Abstract
Voltage-gated K+ (KV) channels govern K+ ion flux across cell membranes in response to changes in membrane potential. They are formed by the assembly of four subunits, typically from the same family. Electrically silent KV channels (KVS), however, are unable to conduct currents on their own. It has been assumed that these KVS must obligatorily assemble with subunits from the KV2 family into heterotetrameric channels, thereby giving rise to currents distinct from those of homomeric KV2 channels. Herein, we show that KVS subunits indeed also modulate the activity, biophysical properties and surface expression of recombinant KV7 isoforms in a subunit-specific manner. Employing co-immunoprecipitation, and proximity labelling, we unveil the spatial coexistence of KVS and KV7 within a single protein complex. Electrophysiological experiments further indicate functional interaction and probably heterotetramer formation. Finally, single-cell transcriptomic analyses identify native cell types in which this KVS and KV7 interaction may occur. Our findings demonstrate that KV cross-family interaction is much more versatile than previously thought—possibly serving nature to shape potassium conductance to the needs of individual cell types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Voltage-gated potassium channels (KV channels) are a diverse family of evolutionarily conserved membrane proteins that allow the flux of K+ ions across cellular membranes. They play a significant role in determining the excitability of tissues including heart, skeletal muscle, brain, and retina. Based on sequence homology, mammalian KV channels are divided into 12 subfamilies (KV1–12) [1]. Functional KV channels are formed as tetramers of pore-forming α-subunits. These may be made up by four identical isoforms (homotetramers) or of α-subunits from distinct members of the same KV subfamily (heterotetramers). Heterotetramerization across family borders is typically not possible. The only known exception to this is the heterotetramerization of KV2 channels with members of the so-called silent modifier (KVS) families, KV5, KV6, KV8, and KV9.
KVS were termed “silent”, as they are unable to form functional homotetramers at the plasma membrane on their own. When expressed alone, they are retained in intracellular compartments, without giving rise to an electrical current [5]. However, when co-expressed with KV2 subunits, they co-assemble into functional KVS/KV2 heterotetramers, with electrophysiological and pharmacological characteristics markedly different to those of KV2 channels alone [5, 8]. Such KV2/KVS heteromerization greatly broadens the functional diversity of KV2 channels. The physiological relevance thereof is highlighted by the fact that mutations in the KCNV2 gene, encoding KV8.2 subunits, cause KCNV2-associated retinopathy [14, 16]. In this condition, the lack of intact KV8.2 causes alterations in a conductance termed IK,x, which counterbalances the dark current in photoreceptors, to an extent that finally photoreceptor death is caused [14, 19, 40]. Similarly, variants in KCNV1 (KV8.1) and KCNV2 are associated with certain forms of epilepsy [4, 22, 49].
Structurally, the KV α-subunit consists of six transmembrane domains (S1–S6) and a pore loop (between S5 and S6) with the signature sequence (TTIGYGD) determining K+ selectivity. The S1–S4 region represents the voltage-sensing domain, and the cytoplasmic N- and C-termini are relevant for subunit assembly, trafficking and functional channel regulation through signalling cascades. Notably, it is well established that KVS affect KV2 currents by altering membrane trafficking, but also by modifying the voltage dependence of activation and inactivation as well as the gating properties [5, 8, 41]. Tetramerization in most KV channels, including the interaction between KV2 and KVS, primarily occurs in the endoplasmic reticulum (ER). This process involves the primary interaction of T1 domains located in the cytoplasmic N-terminus of each subunit [43]. The exact mechanism by which the T1 domain determines subunit specificity remains poorly understood. It is worth noting that certain KV subunits, such as the KV7 channel families, lack the T1 domain but are still capable of forming functional homo- and heteromers [2, 18, 20]. Given the specific co-assembly of closely related family members, it is reasonable to assume that sequence homology is an important determinant of tetramerization. In this regard, we found that KVS (with the exception of KV5) are more closely related to members of the KV7 family than to the other KV subunits including KV2 ([17] and Supplementary Fig. 1). We therefore hypothesised that KVS may constitute modifiers of KV7 channels and investigated this interaction in a series of electrophysiological, molecular and cell biological experiments. We found that KVS modulate current amplitude and voltage-dependence of neuronal KV7 isoforms in a bi directional manner, and we present biochemical and electrophysiological evidence that KV7 and KVS physically assemble into the same protein complex, allowing for direct interaction and possibly even heterotetramerisation.
Overall, this work reveals previously unknown ability of KV7 and KVS to assemble into complexes with unique electrophysiological properties. This may represent a mechanism to extend the native repertoire of K+ currents to maintain cell physiology in various tissues.
Results
Bi-directional modulation of KV7 channels through KVS
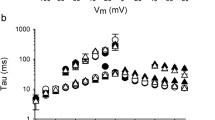
To explore the potential modulation of KV7-mediated currents by KVS, we initiated our analyses by characterising K+ currents in Chinese hamster ovary (CHO) cells transiently co-expressing KV7.2 channel subunits together with different KVS in whole-cell patch-clamp recordings. Depolarising voltage steps (−100 mV to + 60 mV) induced slowly activating outwardly rectifying K+ currents that were completely absent in non-transfected CHO cells and in cells expressing only KVS (not shown). Strikingly, voltage-dependent currents were significantly reduced in cells co-expressing KV7.2 with either KV5.1, KV8.2, KV9.1 or KV9.2 subunits compared to cells expressing KV7.2 alone (Fig. 1A, B, D). Also, in cells co-expressing KV7.2 with either KV5.1, KV8.2, KV9.1 or KV9.2 we observed an alteration of biophysical characteristics in that the steady-state voltage-dependence of whole-cell currents was shifted to significantly more depolarised values as compared to cells expressing KV7.2 only. The magnitude of this shift was in the range of about + 10 mV for Vh values (Fig. 1F, H). The voltage sensitivity, as determined by the slope of the voltage dependence, was not altered by co-expression of KVS. The smaller current amplitudes and altered voltage-dependence were accompanied by significantly more depolarised resting potentials in the cells co-expressing KV7.2 with either KV5.1, KV8.2, KV9.1 or KV9.2 (not shown). In contrast, steady-state current amplitudes were significantly increased in cells co-expressing KV6.1 and KV8.1 channels without however affecting voltage dependence or membrane potentials compared to cells expressing only KV7.2 subunits (Fig. 1A, B, D). Co-expression of KV6.3, KV6.4 or KV9.3 did not affect the characteristics of currents through KV7.2 channels (Fig. 1A, D, H).
Electrophysiological properties of CHO cells expressing KV7 channels are altered by co-expression of KVS. A Voltage stimulus protocol (left) and representative patch-clamp recordings from CHO cells expressing KV7.2 alone (black), and together with KV8.1 (orange) or KV8.2 (green). B, C Voltage-dependent current densities in cells expressing KV7 alone, and together with KV8.1 or KV8.2. D, E Summary statistics for steady-state current densities at + 20 mV obtained in recordings as shown in (B, C) as well as for similar recordings performed on cells expressing KV7 together with other KVS. F–I Normalized tail currents (F, G) and half-maximal activation voltage (H, I) as deduced from Boltzmann fits (solid lines in F, G) for the same cells as show in B–E. Recordings from cells expressing KV7.2 are shown in B, D, F and H while those expressing KV7.4 are shown in C, E, G and I
To assess whether KVS similarly modulated the properties of currents through other KV7 channels, we performed analogous experiments in CHO cells expressing either KV7.4 subunits alone or together with KVS. Notably, the effects of co-expression of KVS on KV7.4 subunits were very similar to those observed for KV7.2 channels, except that no effect was observed for KV5.1 and shifts in Vh were substantially more pronounced (Fig. 1C, E, G, I).
KV8 and KV7 subunits exist in multi-protein complexes in living cells
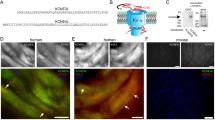
We then turned our attention to investigating how KVS subunits modulates KV7 channels at the molecular level, focusing on KV8.1 and KV8.2 subunits and analysed whether KV7 and KV8 subunits were present in the same protein complex and in close proximity to each other. We hence carried out BioID and co-immunoprecipitation experiments in HEK293 cells. For BioID experiments, we transiently overexpressed BioID2-HA-tagged KVS (KV8.1 or KV8.2) and flag-tagged KV7 subunits (Fig. 2). To ensure that the BioID2 fusion did not affect the expression and subcellular localisation of the KVS subunits, we performed immunostainings on transfected HeLa cells with antibodies against the HA tag at the C-terminal end of KV8.1, KV8.2 BioID2 fusion proteins. This revealed the expected intracellular localisation with a signal pattern typical of the endoplasmic reticulum (ER) within the cells. (Fig. 2B; Supplementary Fig. 6).
KV7 and KVS assemble in close proximity. A BioID assay methodology. The KVS (8.1/8.2) was fused to the promiscuous form (BirA*) of the bacterial biotin ligase BirA and co-expressed with flag-tagged KV7.2 subunits in HEK293 cells. Upon the addition of biotin, proximal proteins (grey) were biotinylated within a labeling radius of ∼10 nm, whereas the distal proteins (green), remained unlabelled. B Representative confocal images of HEK293 cells transiently transfected with BioID2-HA-tagged KV8.1 (first row), BioID2-HA tagged KV8.2 (second row), BioID2-HA alone (third row). Cells were stained with either HA antibody (red, first column) or Alexa Fluor™ 488 streptavidin conjugate (green, second column). Scale bar, 10 μm. C Following biotin labelling, cells were lysed and biotinylated proteins were then purified using streptavidin beads and identified by western blot analysis. Immunoblotting (IB) was performed by using an anti-flag antibody to detect the flag-labelled KV7.2 subunits and an Alexa Fluor™ 488 streptavidin conjugate (SA) to detect the biotinylated proteins. Note the absence of flag-tagged KV7.2 subunits in the AP lane with BioID2-HA alone (indicated by red arrow). However, they are present in both the AP lanes (indicated by green arrows). Overlaying the signals obtained from anti-flag and streptavidin staining revealed a noticeable proximity of these distinct bands (merge). Abbreviations used: Inp (input), Sup (supernatant), AP (affinity purified). Similar results were obtained in n = 3 transfections
Biotinylation of endogenous proteins in cells expressing HA-BioID2-KvS (8.1 and 8.2) with exogenous biotin strongly stimulated a wide range of endogenous proteins on western blots probed with Alexa Fluor™ 488 streptavidin conjugate (data not shown). This indicates that the BioID2 moiety was adequately exposed in the KVS fusion construct, allowing for efficient biotinylation. Our next step was to test whether the Kv7 subunits are in close proximity to the KVS subunits. If they are indeed in close proximity, they should be biotinylated and then precipitated with streptavidin beads. To achieve this, we transiently expressed BioID2 tagged KVS (8.1 or 8.2) and flag-tagged KV7.2 constructs in HEK293 cells in the presence of 50 µM biotin for 24 h and then lysed the cells using a radio-immunoprecipitation assay (RIPA) lysis buffer. HEK293 cells transfected with BioID2-HA alone, processed in parallel, were used as negative controls. As shown in Fig. 2C (anti-flag staining, green arrows), the flag-tagged KV7.2 subunits were robustly precipitated using streptavidin beads, strongly suggesting that the KV7.2 subunits are indeed in close proximity (10 nm distance) to the KVS (8.1 and 8.2) subunits and are therefore biotinylated, whereas no precipitation occurred when BioID2 alone was used as a negative control (red arrow). To complement these data with an independent approach, we performed a proximity ligation assay (PLA) on cells transfected with myc-tagged KVS and flag-tagged KV7. Indeed, PLA signals were only observed in cells co-expressing both, KVS and KV7 (Supplementary Fig. 2).
We then investigated whether the flag-tagged KV7.2 was located in the same complex as the KVS subunits by carrying out co-immunoprecipitation experiments. The protein complexes were precipitated with anti-flag M2 beads, and the subunits in the precipitates were detected in western blots with antibodies directed against myc or flag tags, respectively (Fig. 3A, B). These results demonstrated that the KV7.2 and KVS channels were not only located in close proximity, but also exist in a single multi-protein complex. As a negative control, co-immunoprecipitation experiments were carried out using cell lysates devoid of flag-tagged KV7 subunits (Supplementary Fig. 3), demonstrating the specificity of co-immunoprecipitations.
KV7 and KVS assemble into a protein complex. Co-immunoprecipitation experiments were performed using myc-tagged KV8.1 (left) and KV8.2 (right) subunits in combination with flag-tagged KV7.2 (A) and KV7.4 (B) channel subunits from HEK293 cell lysates using monoclonal anti-flag M2 conjugated agarose beads. The precipitate was then subjected to western blot analysis using monoclonal anti-flag M2 flag antibody and anti-myc antibodies respectively. Note the presence of both KV7 and KVS subunits in the precipitated fraction (indicated by black arrows). Asterisks indicate heavy chains detected by secondary antibodies. Abbreviations used: IP (immunoprecipitation), IB (immunoblotting), Sup (supernatant). Similar results were obtained in n = 3 transfections
KV8 modulate KV7 currents by affecting both membrane trafficking and biophysical properties
We then sought to determine whether KVS modulated KV7 currents through mechanisms similar to those involved in the regulation of KV2 channels. To this end, we measured the plasma membrane expression of both KV7.2 and KV7.4 channels containing an extracellular HA tag, in Xenopus laevis oocytes using a luminometric assay [34]. This method involves the oxidation of luminol by horseradish peroxidase (HRP) in conjunction with antibodies that can detect HA-tagged KV7 channels located on the oocyte surface. The intensity of light emitted directly correlates with the membrane expression of KV7 channels (Fig. 4A). In these experiments, co-expression of KV8.1 significantly increased the membrane expression of both KV7.2 and KV7.4 channels, whereas co-expression of KV8.2 slightly decreased the membrane expression of both channels (Fig. 4B, C), fully in line with the observed effects on current density (c.f., Fig. 1). Taken together, these data indicated that KV8.1 enhances KV7 currents by increasing their membrane expression, whereas co-expression of KV8.2 may reduce current amplitudes, at least in part, by attenuating surface expression of KV7 channels. Therefore, we conclude that KVS modulates KV7 currents by altering their membrane abundance, similar to the mechanisms reported for KV2.1 channels, but, unlike KV2 channels, in a bidirectional manner [5, 8].
KV7 and KVS form complexes with altered membrane trafficking. A Surface expression of HA-tagged KV7 channels measured with a luminometric technique in Xenopus oocytes. Mean surface expression of B HA-tagged KV7.2 channels C HA-tagged KV7.4 channels (measured in relative light units [RLUs]) in Xenopus oocytes after injection of HA-tagged KV7 cRNA either alone or together with KV8.1 or KV8.2. Uninjected oocytes were used as negative control
We then set out to gain insight into whether KVS subunits modulate KV7 channels via heterotetramerization into the same channel complex or by close interaction, e.g., as associated but independent channel entities or as some sort of ancillary beta subunit (Fig. 5A). To determine whether all isoforms contribute to the channel pore (i.e. heterotetramerization), we generated channels containing mutations in the GYG pore motif (GYG/AAA exchange for KV8.1 and GYG/GYS exchange for KV7.2) that render these variants inactive. When co-expressed with wild-type subunits, such variants are known to attenuate whole cell currents via dominant-negative effects [26]. Assuming fully stochastic co-assembly of equally available subunits, co-expression of wild-type and mutant subunits is predicted to reduce whole cell current amplitudes to 1/16 (compared to cells expressing only wild-type subunits), leaving intact a minimal number of channels containing only wild-type subunits (illustrated in Fig. 5A, first row). We performed patch-clamp recordings on CHO cells expressing wild-type and mutant isoforms taking current densities as measure for potential co-assembly of the subunits. In these experiments we used co-transfected KV8.1 and KV7.2 variants at a ratio of 4:1 (KV8.1:KV7.2) to ensure an excess of KV8.1. Co-expression of wild-type KV8.1 with wild-type KV7.2 significantly increased whole cell current amplitudes compared to cells expressing only KV7.2 channels, as we had observed before (Fig. 5B). When we co-expressed pore-mutated KV7.2(GYS) with wild-type KV8.1, current amplitudes were reduced to virtually zero. These experiments demonstrated that KV8.1 subunits alone were not able to form an independent functional pore (Fig. 5B).
KV7 and KVS might form heteromers with a single conducting pore. A Imaginable modes of interaction of KV7 with KVS, consequences of expression of pore mutant subunits and expected membrane conductance. *) The percentage of current reduction depends on the probability of inclusion of the pore-mutant subunit into a heterotetramer. Detailed explanation is given in the main text. B Patch-clamp recordings from CHO cells co-transfected with either combination of wild-type (GYG) and pore-mutated (AAA, GYS, resp.) KV8.1 and KV7.2 or with KV7.2 alone. Top panel shows stimulus protocol (black) and exemplary recordings. Bottom panel shows summary statistics for steady-state current densities at + 20 mV. C Patch-clamp recordings from CHO analogue to those shown in B, but examining the Kv8.1 pore-mutants GYR and W388G. For the experiments shown in B and C KV7.2 and KV8.1 plasmids were co-transfected at a mass ratio of 1:4. Where no KV8.1 was included into the transfection this was substituted by eGFP. D Surface quantification measurements from Xenopus oocytes similar to those shown in Fig. 4B, but using KV8.1 pore-mutants AAA and GYR variants. E Patch-clamp recordings from CHO analogue to those shown in B but performed using a 1:1 mass ratio for transfection. Electrophysiological data are shown as relative current densities normalized to the mean of current densities observed when transfecting wild-type KV7.2 with wild type KV8.1
When wild type KV7.2 was transfected together with pore-mutated KV8.1(AAA), current densities were reduced to approximately 50% of those observed when measuring cells transfected with both wild type constructs, resulting in current densities minimally smaller than those observed in cells transfected with KV7.2 alone (Fig. 5B, note that amount of cDNA encoding KV7.2 was kept constant). These results suggested that functional pore regions of KV8.1 are essential for KVS-dependent modulation of KV7 channels. Noteworthy, the (only) 50% current reduction observed upon KV8.1(AAA) co-transfection may indicate that during co-assembly KV7.2 homotetramer formation is more likely to occur than heterotetramer formation. In particular, the 50% reduction observed herein would be expected if the probability for KV7.2 homomers was approximately fivefold higher than that for KV7.2/KV8.1 heteromers. Yet, our observations clearly rule out alternative modes of interaction: If KV7.2 and KV8.1 co-existed as independent channels (Fig. 5A, second row), a substantial current should have been observable upon co-expression of pore-mutated KV7.2(GYS); If KV8.1was a beta-subunit to KV7.2 (Fig. 5A, third row), mutating the “pore” sequence of KV8.1 would not be expected to have any effect at all.
Notably, the GYG to AAA is a relatively aggressive way of disrupting a KV-channel’s pore, potentially causing the protein to misfold and become unstable. If this were the case, there would be no complex formation between KV7.2 channels and KV8.1(AAA) subunits and there would be no increased surface expression of KV7.2, which could explain the 50% reduction in current, seen with KV8.1(AAA) co-transfection. To rule this out, we first tested other, more conservative variants known to render KVS non, conducting (Fig. 5C). These were G399R, corresponding to the second G in the GYG motif, and W388G, located in the S4 segment. These variants were deduced from KV8.2, where they are naturally occurring pathogenic variants that have been demonstrated to render KV2.1-KV8.2 heterotetramers nonconducting without affecting surface expression [40]. We found that G399R and W388G reduced current amplitudes to 54.05 (± 4.96) % and 44.13 (± 20.27) %, respectively (p < 0.05 for both), and thus even minimally stronger than the AAA mutant. We next tested surface expression for the AAA and G399R variants using Xenopus laevis oocyte system and found that both the variants behave similar to the wild-type KV8.1, indicating no misfolding or stability issues (Fig. 5D). Finally, in CHO cells, we confirmed that also the 4:1 (KV8.1:KV7.2) transfection ratio utilized in the experiments from Fig. 5B, C did not affect our observations. Indeed, results observed with a 1:1 transfection ratio were similar to those obtained with a 4:1 ratio: KV7.2/KV8.1(AAA) co-transfection reduced current amplitudes to 49.4 (± 10) % as compared to co-transfection of wild-type channels (p < 0.05, Fig. 5E).
Taken together, these data suggest that KV7 and KV8.1 subunits can likely heteromerise into functional channels with a slight preference for KV7 homomers.
KV7 and KVS are expressed in the same organs and cells
To investigate whether an interaction between KV7 and KVS might be physiologically relevant, we used reverse transcription quantitative polymerase chain reaction (RT-qPCR) to examine the expression of KV8 in three tissues, where the role of KV7 channels is well established [46]. Indeed, we found that in the hippocampus and dorsal root ganglia, the abundance of Kcnv1 (KV8.1) mRNA was in the same order of magnitude as that of Kcnq2 (KV7.2) mRNA. In both these neural tissues, also Kcnv2 (KV8.2) mRNA was detected, though at much lower levels (Fig. 6A). By contrast, in heart, expression levels of Kcnv2 (KV8.2) were comparable to those of Kcnq1 (KV7.1), while Kcnv1 mRNA was not detected (Fig. 6B).
KV7 and KVS co-express in various tissues in a cell-specific manner. A, B Reverse transcription quantitative PCR for KV7 and KVS transcripts from neuronal (A) and cardiac (B) tissue. For each condition 8 biological replicates with two technical replicates were included into the analysis. Shown are expression levels normalized to the mean expression level of KV7.2 for Hippocampus and Dorsal Root Ganglia (DRG) (A) and KV7.1 for Heart (B), respectively. C Expression dot plots of KV8.1, KV7 and KV2 genes, for comparison, in Hippocampus. Shown are only clusters with KV8.1 expression. The complete dot plot is given in Supplementary Fig. 5. D–G RNAScope of KV8.1 in the mouse hippocampus. D Low-magnification overview. Scale bar: 500 μm. E–G High-magnification confocal micrographs of CA 1 (E), CA 2 (F) regions and Dentate Gyrus (G), respectively. Scale bar: 100 μm. H Correlation coefficients for the single-cell expression of KV7 and KV2 with KV8.1 or KV8.2, respectively. Shown are Pearson’s correlation coefficients for log2 + 1-transformed transcript counts from individual cells as observed in three publicly available singe-cell RNA sequencing datasets. Included into the analysis were only cell types found to express the respective KVS
To further evaluate whether KV7 and KVS transcripts merely coexist in the same tissue, or are actually transcribed in the same cells we analysed publicly available single-cell RNA sequencing (scRNAseq) datasets from the hippocampus [36], dorsal root ganglia (DRG) [13] and the heart [42]. Consistent with our observations in the qPCR experiments we found high read counts for Kcnq2 and Kcnv1 in hippocampal and DRG cells, whereas Kcnv2 was barely detected. In the dataset from cardiac cells, in turn, Kcnq1 and Kcnv2 were predominant, whereas Kv8.1/Kcnv1 was not found (Supplement material file 1, Table S2).
In the hippocampus, Kcnv1 was encountered in seven clusters, with the highest transcript counts being observed in the clusters representing CA1-3 pyramidal neurons, dentate hilus and dentate principal neurons (Fig. 6C). To see if the observed transcriptomic expression pattern would also be supported by methods that provide spatially encoded information, we employed single molecule fluorescence RNA in-situ hybridization (“RNAScope”). Indeed, using RNAScope we found Kcnv1 expression patterns closely matching the observations made using scRNAseq (Fig. 6D–G). By far the highest Kcnv1 signal was obtained from the CA2 region, followed by the dentate gyrus and the CA1 region. In scRNAseq, both KV7.2/Kcnq2 and KV2.1/Kcnb1 were both found in each of these cell types /clusters (Fig. 6H, Supplementary Fig. 5A). We argued that if KV8.1 would interact with KV7.2 rather than a KV2 in hippocampal neurons, one would expect Kcnv1 expression levels to correlate stronger with those of Kcnq2 than with those of Kcnb1 or Kcnb2 at the single cell level. Indeed, Pearson’s correlation coefficient (PCC) for Kcnv1 with Kcnq2 was higher than the correlation of Kcnv1 with 99 [98–99] % of all other transcripts encountered, and in particular higher than that with Kcnb1 (Fig. 6H, left, Supplement material file 1, Table S3).
In dorsal root ganglia, Kcnv1 and Kcnq2 overlapped in two clusters (Supplementary Fig. 5B). In stark contrast to the hippocampus, Kcnv1 expression levels in DRG were more strongly correlated with those of KV2 channel genes rather than with those of KV7 channel genes (Fig. 6H, middle, Supplement material file 1, Table S3). In the heart, Kcnv2 was found exclusively in the cluster representing cardiac myocytes, as was Kcnq1 (Supplementary Fig. 5 C). Here, the PCC for Kcnv2 with Kcnq1 was higher than the correlation of Kcnv2 with 0.99 [0.98–1.00] % of all transcripts encountered, and, again, particularly higher than that with Kcnb1 (Fig. 6H, right, Supplement material file 1, Table S3). Taken together, these transcriptomic analyses suggest that KV7 and KV8 mRNA expression is strongly correlated within individual cells in hippocampus and heart, whereas this was not the case in the DRG.
Discussion
In the present work, we show that KV channels from the silent KV-subfamily (KVS) can interact with KV7 channels to alter their trafficking and biophysical properties. Modulation of KV2 channels through KVS has long been reported [5, 8] and, up until now, this has been considered to be the only case of cross-family interaction among KV channel families. Yet, in analogy to their actions on KV2 channels [5], KVS reduced KV7-mediated currents through an alteration of KV7 membrane targeting and voltage-dependency. Importantly, – and in contrast to their effects on KV2 – certain KVS also increased KV7 current amplitudes and surface expression. Thus, modulation of KV7 by KVS is bidirectional and thereby more diverse than the modulation of KV2. Based on our data, we hypothesise that KV7 co-assemble with KVS into functional heteromers. This further broadens the functional diversity of KV channels and may serve to shape potassium conductance to fit the needs of individual cell types.
Mode and functional aspects of the KV7-KVS interaction
Using PLA and BioID experiments, we herein demonstrated that in living cells KV7 co-exist with KV8.1 and KV8.2 in close physical proximity. In addition, we could show by co-immunoprecipitation that these ion channels assemble into a common protein complex. This interaction dictates trafficking of KV7 channels to the plasma membrane allowing for a bidirectional modulation of KV7-mediated current amplitudes. Indeed, the enhanced surface abundance of KV7.2 and KV7.4 in Xenopus laevis oocytes upon co-expression of KV8.1 was paralleled by increased macroscopic KV7.2 and KV7.4 current amplitudes recorded from CHO cells. Conversely, co-expression of KV8.2 resulted in a reduction in both macroscopic current amplitudes and surface expression. We conclude that the molecular mechanism of the KVS-dependent modulation of KV7 currents may be similar to that of KV2 channels (i.e., modulation of surface expression plus modulation of biophysical properties). However, bidirectional modulation of current amplitudes indicates that the interaction between KV7 and KVS may be somewhat more versatile than their interaction with KV2 channels, which are generally down-regulated by KVS [5]. Taking into account our RNA-sequencing analyses, co-expression of Kv7.2 and Kv8.1 in hippocampal neurons, for example, may lead to a tuned decrease in electrical excitability by increasing the KV7-medited M-current [45]. Modulation of KV7.1 by KV8.2 subunits in cardiac muscle, in turn, may lead to a slower and delayed repolarization process. However, mouse models carrying genetic deletions will be required to unequivocally demonstrate whether this novel interaction is also relevant in native tissues.
Although our data provide clear evidence for the coexistence of KV7 and KVS subunits in a common protein complex, and the functional relevance of the interaction, we are unable to draw a definite conclusion as to whether KV7 and KVS co-assemble into functional heterotetramers, as it has been previously been shown for KV2 channels [6, 32]. Since KVS altered voltage-dependence of KV7 channels, we propose heteromerisation also for KVS and KV7 channels. In support of this notion, we observed that co-transfection of different dominant-negative pore-mutant KV8 channels with wild-type KV7.2 led to a robust (~ 50%) reduction of current amplitudes as compared to transfection of both wild-type subunits while exerting no effect on membrane trafficking. Thus, the functional modulation of KV7.2 currents was abolished via co-expression of KV8 pore mutants. While these findings are in line with a possible heterotetramerization, the dominant-negative effects of KVS pore mutants on KV7 currents were somewhat weaker than expected: Assuming each subunit would be built into a heterotetramer with equal probability a ~ 95% reduction in current amplitude would be anticipated. Importantly, it is unclear whether the potential KV7/KVS heterotetramerization is fully stochastic. Specifically, a roughly fivefold assembly preference for respective homotetramers over heterotetramers would explain the observed 50% current reduction. Potential preference for homomers over heteromer formation might seem surprising, but it has indeed been demonstrated even for the well-studied KV2-KV8.2 interaction, and the degree of current reduction observed for KV2.1 by dominant-negative KV8.2 mutants is only slightly higher than what we observed in our study [9, 40]. Nevertheless, it is important to note that the data presented herein are suggestive of heterotetramerization, yet they are no definite proof of it. There is still room for debate regarding the mode of interaction and studying KV7.4 channels (where changes in the Vh upon co-transfection of dominant-negative KVS) or on KV7-KVS concatemers may help to clarify this issue in future.
Most KV channels, including KVS possess an N-terminal T1 domain that plays an important role in mediating the assembly of individual subunits into a channel complex [50]. Such a T1 domain is absent in KV7. Instead, KV7 assembly is thought to be mainly mediated by C-terminal coiled-coil A-D domains [11]. This imposes the question of how KV7-KVS assembly might occur. It is worth mentioning that although T1 and A-D domains strongly support the formation of heterotetramers, they are not essential for it [3, 50], Thus, interaction of KV7 with the T1 domain of KVS would not be a necessary requirement for complex formation. For the silent-KV channel KV6.4 it has been particularly shown that it’s tetramerization with KV2.1 does not only happen via the N-terminus, but that an additional C-to-N-terminal interaction is required for efficient KV6.4/KV2.1 heterotetramerization [7]. Thus, it appears possible that a non -T1 mediated or C-to-C-terminal mode of interaction could mediate KV7-KVS complex formation. Nevertheless, further research is needed to identify the definitive structural determinants of this interaction.
Further complicating the topic, the A-B domains in KV7 are also the site of Calmodulin (CaM) binding [18]. For example, the mutation (R353G) associated with benign familial neonatal convulsions (BFNC) impairs CaM binding [12], resulting in reduced currents due to retention of KV7.2 subunits in the endoplasmic reticulum. Our experiments show a significant increase in the forward trafficking of both KV7.2 and KV7.4 channels, when co-expressed with KV8.1 subunits, a finding supported by both electrophysiological and surface quantification measurements, suggesting that CaM, if obligatory, should be associated with the KV7/ KV8.1 channel complex. For KV7/KV8.2 heteromers, we observed opposite effects, which could speculatively represent reduced CaM binding. To better understand this, future studies could investigate the presence of native CaM proteins in the co-immunoprecipitates of KV7/KVS heteromers, as both HeLa and HEK cells express sufficient levels of endogenous CaM protein. In addition, association of CaM with KV7/KVS complexes can be investigated by performing experiments with mutant KV7.2 channels (e.g., R353G).
The physiological role of KV7-KVS interaction
In the present study, we used a wide variety of cell types, including cell lines from primates, rodents and amphibian origin. This substantiates the notion that the interaction between KV7 and KVS is not restricted to a particular species or cell line, but instead represents a universally observable and evolutionarily stable phenomenon.
To further explore the presence of KV7-KVS interaction in native systems, we delved into where this interaction might occur. Through RT-qPCR analysis, we detected the expression of KV8.1 and KV8.2 in three tissues, where KV7 play a well-established, prominent role [15, 31]: the hippocampus, dorsal root ganglia, and the heart (Fig. 6A–C). We then analysed single-cell RNA-sequencing datasets, and observed that KV7 and KVS were not just expressed in the same tissues, but actually in the same cell types and cells (Supplementary Fig. 5). Moreover, we found that on the level of individual cells, in several subtypes of hippocampal neurons (e.g. CA1–CA3) and cardiac muscle cells expression levels of KV7 and KVS were robustly correlated. Furthermore, our analysis revealed that in the hippocampus and heart, the expression of KV8.1 and KV8.2, respectively, showed a substantially stronger correlation with KV7 as compared to KV2. Correlation in transcript counts obviously do not immediately proof functional interaction on protein level. Thus, these findings fall short in providing direct evidence for a functional KV7-KVS interaction in the examined tissues. Recent studies, however, show that transcript-level correlation is commonly maintained on protein level and can help to predict protein function [35, 39]. As a word of caution, it needs to be added that ion channel transcripts are typically low in abundance, which poses a challenge to such single-cell transcriptomic correlation analyses. We have therefore selected datasets with a particularly profound read depth for this analysis and were able to obtain narrow confidence intervals for KV7-KVS single-cell transcript level correlation. KV7-KVS correlation coefficients in hippocampus and heart were moreover higher than the correlation of KVS with 99% of all other transcripts encountered, strongly suggesting that the observed correlation is beyond mere coincidence.
To establish definitive evidence for the presence of KV7/KVS complexes in native tissues and unravel their physiological significance, further investigations encompassing both structural and functional studies are warranted. Specific antibodies will be required for immunohistochemical studies as well as for native co-immunoprecipitations. While such antibodies are established for all KV7 channels, the situation is more complex for KVS. To the best of our knowledge, to-date, only antibodies directed against KV8.4 have been successfully used in peer-reviewed research [14]. Functional studies present significant challenges that can only be addressed by establishing knockout mouse models and/or conducting a comprehensive pharmacological characterization of KV7/KVS complexes. In this regard, it is encouraging that a first KV8.2 mouse model is available and has been partially characterized [19, 21]. These approaches will clarify the specific roles and functional properties of KV7-KVS interactions in biological systems.
Translational relevance
While this is primarily a cell physiological study, the herein-described KV7-KVS interaction may hold promise for clinical and translational applications. The newfound KV7-KVS complex may pose unique pharmacological properties that are distinct from KV7 complexes not encompassing KVS. This would enable the development of pharmacological agents specifically targeting KV7-KVS complexes while leaving KV7 homotetramers unaffected. Such an approach could enable highly specific pharmacotherapies e.g., for epilepsy or cardiac arrhythmias.
Indeed, before pursuing such translational directions, a better understanding of the role of KV7-KVS complexes in native tissues, as well as their pharmacological properties is required.
Conclusion
In summary, our study provides evidence for the formation of a common protein complex between KVS and KV7 subunits. These complexes exhibit distinct electrophysiological properties, possibly through heterotetramer formation. Our data suggest that such interactions may also occur in native tissues, particularly in the hippocampus and the heart. KVS/KV7 interactions could therefore represent a mechanism utilized by nature to further increase the functional diversity of KV channels and to fine-tune the electrophysiological properties of individual cell types to their functional needs.
Material and methods
Animal studies
Adult female African clawed frogs (Xenopus laevis) were used for experiments with Xenopus oocytes. The frogs were anaesthetized by placing them in water containing 1 g/l tricaine. Stage V oocytes were collected from the ovarian lobes. Anesthesia and surgery were performed with the approval of the Giessen Regional Animal Health Authority.
C57Bl/6J mice were purchased from Janvier Labs (Le Genest-Saint-Isle, France). Tissue collection was performed in accordance with the Ethics Guidelines of Animal Care (Medical University of Innsbruck).
Molecular cloning and mutagenesis
Supplemental Table S1 summarizes the constructs used in this study. QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, Agilent Technologies, Waldbronn, Germany) was utilized to introduce point mutations. To enhance expression efficiency, all constructs utilized for experiments in Xenopus oocytes were subcloned between the 5' and 3' UTR's of the Xenopus β-globin gene in the modified pSGEM vector. The mMessage mMachine kit (Ambion, Huntingdon, UK) was used to synthesize complementary RNA transcripts. For surface quantification assays, a plasmid containing KCNQ2 [38] or KCNQ4 [24] with an external hemagglutinin (HA) epitope tag was used. All DNA constructs were verified through Sanger sequencing.
Cell culture and transfection
Chinese hamster ovary (CHO) dhFR− cells were maintained as previously described [30]. In brief, cells were kept in MEM Alpha Medium supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (Invitrogen GmbH, Darmstadt, Germany) in a humidified atmosphere at 5% CO2 and 37 °C. Cells were transiently transfected with jetPEI transfection reagent (Polyplus Transfection, Illkirch, France). HeLa and HEK293 cells were cultured in the same way as CHO. Transfection of HeLa cells was performed using jetPRIME transfection reagent (Polyplus Transfection, Illkirch, France). When combining various ion channel coding plasmids into a single transfection, co-transfection was performed usinHeLa and HEK293 cells were cultured in the sameg equal amounts (by mass) of each plasmid, unless otherwise specified.
Electrophysiological recordings
Whole-cell recordings were performed on transiently transfected CHO cells in culture, as previously reported [27, 28, 48]. All experiments were performed approximately 48 h after transfection [10]. During recordings, cells were kept in extracellular solution containing (in mM): 144 NaCl, 5.8 KCl, 1.3 CaCl2, 0.7 Na2HPO4, 0.9 MgCl2, 5.6 glucose, 10 HEPES, pH adjusted to 7.4 (NaOH) (305–310 mOsm/kg). Whole-cell patch clamp recordings were performed at room temperature (19–23˚C) with an HEKA EPC10 USB patch clamp amplifier controlled by PatchMaster software (HEKA, Lambrecht, Germany) or an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Voltage clamp recordings were low-pass filtered at 2.5 kHz and sampled at 5 kHz. Recordings were excluded from analyses, when the series resistance (Rs) was ≥ 7 MΩ, and Rs was compensated through-out the recordings to 80%, with the exception of the data presented in Fig. 5, where no Rs compensation was performed. Patch pipettes were pulled from borosilicate glass (Sutter Instrument Company, Novato, CA, USA) and had a resistance of 2–3.5 MΩ after filling with intracellular solution containing (mM): 135 KCl, 3.5 MgCl2, 2.4 CaCl2 (0.1 free Ca2+), 5 EGTA, 5 HEPES and 2.5 Na2-ATP (pH adjusted with KOH to 7.3; 290–295 mOsm/kg).
Co-immunoprecipitation
To perform immunoprecipitations, anti-FLAG M2 magnetic beads from Sigma-Aldrich (St. Louis, MO) were used in accordance with the manufacturer's instructions. Briefly, lysates from HEK293 cells that expressed flag-tagged KV7 and myc-tagged KVS were utilized. The beads were then incubated with the cell lysates under rotation at 4 °C for 12 h. Beads with antigen–antibody complex were extensively washed (4 times) with wash buffer, followed by elution of the proteins on the beads through boiling at 72 °C for 10 min in 2 × SDS sample loading buffer. The proteins were then separated on a 10–12% SDS-PAGE under reducing conditions, transferred to a nitrocellulose membrane and probed with either a mouse anti-myc antibody (1:1000; Cell Signaling, Danvers, MA) or a monoclonal ANTI-FLAG® M2 antibody (1:1000; Sigma-Aldrich). Fluorescent secondary antibodies (1:5000; Bio-Rad, Hercules, CA) were used to visualize the membrane, which was then imaged with a ChemiDoc MP imaging system from Bio-Rad.
Proximity ligation assay
CHO cells were fixed in 4% ice-cold methanol-free paraformaldehyde as previously described [29]. Proximity ligation assay (PLA) was performed using the Duolink In-Situ Red Starter Kit (Sigma-Aldrich) according to the manufacturer's protocol, with the amplification step being performed by incubation with polymerase at 37 °C for 50 min. Primary antibodies used were rabbit anti myc (71D10, Cell Signaling) at a dilution of 1:200 and mouse anti flag (F1804, Sigma-Aldrich) at 1:400. Fluorescent secondary antibody (Alexa 488 conjugated Donkey anti mouse, ThermoFisher, Waltham, USA, 1:250) was added during the PLA probe incubation step. Image acquisition was performed using an LSM710 confocal laser scanning microscope (LSM, Carl Zeiss, Jena, Germany) as previously described [29].
BioID proximity-dependent biotinylation assay
KVS -BioID2 (HA-tag and biotin-ligase were fused to the C-terminus of KVS subunits) was transfected into 6 cm plates of HEK293 cells. After 24 h of transfection, 50 μM biotin was added to the culture medium to induce biotinylation of proteins in the vicinity of BioID2-KVS in the cells for 18–24 h. Cells were lysed in RIPA lysis buffer (150 mM NaCl; 50 mM Tris-HCl pH = 7.4; 1% Triton X100; 0.1% SDS; 0.5% sodium deoxycholate). The sample was passed through an 18-gauge needle several times to reduce viscosity. Biotinylated proteins were purified using magnetic streptavidin beads (Pierce/Thermo Fisher) and eluted in 6 × SDS-PAGE sample buffer containing 3 mM biotin by boiling at 95 °C for 10 min under reducing conditions. Proteins were transferred to a nitrocellulose membrane and probed with either a flag-tag monoclonal antibody (1:1000; Proteintech, Rosemont, IL) or Alexa Fluor™ 488 streptavidin conjugate (1:3000; Invitrogen, Waltham, MA) for detection of biotinylated proteins.
Single molecule fluorescence in situ hybridization
Brains were rapidly extracted from C57Bl/6J mice and flash-frozen in isopentane on dry ice. Brains were then coronally sectioned at 14 µm thickness in a cryostat. Sections were air-dried for 20 min and subsequently stored at −80 °C until used. The RNAscope Multiplex Fluorescent v2 Assay (Advanced Cell Diagnostics, Newark, CA, USA) was used to visualize mRNA expression following the manufacturer’s instructions except for the washings steps that were extended to 10 min. Kcnv1 mRNA was detected using the target probe Mm-Kcnv1 (Advanced Cell Diagnostics) and the Opal™ 650 dye (1:1500, Akoya Biosciences, Marlborough, MA, USA).
Surface quantification assay
The surface expression of HA-tagged KV7 subunits in Xenopus oocytes was analysed 2 days after injection of the cRNA (10 ng/oocyte of HA-tagged KV7.2 alone or together with 10 ng/oocyte of KV8.1 or KV8.2). To block non-specific antibody binding, oocytes were incubated in ND96 (in mM: NaCl 96, KCl 2, CaCl2 1.8, MgCl2 1, HEPES 20, Na-pyruvate 2.5, and 100U/ml Penicillin–streptomycin) solution containing 1% Bovine serum albumin (BSA) at 4 °C for 30 min. Oocytes were then incubated for 60 min at 4 °C with 100 μg/ml rat monoclonal anti-HA antibody (clone 3F10, Roche Pharmaceuticals, Basel, Switzerland) in 1% BSA/ND96, washed 6 times at 4 °C with 1% BSA/ND96 and incubated with 2 μg/ml of peroxidase-conjugated, affinity-purified, F(ab)2-fragment goat anti-rat immunoglobulin G antibody (Jackson ImmunoResearch, West Grove, PA) in 1% BSA/ND96 for 60 min. Oocytes were washed thoroughly, first in 1% BSA/ND96 (4°C, 60 min) and then in 1 × ND96 without BSA (4 °C, 15 min). Individual oocytes were placed in 20 µL of SuperSignal Elisa Femto solution (Pierce, Chester, UK) and, after an equilibration period of 10 s, chemiluminescence was quantified in a luminometer (Lumat LB9507, Berthold Technologies, Bad Wildbad, Germany). For each construct, the surface expression of 20 oocytes was analysed in one experiment and at least three experiments (∼40 oocytes) were performed. The luminescence produced by uninjected oocytes was used as a negative control.
Quantitative reverse transcriptase-PCR
DRG, hippocampus and heart tissue were isolated from C57Bl/6J mice, snap frozen in liquid nitrogen and stored at −80°C until use. RNA extraction and reverse transcription quantitative polymerase chain reaction (RT-qPCR) were performed as previously described [23, 25]. Briefly, the peqGOLD TriFast reagent (Peqlab) was used to extract total RNA according to manufacturer’s instructions [chloroform (C2432 and absolute ethanol (107017 were obtained from Merck]. RNA pellets were reconstituted in nuclease free water (R0582, ThermoFisher Scientific) and concentration was measured using NanoDrop 2000 (ThermoFisher Scientific). Reverse transcription was performed using MuLV reverse transcriptase (N8080018, ThermoFisher Scientific) according to the supplier’s protocol. Gene expression was estimated using the following TaqMan Gene Expression Assays (ThermoFisher Scientific): Kcnq1 (Mm00434640_m1), Kcnq2 (Mm00440080_m1), Kcnv1 (Mm00550691_m1), Kcnv2 (Mm00807577_m1), Hprt (Mm00446968_m1), Sdha (Mm01352363_m1), and Tfrc (Mm00441941_m1), with Hprt, Sdha and Tfrc serving as reference genes. Reactions were prepared according to TaqMan Gene Expression Assays protocol (20 µL reactions), loaded on MicroAmp Fast Optical 96-well reaction plates (ThermoFisher Scientific) and run on the 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). The protocol details were as follows: an initial 10 min 95 °C step, followed by 40 two-step cycles of 15 s at 95 °C and 1 min at 60 °C. Samples (eight per tissue) were run as technical duplicates alongside non-template controls. Threshold was set manually at 0.1, whereas baselines were estimated automatically. Cq values for each sample were normalized to the respective geometric mean of the Cq values of the three reference genes (Hprt/Sdha/Tfrc) and subsequently expressed in relation to the average expression of either Kcnq2 (for DRG and hippocampus tissues) or Kcnq1 (for heart tissue).
Data analysis and statistics
Patch clamp recordings were analysed with PatchMaster (HEKA) and IgorPro (Wavemetrics, Lake Oswego, OR) or R version 4.1.5 [33] together with the PatchR package (https://github.com/moritzlindner/PatchR). Voltage dependence of activation was derived from tail current amplitudes using voltage protocols indicated: Maximal amplitudes of tail currents were fit with a two-state Boltzmann function with I = Imin + (Imax-Imin)/(1 + exp((V-Vh)/s)), where I is current, V is the membrane voltage, Vh is the voltage at half maximal activation, and s describes the steepness of the curve. Results on voltage dependence are shown as normalised tail current (conductance)-voltage curves, obtained by normalizing to (Imax-Imin), obtained from fits to data of individual experiments. Statistical analysis was performed with two-tailed Student´s, Wilcoxon signed, Dunnett or Scheffé test as appropriate. For surface expression analysis in oocytes, values from three experiments were normalised to oocytes injected with extracellularly HA-tagged KV7 cRNA. Statistical analysis was carried out by ordinary one-way ANOVA with post hoc Dunnett's multiple comparison test. Statistical significance was assigned at P ≤ 0.05 (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). All data are presented as mean ± SEM. In electrophysiological experiments, n represents the number of individual cells recorded from at least three independent experiments (independent transfections or independent tissue, i.e. biological replicates).
Confocal micrographs form PLA experiments were analysed using FIJI (National Institutes of Health, Bethesda, MD, USA, [37]) and a custom-made macro (accessible via: https://www.kvs-liaison.eu/data/PLA_Macro_autothreshold.ijm). This macro enabled unbiased analysis of the micrographs by automatically (1) detecting the boundaries of cells showing flag immunofluorescence, and (2) then counting the PLA dots inside and outside the boundaries of those immunopositive cells separately. Summary statistics were then performed using R version 4.1.5 [33] and ggplot2 [47].
Bioinformatics
Three individual published single-cell RNA sequencing datasets were utilized to analyse the distribution and co-expression of Kv8, Kv7 and Kv2 in hippocampus [36], dorsal root ganglia (DRG, [13]) and heart [42], respectively. Raw gene count matrices were obtained from the Genome Expression Omnibus (GEO) archive (DRG: GSE128276) or author curated archives (hippocampus: dropviz.org, heart: https://figshare.com/projects/Tabula_Muris_Transcriptomic_characterization_of_20_organs_and_tissues_from_Mus_musculus_at_single_cell_resolution/27733). Processing and analysis of the datasets were performed using R 4.1.5 [33] and Seurat 3.1 [44]. Data were log-normalized, centred, and scaled. Where available, cluster assignments lists as published by the authors of the datasets were utilized. For DRG, clustering was performed using the KNN and Louvain algorithms implemented in Seurat after regressing out technical variance. Obtained clusters were validated using a set of unique cluster markers kindly provided by the authors and the DRG cell-type marker genes as reported by Usoskin et al. [44] (Supplementary Fig. 4). Dot plots for KV7, KV2 and KVS expression in the individual cell clusters identified in each dataset were drawn with dot radius representing the percentage of cells in an individual cluster expressing the KV or KVS gene under investigation and dot colour indicating the mean scaled gene count in that cluster, KV/ KVS genes detected in less than 10% of the cells belonging to any individual clusters were not visualized. Finally, correlation of the expression levels of KVS with KV7 and KV2 were analysed using Pearson’s correlation coefficients (PCC). Data were bootstrapped (n = 100, with cells being individual observations) to obtain an estimate of variance. Data are presented as Box-and-Whisker plots with hinges representing quartiles and whiskers extending to the last data point within the 1.5-fold inter quartile ranges. To explore the biological plausibility of this approach correlation coefficients were also calculated for genes of ion-channels that are known to interact with any of the Kv-channels under investigation (Supplementary Fig. 5).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alexander SP, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C et al (2017) The concise guide to pharmacology 2017/18: voltage-gated ion channels. Br J Pharmacol 174(Suppl 1):S160–S194
Bal M, Zhang J, Zaika O, Hernandez CC, Shapiro MS (2008) Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J Biol Chem 283:30668–30676
Bates A, Stowe RB, Travis EM, Cook LE, Dabney-Smith C, Lorigan GA (2023) The role of native cysteine residues in the oligomerization of KCNQ1 channels. Biochem Biophys Res Commun 659:34–39
Bergren SK, Rutter ED, Kearney JA (2009) Fine mapping of an epilepsy modifier gene on mouse Chromosome 19. Mamm Genome 20:359–366
Bocksteins E (2016) Kv5, Kv6, Kv8, and Kv9 subunits: no simple silent bystanders. J Gen Physiol 147:105–125
Bocksteins E, Labro AJ, Mayeur E, Bruyns T, Timmermans JP, Adriaensen D, Snyders DJ (2009) Conserved negative charges in the N-terminal tetramerization domain mediate efficient assembly of Kv2.1 and Kv2.1/Kv6.4 channels. J Biol Chem 284:31625–31634
Bocksteins E, Mayeur E, Van Tilborg A, Regnier G, Timmermans JP, Snyders DJ (2014) The subfamily-specific interaction between Kv2.1 and Kv6.4 subunits is determined by interactions between the N- and C-termini. PloS one 9:e98960
Bocksteins E, Snyders DJ (2012) Electrically silent Kv subunits: their molecular and functional characteristics. Physiology (Bethesda) 27:73–84
Czirjak G, Toth ZE, Enyedi P (2007) Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J Neurophysiol 98:1213–1222
Dierich M, Altoe A, Koppelmann J, Evers S, Renigunta V, Schafer MK, Naumann R, Verhulst S, Oliver D, Leitner MG (2020) Optimized tuning of auditory inner hair cells to encode complex sound through synergistic activity of six independent K(+) current entities. Cell Rep 32:107869
Dixit G, Dabney-Smith C, Lorigan GA (2020) The membrane protein KCNQ1 potassium ion channel: functional diversity and current structural insights. Biochim Biophys Acta Biomembr 1862:183148
Etxeberria A, Aivar P, Rodriguez-Alfaro JA, Alaimo A, Villace P, Gomez-Posada JC, Areso P, Villarroel A (2008) Calmodulin regulates the trafficking of KCNQ2 potassium channels. FASEB J 22:1135–1143
Finno CJ, Peterson J, Kang M, Park S, Bordbari MH, Durbin-Johnson B, Settles M, Perez-Flores MC, Lee JH, Yamoah EN (2019) Single-cell RNA-seq reveals profound alterations in mechanosensitive dorsal root ganglion neurons with vitamin E deficiency. iScience 21:720–735
Gayet-Primo J, Yaeger DB, Khanjian RA, Puthussery T (2018) Heteromeric KV2/KV8.2 Channels Mediate Delayed Rectifier Potassium Currents in Primate Photoreceptors. J Neurosci 38:3414–3427
Greene DL, Hoshi N (2017) Modulation of Kv7 channels and excitability in the brain. Cell Mol Life Sci 74:495–508
Guimaraes TAC, Georgiou M, Robson AG, Michaelides M (2020) KCNV2 retinopathy: clinical features, molecular genetics and directions for future therapy. Ophthalmic Genet 41:208–215
Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W et al (2005) International union of pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57:473–508
Haitin Y, Attali B (2008) The C-terminus of Kv7 channels: a multifunctional module. J Physiol 586:1803–1810
Hart NS, Mountford JK, Voigt V, Fuller-Carter P, Barth M, Nerbonne JM, Hunt DM, Carvalho LS (2019) The role of the voltage-gated potassium channel proteins Kv8.2 and Kv2.1 in vision and retinal disease: insights from the study of mouse gene knock-out mutations. eNeuro. https://doi.org/10.1523/ENEURO.0032-19.2019
Howard RJ, Clark KA, Holton JM, Minor DL Jr (2007) Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron 53:663–675
Jiang X, Rashwan R, Voigt V, Nerbonne J, Hunt DM, Carvalho LS (2021) Molecular, cellular and functional changes in the retinas of young adult mice lacking the voltage-gated K(+) channel subunits Kv8.2 and K2.1. Int J Mol Sci 22:4877
Jorge BS, Campbell CM, Miller AR, Rutter ED, Gurnett CA, Vanoye CG, George AL Jr, Kearney JA (2011) Voltage-gated potassium channel KCNV2 (Kv8.2) contributes to epilepsy susceptibility. Proc Natl Acad Sci U S A 108:5443–5448
Kalpachidou T, Malsch P, Qi Y, Mair N, Geley S, Quarta S, Kummer KK, Kress M (2022) Genetic and functional evidence for gp130/IL6ST-induced transient receptor potential ankyrin 1 upregulation in uninjured but not injured neurons in a mouse model of neuropathic pain. Pain 163:579–589
Kim HJ, Lv P, Sihn CR, Yamoah EN (2011) Cellular and molecular mechanisms of autosomal dominant form of progressive hearing loss, DFNA2. J Biol Chem 286:1517–1527
Kummer KK, Kalpachidou T, Mitric M, Langeslag M, Kress M (2018) Altered gene expression in prefrontal cortex of a fabry disease mouse model. Front Mol Neurosci 11:201
Leitner MG, Feuer A, Ebers O, Schreiber DN, Halaszovich CR, Oliver D (2012) Restoration of ion channel function in deafness-causing KCNQ4 mutants by synthetic channel openers. Br J Pharmacol 165:2244–2259
Leitner MG, Halaszovich CR, Oliver D (2011) Aminoglycosides inhibit KCNQ4 channels in cochlear outer hair cells via depletion of phosphatidylinositol(4,5)bisphosphate. Mol Pharmacol 79:51–60
Leitner MG, Hobiger K, Mavrantoni A, Feuer A, Oberwinkler J, Oliver D, Halaszovich CR (2018) A126 in the active site and TI167/168 in the TI loop are essential determinants of the substrate specificity of PTEN. Cell Mol Life Sci. https://doi.org/10.1007/s00018-018-2867-z
Lindner M, Gilhooley MJ, Palumaa T, Morton AJ, Hughes S, Hankins MW (2020) Expression and Localization of Kcne2 in the Vertebrate Retina. Invest Ophthalmol Vis Sci 61:33
Lindner M, Leitner MG, Halaszovich CR, Hammond GR, Oliver D (2011) Probing the regulation of TASK potassium channels by PI4,5P(2) with switchable phosphoinositide phosphatases. J Physiol 589:3149–3162
Maljevic S, Wuttke TV, Lerche H (2008) Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol 586:1791–1801
Moller L, Regnier G, Labro AJ, Blunck R, Snyders DJ (2020) Determining the correct stoichiometry of Kv2.1/Kv6.4 heterotetramers, functional in multiple stoichiometrical configurations. Proc Natl Acad Sci U S A 117:9365–9376
RCoreTeam (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; 2014.
Renigunta V, Fischer T, Zuzarte M, Kling S, Zou X, Siebert K, Limberg MM, Rinne S, Decher N, Schlichthorl G et al (2014) Cooperative endocytosis of the endosomal SNARE protein syntaxin-8 and the potassium channel TASK-1. Mol Biol Cell 25:1877–1891
Ribeiro DM, Ziyani C, Delaneau O (2022) Shared regulation and functional relevance of local gene co-expression revealed by single cell analysis. Commun Biol 5:876
Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S et al (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174(1015–1030):e1016
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Schwake M, Jentsch TJ, Friedrich T (2003) A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep 4:76–81
Sharan R, Ulitsky I, Shamir R (2007) Network-based prediction of protein function. Mol Syst Biol 3:88
Smith KE, Wilkie SE, Tebbs-Warner JT, Jarvis BJ, Gallasch L, Stocker M, Hunt DM (2012) Functional analysis of missense mutations in Kv8.2 causing cone dystrophy with supernormal rod electroretinogram. J Biol Chem 287:43972–43983
Stas JI, Bocksteins E, Labro AJ, Snyders DJ (2015) Modulation of Closed-State Inactivation in Kv2.1/Kv6.4 Heterotetramers as Mechanism for 4-AP Induced Potentiation. PloS one 10:e0141349
Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators et al (2018) Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562:367–372
Tu L, Santarelli V, Sheng Z, Skach W, Pain D, Deutsch C (1996) Voltage-gated K+ channels contain multiple intersubunit association sites. J Biol Chem 271:18904–18911
Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV et al (2015) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18:145–153
Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D (1998) KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282:1890–1893
Wang JJ, Li Y (2016) KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol Sin 37:25–33
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wilke BU, Lindner M, Greifenberg L, Albus A, Kronimus Y, Bunemann M, Leitner MG, Oliver D (2014) Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat Commun 5:5540
Winden KD, Bragin A, Engel J, Geschwind DH (2015) Molecular alterations in areas generating fast ripples in an animal model of temporal lobe epilepsy. Neurobiol Dis 78:35–44
Zerangue N, Jan YN, Jan LY (2000) An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1. Proc Natl Acad Sci U S A 97:3591–3595
Acknowledgements
The authors acknowledge the kind gift of plasmids (KVS channels) form Regina Preisig-Muller and Elke Bocksteins as well as Thomas Jentsch (KV7.2-HA) and thank Olga Ebers for outstanding technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. Supported by the German Research Foundation (LI 2846/5-1 to ML and RE 4617/2-1 to VR), the Austrian Science Fund FWF (ZFI-053640 to KK and MGL) and by research Grants of the University Medical Centre Giessen und Marburg to MGL (UKGM 13/2016) and ML (UKGM 15/2020).
Author information
Authors and Affiliations
Contributions
Participated in research design: VR, DO, MGL, ML. Conducted experiments: VR, NX, IGS, JS, RB, IS, KK, MGL, ML. Performed data analysis: VR, NX, IGS, JS, RB, IS, KK, MGL, ML. Wrote or contributed to the writing of the manuscript: VR, KK, MGL, ML.
Corresponding authors
Ethics declarations
Conflict of interest
MGL is employed by Boehringer Ingelheim Pharma GmbH & Co. KG (this study is not linked to this employer). ML has received Grants from Bayer Healthcare outside the submitted work. The other authors declare no competing interests.
Consent to publish
Not applicable.
Ethics approval
This study does not involve human participants. Animal work was performed with approval of the relevant authorities and in accordance with the institutional Ethics Guidelines of Animal Care. Further details are provided in the Methods section.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Renigunta, V., Xhaferri, N., Shaikh, I.G. et al. A versatile functional interaction between electrically silent KV subunits and KV7 potassium channels. Cell. Mol. Life Sci. 81, 301 (2024). https://doi.org/10.1007/s00018-024-05312-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05312-1