl-2/Bax protein ratio and flow cytometry (Fig. 6e-h). Moreover, the incubation of Ro 67-7476 partially offset the protective effects of Hp. That is to say, Hp + Ro 67-7476 group displayed more apoptotic cells and a lower Bcl-2/Bax protein ratio compared to the Hp treatment group (Fig. 6e-h). Meanwhile, oxidative stress levels were dramatically attenuated by Hp but reversed by Ro 67-7476. The simultaneous application of Ro 67-7476 and Hp on HKC cells increased the fluorescence intensity of DCFH-DA staining, the levels of MDA in cell lysates, the levels of LDH in cell supernatants, while decreased the levels of SOD in cell lysates, compared to the Hp-treatment group (Fig. 6i-m). Taken together, our results demonstrated that Hp suppressed apoptosis and oxidative stress by inhibiting ERK signaling pathway, while Ro 67-7476 offset its anti-apoptotic and antioxidant effects.
Discussion
Kidney transplantation is invariably associated with renal damage, including IRI [40]. Renal IRI is the major cause of significant AKI, associated with high mortality and adverse long-term outcomes in kidney transplantation [1, 34]. Despite major progress has been made in the understanding of IRI, effective strategies to prevent allograft damage are still lacking and urgently needed [14, 35]. Intensive studies of MSCs have provided promising therapeutic strategies for the treatment of AKI [41]. However, concerns about the safety and effectiveness of MSCs therapy have been raised. MSCs might embed in the lung and fail to migrate to the injured kidney [42]. Most of MSCs undergo apoptosis after systemic infusion, due to the adverse local microenvironment, which impairs the positive effects of MSCs [43]. The transplanted BMSCs may acquire malignancy and induce tumors in mice models [44]. The administration of MSCs in human subjects with AKI has shown safety but limited efficacy [10]. Hence, further investigations are in great need of to confirm specific therapeutic mechanisms and promote the safe use of MSCs-based cell therapy. Numerous studies demonstrated that MSCs with gene modification, pretreatment, or cultured in hypoxic conditions not only could enhance the survival and migration, paracrine effects, and injury repair efficacy, but also possess minimal adverse impacts and systemic toxicity, which provide the novel strategy to boost MSCs into clinical use [17, 25, 45,46,47,48].
ISL1 has been shown the pivotal role in embryogenesis of heart and pancreatic islets [49]. ISL1 expression in endothelial cells promoted their angiogenic properties and IL-1b and VEGF secretion [50]. Our previous work demonstrated that BMSCs overexpressing ISL1 promoted the survival of grafted islets through paracrine function [29]. Besides, ISL1 overexpression MSCs and their derived exosomes have been shown to promote angiogenesis and protect endothelial cells in the myocardial infarction model [30, 31]. In this study, we demonstrated that BMSCs-ISL1 significantly improved renal function, inhibited the mRNA expression levels of inflammatory factors, inhibited apoptosis and oxidative stress induced by IRI. The dose of BMSCs we used in this study (2 × 105 cells) was lower than previously published studies [25, 43, 51], which implied that BMSCs-ISL1 were more beneficial than BMSCs-Scrambled whose therapeutic effects were rather limited. Whether low dose of MSCs could be effective is still controversial, while high dose infusion may cause side effects such as microvascular embolization and tumorigenesis. The appropriate transplantation dose of MSCs is still necessary to further explore [41].
Since most infused MSCs are entrapped in lung and only a small portion of infused cells are able to migrate to the injured sites, the beneficial effects MSCs exerted are primarily due to their paracrine actions [48, 52]. Previous study reported that ISL1-hMSCs expressed elevated levels of monocyte chemoattractant protein-3 (MCP3), thus enhanced the survival and angiogenesis properties of human umbilical vein endothelial cells [53]. Insulin-like growth factor binding protein 3 (IGFBP3) was found higher in the CM of ISL1-hMSCs, which played an important role in the anti-apoptosis efficacy [30]. Our previous work comprehended the impact of ISL1 on the paracrine actions of BMSCs based on proteomic and metabolomic analyses, revealed that BMSCs-ISL1 reduced islet cells apoptosis through exosomes carrying anillin (ANLN), secretory proteins inhibin beta A chain (INHBA), and metabolites caffeine, verified by ChIP-qPCR [29]. In this study, the CM of BMSCs-ISL1 or BMSCs-Scrambled was collected and then used to treat HKC cells. The sudden increase of oxygen could cause rapid injury to the kidney grafts after the restoration of circulation, thus H2O2, a primary kind of ROS, was used to stimulate HKC cells to simulate the excess ROS production in vivo during IRI [6, 54, 55]. Surprisingly, ISL1-CM was proved to decrease the apoptotic effect and oxidative stress induced by H2O2 in HKC cells. In brief, previous studies and our work demonstrated that the paracrine actions played a vital role in the beneficial effects of MSCs-ISL1.
Hp is an acute phase plasma protein that captures and binds hemoglobin (Hb) with the highest affinity [56]. The Hp-Hb complex is subsequently cleared from circulation, preventing the oxidative damage in tissues and cells [57, 58]. Besides, Hp is also an angiogenic factor and anti-inflammatory modulator, plays a critical role in various biological processes [59, 60]. Hp is also known as the scavenger of cell-free hemoglobin (CFH), while studies have demonstrated that administration of CFH could lead to severe AKI in renal IRI and septic mice models [61, 62]. The infusion of Hp prevents kidney dysfunction, kidney injury after acute hemolysis as well as blood transfusion [63, 64]. It has been reported that Hp was a cytoprotective protein in kidney, whose mRNA and protein expression levels were applied to assess the protective impact of the agents [65, 66]. Undetectable plasmatic Hp is an independent risk factor for major adverse kidney events (MAKE) and AKI in patients with severe burns, which may be considered as a biomarker for predicting AKI [67]. While high plasma concentrations of Hp might play a protective role from AKI in critically ill patients [68]. In Japan, plasma-derived Hp was approved and clinically used for the treatment of hemolysis due to extracorporeal circulation, burn injuries, trauma, and blood transfusions that could lead to AKI [69, 70]. A retrospective observational study indicated that Hp administration was independently associated with lower risk of AKI in cardiovascular surgery patients, suggesting the renoprotective role of Hp [71]. The low dose administration of Hp (0.5 mg on days 0 and 3) to Shiga-toxin induced mice displayed partial beneficial effects by only reducing the platelet deposition and neutrophil recruitment in kidney, but had no significant influences on the NGAL plasma levels, the PAS staining, and the immunohistochemical analysis of KIM-1 as well as cleaved caspase-3, compared to the mice subjected to Shiga-toxin which were treated with vehicle [72]. Whether Hp may serve as a therapeutic approach in renal IRI has not been investigated, therefore, we will explore the underlying mechanism of Hp in IRI in vivo in the future.
Numerous studies have shown the anti-inflammatory effects and antioxidative properties of Hp, however, the effect of Hp on H2O2-induced injury in HKC cells still remains unclear [73, 74]. In this study, Hp was found to be one of the secretory proteins that upregulated in ISL1-CM compared to the Scrambled-CM. ELISA and Western blot showed that the release of Hp increased in the ISL1-CM, consistent with proteomic data. The pretreatment of Hp elevated cell viability of HKC cells with or without H2O2 stimulation. Furthermore, Hp sharply decreased the apoptotic rate and protected HKC cells from oxidative stress that induced by H2O2. All aforementioned results demonstrated that Hp was an anti-apoptotic and antioxidant paracrine factor from ISL1-BMSCs. ERK has been implicated in cell survival and apoptosis in response to IRI and the H2O2-induced injury [75, 76]. For instance, taraxasterol protected renal IRI from oxidative stress, inflammation, and apoptosis by suppressing the phosphorylation of ERK and JNK signaling pathways [77]. Dexmedetomidine attenuated hepatic IRI by inhibiting ERK, JNK and p38 phosphorylation [78]. In this study, the decreased phosphorylation level of ERK after Hp treatment was observed, while there is no significant change in the phosphorylation level of JNK. In addition, we conducted ERK agonist (Ro 67-7476) to HKC cells exposed to H2O2. The results showed that the activation of ERK impaired the protective effects of Hp. Taken these together, our results demonstrated that Hp inhibited apoptosis and oxidative stress via the inhibition of ERK pathway. There may be a complicated crosstalk of the anti-apoptotic and antioxidant mechanisms of Hp in H2O2-induced injury. The properties and potential molecular signaling mechanisms of Hp in various pathological conditions still need to be further explored.
In conclusion, our findings demonstrated that the administration of BMSCs-ISL1 improved renal function, suppressed apoptosis and oxidative stress in the rat IRI model. However, the tumorigenic risk of MSCs therapy is still a major concern. Long-term studies to verify the safety and effectiveness are still of great necessity. ISL1-CM was proved to attenuate apoptosis and oxidative stress induced by H2O2 in HKC cells. Our proteomics analysis and subsequent in vitro experiments demonstrated that Hp was the important paracrine factor that protected HKC cells, which suggested that Hp might be a novel therapeutic approach that limits apoptosis and oxidative stress in IRI models, thus providing insights to allow cell-free treatments to address lone-term adverse impacts.
Data availability
All data associated with this study are present in the paper and/or the Supplementary Materials.
References
Bellomo R, Kellum JA, Ronco C (2012) Acute kidney injury. Lancet 380:756–766. https://doi.org/10.1016/s0140-6736(11)61454-2
Su L, Zhang J, Gomez H, Kellum JA, Peng Z (2023) Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 19:401–414. https://doi.org/10.1080/15548627.2022.2084862
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS (2018) Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14:607–625. https://doi.org/10.1038/s41581-018-0052-0
Jang HR, Rabb H (2015) Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11:88–101. https://doi.org/10.1038/nrneph.2014.180
Reid S, Scholey JW (2021) Recent approaches to Targeting Canonical NFκB Signaling in the early inflammatory response to renal IRI. J Am Soc Nephrol 32:2117–2124. https://doi.org/10.1681/asn.2021010069
Ponticelli C (2014) Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transpl 29:1134–1140. https://doi.org/10.1093/ndt/gft488
Smith SF, Hosgood SA, Nicholson ML (2019) Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int 95:50–56. https://doi.org/10.1016/j.kint.2018.10.009
Zhao H, Alam A, Soo AP, George AJT, Ma D (2018) Ischemia-Reperfusion Injury reduces Long Term Renal Graft Survival: mechanism and Beyond. 28:31–42. https://doi.org/10.1016/j.ebiom.2018.01.025
Andrzejewska A, Lukomska B, Janowski M (2019) Concise Review: mesenchymal stem cells: from roots to Boost. Stem Cells 37:855–864. https://doi.org/10.1002/stem.3016
Fazekas B, Griffin MD (2020) Mesenchymal stromal cell-based therapies for acute kidney injury: progress in the last decade. Kidney Int 97:1130–1140. https://doi.org/10.1016/j.kint.2019.12.019
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32:252–260. https://doi.org/10.1038/nbt.2816
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709–716. https://doi.org/10.1016/j.stem.2012.05.015
Perico L, Morigi M, Rota C, Breno M, Mele C, Noris M, Introna M, Capelli C, Longaretti L, Rottoli D et al (2017) Human mesenchymal stromal cells transplanted into mice stimulate renal tubular cells and enhance mitochondrial function. Nat Commun 8:983. https://doi.org/10.1038/s41467-017-00937-2
Tögel FE, Westenfelder C (2010) Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol 6:179–183. https://doi.org/10.1038/nrneph.2009.229
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14:493–507. https://doi.org/10.1038/s41581-018-0023-5
Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J et al (2019) Mesenchymal stromal cell therapeutic delivery: Translational challenges to clinical application. Front Immunol 10:1645. https://doi.org/10.3389/fimmu.2019.01645
Liang W, Chen X, Zhang S, Fang J, Chen M, Xu Y, Chen X (2021) Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol Biol Lett 26:3. https://doi.org/10.1186/s11658-020-00246-5
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L (2021) Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol 14:136. https://doi.org/10.1186/s13045-021-01141-y
Toh WS, Lai RC, Hui JHP, Lim SK (2017) MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol 67:56–64. https://doi.org/10.1016/j.semcdb.2016.11.008
Miceli V, Bulati M, Gallo A, Iannolo G, Busà R, Conaldi PG, Zito G (2023) Role of mesenchymal Stem/Stromal cells in modulating Ischemia/Reperfusion Injury: current state of the art and future perspectives. Biomedicines 11. https://doi.org/10.3390/biomedicines11030689
Miceli V, Bertani A, Chinnici CM, Bulati M, Pampalone M, Amico G, Carcione C, Schmelzer E, Gerlach JC, Conaldi PG (2021) Conditioned medium from human amnion-derived mesenchymal Stromal/Stem cells attenuating the effects of Cold Ischemia-Reperfusion Injury in an in vitro model using human alveolar epithelial cells. Int J Mol Sci 22. https://doi.org/10.3390/ijms22020510
Zito G, Miceli V, Carcione C, Busà R, Bulati M, Gallo A, Iannolo G, Pagano D, Conaldi PG (2022) Human amnion-derived mesenchymal Stromal/Stem cells pre-conditioning inhibits inflammation and apoptosis of Immune and Parenchymal cells in an in vitro model of liver Ischemia/Reperfusion. Cells 11. https://doi.org/10.3390/cells11040709
Su VY, Lin CS, Hung SC, Yang KY (2019) Mesenchymal stem cell-conditioned medium induces Neutrophil apoptosis Associated with inhibition of the NF-κB pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci 20. https://doi.org/10.3390/ijms20092208
Hu C, Wu Z, Li L (2020) Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med 24:40–49. https://doi.org/10.1111/jcmm.14788
Tseng WC, Lee PY, Tsai MT, Chang FP, Chen NJ, Chien CT, Hung SC, Tarng DC (2021) Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem Cell Res Ther 12:367. https://doi.org/10.1186/s13287-021-02374-x
Li M, Sun C, Bu X, Que Y, Zhang L, Zhang Y, Zhang L, Lu S, Huang J, Zhu J et al (2021) ISL1 promoted tumorigenesis and EMT via Aurora kinase A-induced activation of PI3K/AKT signaling pathway in neuroblastoma. Cell Death Dis 12:620. https://doi.org/10.1038/s41419-021-03894-3
Galang G, Mandla R, Ruan H, Jung C, Sinha T, Stone NR, Wu RS, Mannion BJ, Allu PKR, Chang K et al (2020) ATAC-Seq reveals an Isl1 enhancer that regulates sinoatrial Node development and function. Circ Res 127:1502–1518. https://doi.org/10.1161/circresaha.120.317145
Bohuslavova R, Fabriciova V, Lebrón-Mora L, Malfatti J, Smolik O, Valihrach L, Benesova S, Zucha D, Berkova Z, Saudek F et al (2023) ISL1 controls pancreatic alpha cell fate and beta cell maturation. Cell Biosci 13:53. https://doi.org/10.1186/s13578-023-01003-9
Wang Y, Zhang JW, Wang JW, Wang JL, Zhang SC, Ma RY, Zhang J, Li Y, Liu PJ, Xue WJ et al (2022) BMSCs overexpressed ISL1 reduces the apoptosis of islet cells through ANLN carrying exosome, INHBA, and caffeine. Cell Mol Life Sci 79:538. https://doi.org/10.1007/s00018-022-04571-0
Xiang Q, Liao Y, Chao H, Huang W, Liu J, Chen H, Hong D, Zou Z, Xiang AP, Li W (2018) ISL1 overexpression enhances the survival of transplanted human mesenchymal stem cells in a murine myocardial infarction model. Stem Cell Res Ther 9. https://doi.org/10.1186/s13287-018-0803-7
Hu X, Ning X, Zhao Q, Zhang Z, Zhang C, Xie M, Huang W, Cai Y, Xiang Q, Ou C (2022) Islet-1 mesenchymal stem cells-Derived Exosome-Incorporated Angiogenin-1 hydrogel for enhanced Acute myocardial infarction therapy. ACS Appl Mater Interfaces 14:36289–36303. https://doi.org/10.1021/acsami.2c04686
Fang J, Zhao X, Li S, Xing X, Wang H, Lazarovici P, Zheng W (2019) Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90(rsk)-CREB pathway. Stem Cell Res Ther 10:312. https://doi.org/10.1186/s13287-019-1419-2
Jakobs C, Bartok E, Kubarenko A, Bauernfeind F, Hornung V (2013) Immunoblotting for active caspase-1. Methods Mol Biol 1040:103–115. https://doi.org/10.1007/978-1-62703-523-1_9
Pefanis A, Ierino FL, Murphy JM, Cowan PJ (2019) Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int 96:291–301. https://doi.org/10.1016/j.kint.2019.02.009
Granata S, Votrico V, Spadaccino F, Catalano V, Netti GS, Ranieri E, Stallone G, Zaza G (2022) Oxidative stress and Ischemia/Reperfusion Injury in kidney transplantation: focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxid (Basel) 11. https://doi.org/10.3390/antiox11040769
Liu M, Wang RB, Xing JH, Tang YX (2021) Atractylenolide inhibits apoptosis and oxidative stress of HTR-8/SVneo cells by activating MAPK/ERK signalling in preeclampsia. Phytomedicine 93:153773. https://doi.org/10.1016/j.phymed.2021.153773
Yu F, Wei J, Cui X, Yu C, Ni W, Bungert J, Wu L, He C, Qian Z (2021) Post-translational modification of RNA m6A demethylase ALKBH5 regulates ROS-induced DNA damage response. Nucleic Acids Res 49:5779–5797. https://doi.org/10.1093/nar/gkab415
Sheffler DJ, Conn PJ (2008) Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology 55:419–427. https://doi.org/10.1016/j.neuropharm.2008.06.047
Feng Q, Yang P, Wang H, Li C, Hasegawa T, Liu Z, Li M (2022) ID09, a newly-designed tubulin inhibitor, regulating the Proliferation, Migration, EMT process and apoptosis of oral squamous cell carcinoma. Int J Biol Sci 18:473–490. https://doi.org/10.7150/ijbs.65824
Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, Zhang ZX, Jevnikar AM (2013) RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transpl 13:2805–2818. https://doi.org/10.1111/ajt.12447
Wanyan P, Wang X, Li N, Huang Y, She Y, Zhang L (2023) Mesenchymal stem cells therapy for acute kidney injury: a systematic review with meta-analysis based on rat model. Front Pharmacol 14:1099056. https://doi.org/10.3389/fphar.2023.1099056
Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q et al (2013) Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int 84:521–531. https://doi.org/10.1038/ki.2013.114
Zhou S, Qiao YM, Liu YG, Liu D, Hu JM, Liao J, Li M, Guo Y, Fan LP, Li LY et al (2020) Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerate the repair of acute kidney injury. Cell Biosci 10:130. https://doi.org/10.1186/s13578-020-00492-2
Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS (2011) Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 108:1340–1347. https://doi.org/10.1161/circresaha.110.239848
Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, Bai X, Feng Z, Sun S, Chen X (2018) IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int 93:814–825. https://doi.org/10.1016/j.kint.2017.08.030
Liu N, Tian J, Cheng J, Zhang J (2013) Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem 114:2677–2689. https://doi.org/10.1002/jcb.24615
Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y, Cheng H, Kong M, Zhang F, Chen Q et al (2016) A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in Nonhuman Primates: paracrine activity without remuscularization. Circ Res 118:970–983. https://doi.org/10.1161/circresaha.115.307516
Xu H, Chen C, Hu L, Hou J (2017) Gene-modified mesenchymal stem cell-based Therapy in Renal Ischemia- Reperfusion Injury. Curr Gene Ther 17:453–460. https://doi.org/10.2174/1566523218666180214094253
Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S et al (2006) Multipotent embryonic isl1 + progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165. https://doi.org/10.1016/j.cell.2006.10.029
Barzelay A, Ben-Shoshan J, Entin-Meer M, Maysel-Auslender S, Afek A, Barshack I, Keren G, George J (2010) A potential role for islet-1 in post-natal angiogenesis and vasculogenesis. Thromb Haemost 103:188–197. https://doi.org/10.1160/th09-07-0433
Liu N, Wang H, Han G, Cheng J, Hu W, Zhang J (2018) Enhanced proliferation and differentiation of HO-1 gene-modified bone marrow-derived mesenchymal stem cells in the acute injured kidney. Int J Mol Med 42:946–956. https://doi.org/10.3892/ijmm.2018.3670
Wu Y, Zhao RC (2012) The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev Rep 8:243–250. https://doi.org/10.1007/s12015-011-9293-z
Liu J, Li W, Wang Y, Fan W, Li P, Lin W, Yang D, Fang R, Feng M, Hu C et al (2014) Islet-1 overexpression in human mesenchymal stem cells promotes vascularization through monocyte chemoattractant protein-3. Stem Cells 32:1843–1854. https://doi.org/10.1002/stem.1682
Carcy R, Cougnon M, Poet M, Durandy M, Sicard A, Counillon L, Blondeau N, Hauet T, Tauc M, D FP (2021) Targeting oxidative stress, a crucial challenge in renal transplantation outcome. Free Radic Biol Med 169:258–270. https://doi.org/10.1016/j.freeradbiomed.2021.04.023
Feng S, Qu Y, Chu B, Chen X, Yang Z, Li P, Wang P, He Q, He Y, Lin T et al (2022) Novel gold-platinum nanoparticles serve as broad-spectrum antioxidants for attenuating ischemia reperfusion injury of the kidney. Kidney Int 102:1057–1072. https://doi.org/10.1016/j.kint.2022.07.004
Andersen CBF, Stødkilde K, Sæderup KL, Kuhlee A, Raunser S, Graversen JH, Moestrup SK (2017) Haptoglobin Antioxid Redox Signal 26:814–831. https://doi.org/10.1089/ars.2016.6793
Sultan A, Raman B, Rao CM, Tangirala R (2013) The extracellular chaperone haptoglobin prevents serum fatty acid-promoted amyloid fibril formation of β2-microglobulin, resistance to lysosomal degradation, and cytotoxicity. J Biol Chem 288:32326–32342. https://doi.org/10.1074/jbc.M113.498337
di Masi A, De Simone G, Ciaccio C, D’Orso S, Coletta M, Ascenzi P (2020) Haptoglobin: from hemoglobin scavenging to human health. Mol Aspects Med 73:100851. https://doi.org/10.1016/j.mam.2020.100851
Naryzny SN, Legina OK (2021) Haptoglobin as a Biomarker. Biochem Mosc Suppl B Biomed Chem 15:184–198. https://doi.org/10.1134/s1990750821030069
Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R et al (2010) Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 12:293–304. https://doi.org/10.1089/ars.2009.2793
Greite R, Wang L, Gohlke L, Schott S, Kreimann K, Doricic J, Leffler A, Tudorache I, Salman J, Natanov R et al (2022) Cell-free hemoglobin in Acute kidney Injury after Lung Transplantation and experimental renal Ischemia/Reperfusion. Int J Mol Sci 23. https://doi.org/10.3390/ijms232113272
Shaver CM, Paul MG, Putz ND, Landstreet SR, Kuck JL, Scarfe L, Skrypnyk N, Yang H, Harrison FE, de Caestecker MP et al (2019) Cell-free hemoglobin augments acute kidney injury during experimental sepsis. Am J Physiol Ren Physiol 317:F922–f929. https://doi.org/10.1152/ajprenal.00375.2018
Hashimoto K, Nomura K, Nakano M, Sasaki T, Kurosawa H (1993) Pharmacological intervention for renal protection during cardiopulmonary bypass. Heart Vessels 8:203–210. https://doi.org/10.1007/bf01744743
Yang H, Wang H, Levine YA, Gunasekaran MK, Wang Y, Addorisio M, Zhu S, Li W, Li J, de Kleijn DP et al (2016) Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight 1. https://doi.org/10.1172/jci.insight.85375
Zager RA (2015) Marked protection against acute renal and hepatic injury after nitrited myoglobin + tin protoporphyrin administration. Transl Res 166:485–501. https://doi.org/10.1016/j.trsl.2015.06.004
Zager RA, Johnson AC, Frostad KB (2016) Combined iron sucrose and protoporphyrin treatment protects against ischemic and toxin-mediated acute renal failure. Kidney Int 90:67–76. https://doi.org/10.1016/j.kint.2016.01.022
Dépret F, Dunyach C, De Tymowski C, Chaussard M, Bataille A, Ferry A, Moreno N, Cupaciu A, Soussi S, Benyamina M et al (2017) Undetectable haptoglobin is associated with major adverse kidney events in critically ill burn patients. Crit Care 21:245. https://doi.org/10.1186/s13054-017-1837-4
Graw JA, Hildebrandt P, Krannich A, Balzer F, Spies C, Francis RC, Kuebler WM, Weber-Carstens S, Menk M, Hunsicker O (2022) The role of cell-free hemoglobin and haptoglobin in acute kidney injury in critically ill adults with ARDS and therapy with VV ECMO. Crit Care 26. https://doi.org/10.1186/s13054-022-03894-5
Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM (2013) Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121:1276–1284. https://doi.org/10.1182/blood-2012-11-451229
Irwin DC, Baek JH, Hassell K, Nuss R, Eigenberger P, Lisk C, Loomis Z, Maltzahn J, Stenmark KR, Nozik-Grayck E et al (2015) Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 82:50–62. https://doi.org/10.1016/j.freeradbiomed.2015.01.012
Kubota K, Egi M, Mizobuchi S (2017) Haptoglobin Administration in Cardiovascular surgery patients: its Association with the risk of postoperative acute kidney Injury. Anesth Analg 124:1771–1776. https://doi.org/10.1213/ane.0000000000002093
Pirschel W, Mestekemper AN, Wissuwa B, Krieg N, Kröller S, Daniel C, Gunzer F, Tolosano E, Bauer M, Amann K et al (2022) Divergent roles of haptoglobin and hemopexin deficiency for disease progression of Shiga-toxin-induced hemolytic-uremic syndrome in mice. Kidney Int 101:1171–1185. https://doi.org/10.1016/j.kint.2021.12.024
Koami H, Sakamoto Y, Miyasho T, Noguchi R, Sato N, Kai K, Chris Yamada K, Inoue S (2017) Haptoglobin reduces inflammatory cytokine INF-γ and facilitates clot formation in Acute severe burn rat model. J Nippon Med Sch 84:64–72. https://doi.org/10.1272/jnms.84.64
Jelena A, Mirjana M, Desanka B, Svetlana IM, Aleksandra U, Goran P, Ilijana G (2013) Haptoglobin and the inflammatory and oxidative status in experimental diabetic rats: antioxidant role of haptoglobin. J Physiol Biochem 69:45–58. https://doi.org/10.1007/s13105-012-0186-7
Kumar S, Allen DA, Kieswich JE, Patel NS, Harwood S, Mazzon E, Cuzzocrea S, Raftery MJ, Thiemermann C, Yaqoob MM (2009) Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol 20:2412–2425. https://doi.org/10.1681/asn.2008080868
Incani A, Deiana M, Corona G, Vafeiadou K, Vauzour D, Dessì MA, Spencer JP (2010) Involvement of ERK, Akt and JNK signalling in H2O2-induced cell injury and protection by hydroxytyrosol and its metabolite homovanillic alcohol. Mol Nutr Food Res 54:788–796. https://doi.org/10.1002/mnfr.200900098
Li C, Zheng Z, Xie Y, Zhu N, Bao J, Yu Q, Zhou Z, Liu J (2020) Protective effect of taraxasterol on ischemia/reperfusion-induced acute kidney injury via inhibition of oxidative stress, inflammation, and apoptosis. Int Immunopharmacol 89:107169. https://doi.org/10.1016/j.intimp.2020.107169
Zhang S, Tang J, Sun C, Zhang N, Ning X, Li X, Wang J (2023) Dexmedetomidine attenuates hepatic ischemia-reperfusion injury-induced apoptosis via reducing oxidative stress and endoplasmic reticulum stress. Int Immunopharmacol 117:109959. https://doi.org/10.1016/j.intimp.2023.109959
Acknowledgements
We are thankful for the support from the experimental animal center of Xi’an Jiaotong University for the technical help.
Funding
This work was supported by the National Natural Science Foundation of China (82370802), The Key Research and Development Program of Shaanxi Province (2023-YBSF-259) and Natural Science Foundation of Shaanxi Province (2023JC-QN-1000).
Author information
Authors and Affiliations
Contributions
Jiale Wang, Jingwen Wang and Xiaoming Ding conceptualized the study. Cuinan Lu and Ying Wang were responsible for the data curation and formal analysis. Jiale Wang and Jingwen Wang were responsible for visualization. Jiale Wang wrote the original draft. Huanjing Bi was responsible for funding acquisition. Jin Zheng and Xiaoming Ding were responsible for methodology and provided supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All animal procedures were approved by the Xi’an Jiaotong University Animal Experimentation Ethics Committee.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Wang, J., Lu, C. et al. ISL1-overexpressing BMSCs attenuate renal ischemia-reperfusion injury by suppressing apoptosis and oxidative stress through the paracrine action. Cell. Mol. Life Sci. 81, 312 (2024). https://doi.org/10.1007/s00018-024-05354-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05354-5