Abstract
Metastasis accounts for 90% of cancer-related deaths among the patients. The transformation of epithelial cells into mesenchymal cells with molecular alterations can occur during epithelial–mesenchymal transition (EMT). The EMT mechanism accelerates the cancer metastasis and drug resistance ability in human cancers. Among the different regulators of EMT, Wnt/β-catenin axis has been emerged as a versatile modulator. Wnt is in active form in physiological condition due to the function of GSK-3β that destructs β-catenin, while ligand–receptor interaction impairs GSK-3β function to increase β-catenin stability and promote its nuclear transfer. Regarding the oncogenic function of Wnt/β-catenin, its upregulation occurs in human cancers and it can accelerate EMT-mediated metastasis and drug resistance. The stimulation of Wnt by binding Wnt ligands into Frizzled receptors can enhance β-catenin accumulation in cytoplasm that stimulates EMT and related genes upon nuclear translocation. Wnt/β-catenin/EMT axis has been implicated in augmenting metastasis of both solid and hematological tumors. The Wnt/EMT-mediated cancer metastasis promotes the malignant behavior of tumor cells, causing therapy resistance. The Wnt/β-catenin/EMT axis can be modulated by upstream mediators in which non-coding RNAs are main regulators. Moreover, pharmacological intervention, mainly using phytochemicals, suppresses Wnt/EMT axis in metastasis suppression.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
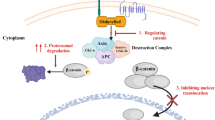
Wnt/β-catenin is an evolutionary conserved pathway essential for embryonic development and regulation of adult stem cell, homeostasis and tissue regeneration [1,2,3,4,5]. Pre-clinical and clinical evidences have confirmed the vital role of Wnt in the initiation and development of human diseases, particularly cancer [6]. Up to date, 19 glycoproteins have been identified in Wnt ligand family to function in a paracrine or autocrine manner and demonstrate various spatiotemporal expression. Upon their synthesis, Wnt proteins are transferred to lumen of endoplasmic reticulum (ER) to undergo palmitoylation by PORCN in ER. Then, Wnt ligand interacts with Wntless (WLS) to accelerate release of Wnt proteins [4]. Passive diffusion or secretion in membrane-enclosed vesicles or traveling by cytonemes are responsible for cell–cell transfer of Wnt ligands [5, 7]. Wnt has two pathways including canonical and non-canonical. The canonical pathway occurs in self-renewing and undifferentiated state triggered by addition of Wnt3a to cell culture [8,9,10,11], while in the non-canonical pathway, Wnt5 ligand is capable of promoting differentiation and migration, curtailing proliferation [12, 13]. The interaction of Wnt receptor with ligands is responsible for its stimulation accounting for transmission of cell signaling information from extracellular environment to intracellular compartments and downstream targets [14]. Wnt is tightly controlled in cells and is an important participant in physiological processes including organism axis differentiation, tissue formation, brain formation and stem cell maintenance [15, 16]. Accumulating data has shown association of Wnt dysregulation with the development of pathological events [17, 18]. On the cell surface, there are receptors including LRP5/6 and FZD that can interact with Wnt ligands to induce Dishevelled protein, a member of complex with other proteins including GSK-3β, Axin2 and APC. Then, accumulated β-catenin transfers to nucleus to stimulate TCF and LEF family members in further modulation of gene targets [19]. However, there is non-canonical and β-catenin-independent pathway including Wnt/Ca2+ signaling, mTOR signaling, Ras homolog gene family, RhoA/ROCK and JNK signaling cascades [20]. The Wnt inhibitory factor (WIF) or SFRPs can bind to Wnt ligands or interaction of DKK1 or SOST with LRP5/6 receptor can occur to suppress Wnt signaling [21].
Figure 1 represents Wnt and related molecular interactions. Wnt has been associated with the development of various human cancers. Wnt/β-catenin overexpression occurs in thyroid cancer due to KDM1A upregulation to increase cancer malignancy and promote stemness [22]. Upregulation of Wnt can lead to resistance of tumor cells to cell death. For instance, overexpression of Wnt/β-catenin promotes GPX4 expression to induce ferroptosis resistance in gastric cancer cells. Silencing GPX4 enhances sensitivity to ferroptosis [23]. LINC01606 also adopts a similar manner in the regulation of cell death in colon cancer. By promoting SCD1 expression, LINC01606 induces Wnt to prevent ferroptosis in tumor cells [24]. Interestingly, metabolism of tumor cells can be controlled by Wnt. NME7 as a protein kinase is able to induce Wnt/β-catenin to enhance one-carbon metabolism in hepatocellular carcinoma [25]. A synergy between Wnt/β-catenin and PI3K/Akt is able to enhance HIF-1α expression in glycolysis induction and stimulation of 5-flourouracil resistance in colorectal tumor [26]. The interactions of upstream mediators can determine progression of tumor cells via Wnt/β-catenin control. Circ-EIF6 is capable of encoding EIF6-224aa in enhancing tumor progression. The downstream signaling is unique and by stabilizing MYH9, circ-EIF6 participates in the induction of Wnt/β-catenin and elevation of breast cancer progression [27]. Both carcinogenesis and drug resistance can be modulated by Wnt/β-catenin in cancers. N7-methylguanosine tRNA modification stimulates Wnt/β-catenin in inducing drug resistance and increasing progression in nasopharyngeal cancer [28]. Upon deficiency of TET, Wnt is reprogrammed to impair its promoter demethylation and enhance lung cancer malignancy [29]. Based on these findings, interaction of Wnt with other molecular pathways determines cancer progression and its targeting is of importance in cancer therapy [30,31,32,33]. Table 1 summarizes the recent findings of Wnt dysregulation in human cancers. In the current review, we will focus on revealing function of Wnt in regulating tumor metastasis by affecting a well-known mechanism, epithelial–mesenchymal transition (EMT).
The presence of Wnt ligand mediates the function of Frizzled and LRP5/6 receptors to suppress GSK-3β, Dishevelled, CK1α and Axin. Then, it enhances the intracellular accumulation of β-catenin to transfer into nucleus and enhance gene transcription. However, lack of Wnt ligand mediates the phosphorylation of β-catenin to enhance its proteasomal degradation
EMT mechanism: general aspects and carcinogenic function
Complexity in metastasis process has been intriguing to researchers and the mechanism of metastasis can be observed in various human cancers to disseminate in other tissues and increase their population. Moreover, metastasis has reverse relationship with prognosis and overall survival of patients. The metastasis program can be simply defined as detachment of some tumor populations from their colony to enter into bloodstream and then, exit from circulation to a new tissue and establishing a new colony. In contrast to simple definition of metastasis, molecular interactions participating in metastasis are rather complicated. Despite the recent advances in metastasis research, there are still many unidentified aspects of cancer metastasis at molecular and cellular levels. In order to better understand metastasis, key molecular processes have come into attention, among which epithelial–mesenchymal transition (EMT) being an attracting mechanism. Generally, loss of polarity in epithelial cells and obtaining mesenchymal-like features are known as EMT that there are different categories of EMT based on occurring in embryogenesis, wound healing/tissue fibrosis and tumor cells [44, 45]. The tight junctions, desomosomes, gap junctions and adheren junctions are responsible for attaching epithelial cells to basement membrane [46]. However, during maturation in the developmental process, these cells become more motile and lose their polarity [47]. A similar process can be observed in cancer cells where they are separated from basement membrane and enter into bloodstream, resulting in increased migration and metastasis [48]. E-cadherin down-regulation, and vimentin and N-cadherin upregulation can be observed during loss of polarity in epithelial cells. The EMT-inducing transcription factors (EMT-TFs) are present and demonstrate high expression in tumor cells such as TGF-β, ZEB proteins, Slug and Twist to increase cancer metastasis [49, 50].
In the recent years, the factors regulating EMT in cancer metastasis have been of interest with new insights and ideas being offered towards the EMT modulators. During hypoxia, expression level of Nrf2 increases through down-regulation of miR-27a. Nrf2 participates in EMT induction and elevating metastasis of lung tumor cells [51]. Loss of expression of iNOS/NO is involved in elevating invasion of colorectal tumor cells via EMT induction [52]. The interesting point is that EMT is not certain to a tumor type and its regulation by various factors can affect carcinogenesis and metastasis. PODNL1 exhibits high levels of expression in bladder cancer. By reducing E-cadherin levels, PODNL1 promotes EMT in tumor cells [53]. On the other hand, there are factors that can suppress EMT in cancers. For instance, FOXA2 has been in favor of decreasing cancer metastasis and it suppresses EMT [54]. Since molecular pathways regulating EMT mechanism have been somewhat well understood [55], studies have focused on EMT targeting in cancer therapy [52]. Decrease in N-cadherin and vimentin levels, and increase in E-cadherin levels can be observed after administration of β-elemene in inhibiting EMT in colorectal tumor [56]. As mentioned, hypoxia is involved in EMT induction and facilitated cancer invasion which can be overcome by β-patchoulene [57]. Moreover, there is a close association between EMT induction and drug resistance in human cancers [58]. Noteworthy, inhibition of Wnt/β-catenin by fucoxanthin can suppress EMT in lung cancer, suggesting the role of Wnt as a regulator of EMT [59] (will be discussed in details in next sections). Table 2 summarizes the role of EMT in human cancer invasion.
Wnt/β-catenin signaling as a regulator of EMT in human cancers
Brain tumors
Glioblastoma (GBM) is a cancer of central nervous system [69] with an incidence rate of 7.2/100,000 [70]. GBM causes high mortality rate and the survival rate of patients appears to be less than 14 months [71]. Surgery, radiotherapy and chemotherapy are considered as current treatments for GBM [72] and in addition to poor efficacy of current therapies, it is quite troublesome to eliminate GBM by surgical resection [73]. The aggressiveness of GBM cells depends on EMT induction and invasion, and STAT1 upregulation is essential in this case. STAT1 is capable of evoking Wnt/β-catenin axis in EMT induction and elevating invasion and metastasis of GBM cells [74]. Since stimulation of Wnt enhances GBM metastasis, its inhibition will have negative impact on the aggression of tumor cells. This has been evaluated in a recent experiment that LINC-PINT impairs Wnt/β-catenin axis to suppress EMT in decreasing metastasis of GBM [75]. More importantly, when transcription of FZD7 occurs, it can interact with Wnt ligand to induce β-catenin translocation to nucleus. If its transcription is suppressed, there will be little receptor for ligand recognition. miR-504 reduces FZD7 expression to suppress β-catenin in suppressing EMT and stemness in GBM [76]. In order to disrupt progression of GBM cells, studies have focused on knock-down of factors responsible for EMT stimulation in cancers. For instance, when expression level of GOLM1 decreases and it is silenced, suppression of Wnt/β-catenin axis occurs that is vital for reducing metastasis of GBM cells [77]. Therefore, knock-down and upregulation of certain upstream factors regulating Wnt/EMT axis can help in highlighting the mechanisms involved in GBM aggression [78]. Interestingly, high expression of CTNND1 is vital for the induction of Wnt/β-catenin in GBM and subsequent EMT induction [79]. Therefore, studies have highlighted the function of Wnt/EMT axis in aggravating GBM progression. However, GBM is not the only brain tumor and glioma is another one that researchers have also focused on Wnt/EMT function in its progression. Upregulation of LGR5 results in unfavorable prognosis in glioma. By inducing Wnt/β-catenin axis, LGR5 mediates EMT and promotes cancer invasion and metastasis [80]. One of the important targets in the treatment of brain tumors is CBX7 that its expression level is epigenetically reduced in tumor cells. Moreover, it is able to suppress cancer metastasis via inhibiting YAP/TAZ axis [81]. Furthermore, CBX7 can be considered as a prognostic factor in cancers. By reducing CCNE1 expression, CBX7 stimulates cell cycle arrest in glioma [82]. Similar to the approach that was followed in GBM therapy, for instance, silencing TRIM47 impairs Wnt pathway that is in favor of reducing vimentin and N-cadherin levels [83]. Hence, increasing evidence demonstrates that upregulation of Wnt can increase glioma progression via EMT induction [84, 85].
Gastrointestinal tumors
The aim of current section is to evaluate role of Wnt/EMT axis in metastasis of gastrointestinal tumors. Gastric cancer is the third most common tumor around the world and the most common malignant of gastrointestinal tract in China [86,87,88]. However, metastasis results in recurrence in gastric cancer and that is why 5-year survival rate of patients is poor [89]. Samples obtained from gastric cancer patients revealed that ADMA serves as a potential factor for increased metastasis of tumor cells. By inducing Wnt/β-catenin, ADMA mediates EMT-induced metastasis [90]. The intriguing point is that factors and molecular pathways can regulate expression level of Wnt and nuclear translocation of β-catenin. However, a recent experiment has shown that Zic1 is a suppressor of β-catenin/TCF4 complex and does not affect the nuclear translocation of β-catenin. Then, it reduces expression levels of c-Myc and cyclin D1 as targets of Wnt and suppresses EMT in gastric tumor cells [91]. TIPE1 is a member of TNFAIP8 family and despite similarities in structure and sequence of TIPE family, their biological functions are distinct. TIPE3 and TNFAIP8 participate in the process of tumorigenesis [92, 93]; TIPE2 is a negative modulator of immune system and it is involved in inflammatory disease regulation [94, 95]; TIPE1 is a tumor suppressor-mediating apoptosis in cancer cells [96]. In gastric tumor, TIPE1 is an inhibitor of metastasis and for this purpose, it impairs Wnt/β-catenin to reduce levels of MMP-2 and MMP-9 in EMT inhibition and reducing malignancy [97]. The confirmation for role of Wnt in gastric cancer metastasis is that function of TFEB in enhancing invasion of gastric cancer and EMT induction is essential based on stimulation of Wnt [98]. Therefore, Wnt/EMT axis is an important pathway involved in enhanced invasion and metastasis of gastric tumor cells [99].
Colorectal cancer (CRC) is another important malignancy of gastrointestinal tract. Its morbidity and mortality ranked the 3rd and 2rd, respectively, among cancers, and is considered as a main threat to human health [100]. The incidence rate of CRC in young patients has demonstrated an increase [101] and survival rate of patients at stage I and II of CRC is 91% and 82%, for stage IV is 12% [102]. Similar to gastric tumor, one of the main problems of CRC is metastasis and therefore, studies have focused on understanding the role of Wnt/EMT axis in its progression and invasion. NCAPG may increase metastasis of CRC cells and when its expression enhances, it induces Wnt/β-catenin to mediate EMT in enhancing tumor invasion [103]. When Wnt undergoes activation in CRC, it can affect EMT-TFs including ZEB1. RHBDD1 upregulation in CRC results in phosphorylation of β-catenin to induce Wnt pathway for increasing ZEB1 expression and mediating EMT [104]. Moreover, Snail expression increases by Wnt in CRC cells and CEMIP down-regulation results in Wnt/β-catenin inhibition to reduce Snail expression in EMT inhibition [105].
Hepatocellular carcinoma (HCC) is another malignancy with an escalating incidence rate. It is one of the leading causes of death in Chinese population [106, 107]. Hepatitis B virus (HBV) is a leading cause of HCC around the world and up to 50–80% of cases are due to this infection [108]. Furthermore, by increase in number of NAFLD, incidence rate of HCC also increases [109]. High expression of Wnt in HCC can increase tumor progression, and aquaporin 9 impairs Wnt/β-catenin to suppress invasion and EMT in tumor cells [110]. A recent study revealed that increased expression of Wnt can be mediated by borealin (which induces β-catenin in EMT induction and promotes HCC invasion and metastasis) [111]. OCT4 is another factor related to stemness, but by activating LEF1/β-catenin axis, it mediates EMT in a Wnt-dependent manner [112].
Urological tumors
Prostate cancer (PCa) is the sixth most common tumor in men and causes high mortality [113]. The major reason of PCa-related death is metastasis and inefficacy of current therapeutics [114]. FOXO3a is an inhibitor of metastasis in PCa and for this purpose, it suppresses EMT. The FOXO3a increases transcription of miR-34b/c in the nucleus and then, mature miR-34b/c in cytoplasm suppresses β-catenin to reduce ZEB/EMT. Moreover, FOXO3a can directly inhibit β-catenin. Therefore, FOXO3a may directly and indirectly suppress β-catenin/ZEB/EMT in reducing PCa progression [115]. GPX2, another factor mediating recurrence in PCa, has important function to promote metastasis of tumor cells mediated via β-catenin induction and subsequent increased cancer metastasis through EMT stimulation [116]. When silencing Wnt/β-catenin occurs, EMT-mediated metastasis in PCa is suppressed [117]. Interestingly, both TGF-β1 and Wnt can induce EMT in enhancing PCa metastasis. CD82/KAI1 functions as tumor suppressor that by inhibiting Wnt and TGF-β1, impairs metastasis in PCa cells and EMT mechanism [118]. Seventy percent of BC cases are non-muscle-invasive and metastasis is an increasing challenge in this case. Upregulation of Wnt results in EMT in BC, and EFEMP2 is capable of suppressing Wnt/β-catenin in reducing metastasis via EMT inhibition [119]. Another malignant urological tumor is renal cancer, although only one study has evaluated function of Wnt in the modulation of metastasis. In this case, Wnt increases levels of ARL4C to enhance cyclin D1 and c-Myc levels, leading to EMT induction and enactment in progression of tumor cells [120].
Hematological cancers
Leukemia, lymphoma and myeloma are three important hematological tumors, but one of the drawbacks of current studies is ignorance towards understanding function of Wnt/EMT axis and only a few experiments have investigated that are included here. Since Wnt is an important regulator of tumorigenesis in these cancers, future studies should be engaged towards the role of Wnt/EMT axis in hematological tumor progression. PLAGL2 shows upregulation in lymphoma and by inducing Wnt/β-catenin axis, it mediates EMT to enhance invasion and metastasis of tumor cells [121]. Moreover, expression level of SOX12 decreases by miR-744-5p in myeloma and this results in Wnt/β-catenin inhibition in reversing EMT and decreasing invasion of tumor cells [122].
Gynecological tumors
Ovarian cancer (OC) is another malignancy of gynecological tumors and the second leading cause of death [123]. The 5-year survival of patients in last 30 years has been less than 30% and up to 70% of females do not demonstrate symptoms in early stages; hence, peritoneal cavity metastasis is one of the major complications of OC [124]. The invasion of OC cells that can be accelerated by EMT is one of the reasons of OC malignancy. Inhibition of Wnt/LRP5 axis results in EMT inhibition and subsequent suppression of migration in vitro and in vivo [125]. One of the factors involved in cancer development is the exposure to chemicals and toxic agents such as benzophenone-1 (BP-1) with the ability of inducing Wnt/β-catenin and ERα expression to mediate EMT biomarkers [126]. Moreover, high levels of IL-8 as an inflammatory factor in OC can result in EMT stimulation via inducing Wnt/β-catenin [127]. Hence, targeting Wnt/EMT is promising in retarding OC progression. Another important gynecological malignancy is cervical cancer accounting for 6.5% of current cancer cases and 7.7% of female mortalities [128]. Inhibition of both Wnt and EMT can significantly diminish progression of cervical tumor cells [129]. However, the important part is related to role of Wnt/EMT as a novel axis in regulating cervical cancer progression. APMAP is deemed one of the key factors in cervical cancer metastasis, and stimulates EMT through induction of Wnt/β-catenin signaling [130]. Hence, both Wnt and EMT play a vital role in facilitated progression of cervical cancer cells [131]. The aim of “Wnt/β-catenin signaling as a regulator of EMT in human cancers” was to discuss the role of Wnt/EMT axis in various cancer types (as summarized in Table 3, Fig. 2).
Wnt/β-catenin signaling and cancer drug resistance via regulation of EMT
Drug resistance is an evolving field and imposes a major challenge for physicians around the world. Moreover, hallmarks of cancer can display association with chemoresistance. Since EMT increases metastasis as an important hallmark of tumors, the association of this pathway with drug resistance development has been evaluated in various studies. Moreover, overexpression of ZEB1 results in EMT-mediated drug resistance in ovarian cancer [145]. Therefore, if some factors suppress EMT, sensitivity of tumor cells to chemotherapy enhances. Upregulation of Par-4 decreases MDM-2 expression to suppress EMT-mediated drug resistance [146]. Moreover, miR-128-3p-mediated suppression of c-Met/EMT axis leads to increased temozolomide sensitivity in glioblastoma [147]. Therefore, EMT induction leads to chemoresistance, and this section focuses on the EMT regulation by Wnt in tumor cells and determines their sensitivity to chemotherapy. However, it should be noted that in all studies, the role of Wnt/EMT axis in the regulation of drug resistance has not been evaluated. For instance, it has been reported that endothelin A receptor/β-arrestin integrates with Wnt pathway to induce EMT and drug resistance in ovarian tumor [148]. Although previous study evaluated the role of Wnt and its correlation with other signaling pathways in EMT induction and chemoresistance, it is not certain that EMT leads to malignant progression and chemoresistance or not. However, since accumulating data has shown potential of EMT in increasing cancer aggressiveness, it can be indirectly concluded that EMT induction causes drug resistance. A similar approach has been followed in which METTL1 is able to induce Wnt/β-catenin in mediating chemoresistance and EMT nasopharyngeal cancer, but association of EMT and chemoresistance has not been evaluated [28]. However, there are studies showing that dysregulation of Wnt/EMT axis can lead to chemoresistance in human cancers. In pancreatic cancer, EMT and extracellular matrix (ECM) cooperate in the development of resistance to chemotherapy. Using an oncolytic adenovirus for expressing both Wnt decoy and decorin leads to ECM degradation and EMT suppression in suppressing gemcitabine resistance in tumor cells [149]. This experiment clearly demonstrates that Wnt inhibition can suppress EMT-induced chemoresistance. In fact, when onco-suppressor factors display low expression, chance of EMT-mediated chemoresistance increases. An interesting experiment has evaluated the function of SFRP5 in drug resistance in ovarian tumor; based on this experiment, epigenetic silencing of SFRP5 results in hyperactivation of Wnt to mediate EMT via TWIST upregulation and increases AKT2 levels in favor of drug resistance in ovarian tumor [150]. The interesting point is that even pathways related to EMT and Wnt can exhibit certain associations with the development of chemoresistance. Down-regulation of FILIP1L, an inhibitor of WNT, in ovarian tumor leads to SLUG upregulation and subsequent drug resistance development [151]. The interesting point is that when activation of Wnt occurs, it leads to 5-flourouracil resistance in colorectal tumor. The cadherin–catenin complex leads to simulation of β-catenin. However, Sanguisorba officinalis L. impairs nuclear translocation of β-catenin to diminish N-cadherin, vimentin and Snail levels, and enhance E-cadherin levels in increasing 5-flourouracil sensitivity in colorectal tumor [152]. Therefore, Wnt/EMT axis can modulate response of tumor cells to chemotherapy (Fig. 3).
Pharmacological targeting of Wnt/β-catenin/EMT axis
Pharmacological inhibition of Wnt/EMT axis has offered new insight for cancer treatment to counter invasion and metastasis. Therefore, if molecular pathways related to Wnt/EMT are affected by these compounds, it can suppress invasion, as a factor mediating death in patients. O2-(2,4-dinitrophenyl) diazeniumdiolate is a new emerged compound in the treatment of HCC that its anti-tumor activity regarding Wnt regulation has been evaluated. O2-(2,4-dinitrophenyl) diazeniumdiolate suppresses Wnt/β-catenin axis to suppress EMT via reducing vimentin, Slug, Snail levels and increasing E-cadherin levels [153]. In order to increase potential in the modulation of Wnt/EMT axis, the combination of pharmacological compounds have been utilized in suppressing metastasis. Phytocannabinoid along with PARP1 inhibitor are capable of suppressing Wnt/β-catenin to minimize metastasis and mesenchymal phenotype of OC cells and EMT [154]. Cannabidiol (CBD) is well known in traditional Chinese medicine and there has been focus on its anti-tumor activity [155, 156]. One of the interesting points regarding CBD is its ability in the regulation of Wnt pathway. CBD promotes expression level of Axin1, suppresses Wnt/β-catenin pathway and reduces expression level of β-catenin target genes including APC and CK1 in EMT suppression in colorectal tumor [157].
In “Wnt/β-catenin signaling as a regulator of EMT in human cancers”, it was previously mentioned that increase in expression level of Wnt and induction of β-catenin can lead to EMT induction in HCC cells. Furthermore, related molecular pathways regulating Wnt/EMT axis in HCC were evaluated. Paris saponin H suppresses Wnt pathway in vitro and in vivo in HCC that changes expression level of EMT-related proteins such as vimentin and E-cadherin in favor of cancer invasion reduction [158]. However, one of the notes that should be considered during evaluation of experiments is that some of the studies have only focused on the regulation of EMT and Wnt separately in tumor cells and have not mentioned their association. For instance, wogonoside and Oldhamianoside II can suppress both Wnt/β-catenin and EMT in human cancers [159, 160], although future studies should focus on their association and modulation by these anti-tumor compounds.
As mentioned, one of the main reasons of drug resistance is the induction of Wnt/EMT. In PCa, high expression of Wnt can result in malignant behavior and enzalutamide resistance. A combination of enzalutamide and 3,3ʹ-diindolylmethane can inhibit Wnt/β-catenin axis in EMT inhibition and suppressing EMT via enhancing E-cadherin levels and decreasing vimentin levels [161]. In fact, progression of PCa cells mainly depends on EMT and their enhanced metastasis relies on changes in phenotype and levels of mesenchymal and epithelial markers. 2'‑Hydroxyflavanone is capable of downregulating level of Wnt/β-catenin to suppress EMT in reducing progression of PCa cells [162]. Thymoquinone (TQ) is isolated from Nigella sativa and its pharmacological activities encompass antioxidant, anti-inflammation, immunomodulatory and anti-cancer [163,164,165,166]. Recently, TQ has been applied in the treatment of bladder cancer and by inhibiting Wnt/β-catenin/EMT axis, the metastasis of tumor cells significantly reduces [167]. Although bladder cancer is a multifactorial disease, tobacco smoke (TS), parasitic infection and radiation or chemical exposure have been considered as possible factors involved in its development. Accumulating evidence has shown an association between TS and development of bladder cancer [168]. Curcumin has been shown to suppress urocystic EMT and prevents acquisition of stemness in tumor cells. Curcumin suppresses Wnt/β-catenin axis to impair EMT, thus eliminating the development of TS-mediated bladder cancer [169]. Therefore, modulation and inhibition of Wnt/EMT axis by pharmacological compounds can greatly help in suppressing cancer invasion and metastasis [170,171,172] (Fig. 4).
The role of Wnt/EMT in cancer drug resistance and its suppression by anti-cancer drugs. The application of oncolytic adenovirus allows to suppress the Wnt/EMT axis for reversing gemcitabine resistance. When Wnt expression increases, it stimulates Twist/EMT axis in mediating drug resistance. Moreover, suppression of Wnt by thymoquinone and other compounds can prevent cancer metastasis and chemoresistance
Nanoparticle-mediated regulation of Wnt/EMT axis in cancer metastasis: new visions
The introduction of nanostructures into the treatment of cancer revolutionized the ability to eradicate tumor cells. The pharmacological compounds suffer from poor pharmacokinetic profile and in spite of high potential in tumor suppression in vitro, their anti-cancer function reduces in vivo. Then, translation of such findings into clinic is a difficulty. Therefore, the targeted delivery of therapeutic for improving their accumulation at the tumor site has been suggested. Currently, the several conventional therapies have been introduced for cancer including chemotherapy and radiotherapy. Moreover, immunotherapy has been emerged for tumor suppression. However, the cancer cells stimulate the alternative molecular pathways to stimulate resistance into these therapies. The increasing evidences have shown that nanostructures can improve the ability of conventional therapies in cancer suppression, they reverse drug resistance and augment immunotherapy [173,174,175,176]. Since Wnt has been associated with EMT induction, the application of nanoparticles for the regulation of Wnt/EMT axis has been provided. The sweroside nanostructures have been introduced for the treatment of prostate cancer through increasing ROS generation and apoptosis. Moreover, these nanoparticles impair growth and invasion of tumor cells. They disrupt the stem cell features including CD33 and CD44. Moreover, sweroside nanoparticles impair TTCF/LEF activity to suppress β-catenin resulting down-regulation of c-Myc, cyclin D1, survivin and MMP-7. The down-regulation of Wnt/β-catenin by sweroside nanoparticles can also suppress the EMT-related markers in prostate cancer [177].
Noncoding RNAs in regulation of Wnt/β-catenin
microRNAs
microRNAs (miRNAs) are short and endogenous noncoding RNAs (ncRNAs) with length of 22–24 nucleotides. miRNAs are capable of binding to complementary components (3’-UTR) of mRNAs for degradation or translation suppression. This is known as post-transcriptional regulation of gene expression [178]. The dysregulation of miRNAs has been frequently observed in cancer and it can cause progression or suppression, based on function of miRNAs [179]. Since GSK-3β suppresses β-catenin, studies have focused on if there is any relationship between miRNAs and GSK-3β in EMT modulation in tumors. miR-1246 decreases GSK-3β levels by binding to its 3’-UTR in inducing β-catenin/EMT, resulting in enhanced cancer metastasis [180]. In KRAS-mutated colorectal tumor cells, expression level of miR-139-5p reduces and when poor expression of miR-139-5p occurs in cancer cells, their phenotype changes to an aggressive form. Interestingly, β-catenin may reduce miR-139-5p expression to induce EMT, resulting in elevated colorectal cancer invasion [181].
miR-27a is one of the factors that its exact function in cancer is not certain and miR-27a-3p sponging by circ-BCAR3 results in esophageal tumor progression [182], while down-regulation of miR-27a and miR-27b by circ-0000994 results in pancreatic tumor suppression [183], confirming dual function of miR-27a in cancers. miR-135 shows a contrast function compared to miR-27a and by decreasing SMAD3 expression, miR-135 suppresses TGF-β-mediated EMT in breast tumor [184]. Moreover, glycolysis and metabolic reprogramming in pancreatic tumor can be suppressed by miR-135 [185]. The EMT-TFs can be regulated by miRNAs in affecting progression of tumor cells. miR-519d inhibits Wnt/β-catenin and it reduces Twist1 expression to suppress EMT in gastric tumor [186]. However, the limitation of previous experiment is that it has not evaluated the correlation between Wnt and EMT regulated by miR-519d that can be focus of future studies.
In gastric tumor, high expression level of Wnt results in angiogenesis and metastasis, and silencing MED27 leads to inactivation of Wnt in retarding cancer progression [187]. FOXC1 upregulation results in induction of β-catenin in enhancing gastric tumor invasion [188]. Hence, metastasis of gastric tumor cells can be regulated by Wnt and a recent experiment has evaluated function of miR-330-3p in modulating invasion of gastric tumor. miR-330-3p is capable of impairing PRRX1-induced Wnt/β-catenin axis in reducing EMT and invasion of gastric tumor [189]. One of the interesting points is regulation of miRNAs by others factors in cancer cells. SOX17 and PAX8 physically cooperate in increasing ovarian tumor progression that it can be suppressed by small molecule inhibitors [190]. Moreover, reduced SOX17 expression by miR-200a-3p can result in increase in proliferation and invasion of tumor cells [191].
One important feature of miRNAs is their enrichment in exosomes, as small extracellular vesicles with 40–120 nm in diameter and secreted from many cells into body fluids [192,193,194,195]. When exosomes were discovered, it was believed that these structures contribute to removal of intracellular wastes [196], while recent studies have shown that exosomes are potential regulators of carcinogenesis [197]. Low expression level of miR-7-5p in exosomes derived from breast tumor cells can result in enhanced metastasis. However, if exosomes derived from breast tumor cells have high levels of miR-7-5p, it can lead to the regulation of atypical WNT in which reducing RYK expression to favor JNK phosphorylation, resulting in c-Jun protein enhancement and subsequent EMT inhibition in decreasing cancer metastasis [198]. The catch point is that exosomal miRNAs can regulate TME components such as cancer-associated fibroblasts (CAFs). miR-146a demonstrates enrichment in exosomes derived from breast tumor cells. Through reduction in TXNIP levels, miR-146a induces Wnt/β-catenin axis in the promotion of metastasis and EMT-related protein expression such as vimentin and N-cadherin [199].
Long noncoding RNAs
Long non-coding RNAs (lncRNAs) are transcribed by RNA polymerase II transcripts with length more than 200 nucleotides [200]. In addition to implication in the modulation of biological processes, their function in cancer is also of importance [201, 202]. LncRNAs can exert sponging effect in reducing miRNA expression and modulating biological events. Furthermore, various molecular pathways are affected by lncRNAs present in cytoplasm and nucleus, and function of lncRNAs is different based on the location that those in nucleus provide chromatin remodeling and cytoplasmic lncRNAs mediate miRNA interaction. Wnt regulation by lncRNAs can affect metastasis of tumors. miR-106a-3p suppresses APC expression in gastric tumor, while LINC01133 sponges miR-106a-3p to increase APC expression. Then, APC suppresses β-catenin in reducing invasion and EMT [203]. LINC01089 suppresses EMT and invasion in lung tumor and for this purpose, it follows a complicated pathway in which LINC01089 sponges miR-27a to increase SFRP1. Then, suppression of Wnt/β-catenin axis is observed to inhibit EMT in reducing cancer invasion and metastasis [204]. In contrast, lncRNAs can promote invasion of tumor cells through induction of EMT mechanism. For instance, lncRNA NORAD decreases miR-30a-5p expression as a way to promote RAB11A levels in the induction of Wnt/β-catenin, resulting in EMT and enhanced cancer invasion [205]. Besides, lncRNA HOXD-AS1 sponges miR-133a-3p to induce Wnt/β-catenin for EMT induction and promoting metastasis of ovarian tumor [206]. Therefore, lncRNAs are important regulators of Wnt/EMT axis in human cancers.
Circular RNAs
Circular RNAs (circRNAs) are other factors that are considered as special ncRNAs and they are ubiquitous in human cells [207]. CircRNAs are new endogenous ncRNAs that were adjusted to be pivotal regulators of various biological mechanisms [208] and their unique roles in tumors have been interesting. Increasing evidence reveals that circRNAs may modulate EMT in cancers [209]. More importantly, circRNAs can decrease miRNA expression via sponging [210]. The overall aim of current section is to evaluate role of circRNAs in EMT regulation via targeting Wnt. Circ-0007059 shows function in suppression of lung tumor growth and metastasis, and its ability in decreasing metastasis is due to EMT suppression. The hsa-circ-0007059 decreases miR-378 expression to inhibit Wnt/β-catenin and ERK1/2 in reducing vimentin, Twist, and ZEB1 levels to suppress EMT [211]. On the other hand, high expression level of circ-000984 results in enhanced invasion of lung tumor that is related to increasing β-catenin levels in mediating EMT [212]. SP1 is a transcription factor that modulates intracellular gene expression, and its dysregulation has resulted in increased carcinogenesis, especially colorectal cancer [213]. Since colorectal tumor is one of the leading causes of death, ample efforts have been engaged towards understanding circRNAs in the regulation of Wnt and EMT in this malignant tumor. Hsa-circ-0001666 can diminish malignancy of colorectal tumor and mechanistically, circ-0001666 decreases miR-576-5p expression to upregulate PCDH10, to induce Wnt pathway and to increase EMT [214]. Circ-0067934 is one of the new emerging factors in cancer that its upregulation prevents ferroptosis in thyroid tumor [215] and by decreasing JNK phosphorylation, it can result in cisplatin resistance [216]. More than one molecular pathway can be simultaneously regulated by circ-0067934 and notably, this circRNA induces Wnt/β-catenin pathway and promotes KLF8 expression via miR-1182 down-regulation to induce EMT and metastasis in lung tumor [217]. Although each study provides a new insight and pathway in which Wnt/EMT axis is regulated by circRNAs, many of them share a similar function and that is miRNA sponging by circRNAs. Circ-0082182 promotes progression and metastasis in colorectal tumor that is mediated by miR-411 and miR-1205 suppression via sponging to stimulate Wnt/β-catenin pathway in EMT induction [218]. Table 4 and Fig. 5 summarize the role of ncRNAs in Wnt/EMT axis regulation in human cancers.
Conclusion and remarks
The regulation of molecular pathways in cancer represents one of the important aspects of research for biologists to track down the interplay among these signaling cascades in the regulation of carcinogenesis. A given form of cancer can display distinct features and characteristics such as growth, invasion and chemoresistance. Each hallmark of cancer may intertwin with one another. For instance, increase in proliferation and invasion of tumor cells promotes their malignancy, resulting in the development of therapeutic resistance. However, among the different hallmarks, increase in metastasis is an important aspect that causes high death among patients and can mediate therapy failure. The process of metastasis is complicated and one important mechanism facilitating tumor invasion is EMT mechanism. Wnt/β-catenin is the most well-known one among various regulators of EMT. Due to tumor-promoting property of Wnt, it can induce EMT in increasing tumor progression. High expression level of Wnt occurs during progression of tumor cells and this is in favor of cancer invasion. Wnt/β-catenin can mediate EMT in various cancers including brain, hematological, urological, gynecological and gastrointestinal tumors. Therefore, an optimal strategy is to directly suppress Wnt pathway or inhibit EMT as another key player in the progression of tumor. Since both Wnt and EMT mechanisms result in the drug resistance in human cancers, studies demonstrated that EMT induction by Wnt can also lead to chemoresistance. Therefore, inhibition of Wnt/EMT axis would be the important next step to overcome drug resistance in cancers. The modulation of Wnt/EMT axis by non-coding RNAs is a hot topic recent year and since Wnt has bindings site for non-coding RNAs, Wnt/EMT axis can be modulated by these RNA transcripts. Furthermore, pharmacological compounds and nanostructures are capable of Wnt/EMT suppression in reducing invasion and metastasis of tumor cells.
Availability of data and materials
Not applicable.
References
Aros CJ et al (2020) Distinct spatiotemporally dynamic Wnt-secreting niches regulate proximal airway regeneration and aging. Cell Stem Cell 27(3):413-429. e4
Baarsma H, Königshoff MJT (2017) ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax 72(8):746–759
Hashimoto S et al (2012) β-Catenin–SOX2 signaling regulates the fate of developing airway epithelium. J Cell Sci 125(4):932–942
Jung Y-S, Park J-IJE, Medicine M (2020) Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med 52(2):183–191
Steinhart Z, Angers SJD (2018) Wnt signaling in development and tissue homeostasis. Development 145(11):dev146589
Chatterjee A et al (2022) Advances in targeting the WNT/β-catenin signaling pathway in cancer. Drug Discovery Today 27(1):82–101
Moti N et al (2019) Wnt traffic from endoplasmic reticulum to filopodia. PLoS One 14(2):e0212711
Maeda K et al (2019) The regulation of bone metabolism and disorders by wnt signaling. Int J Mol Sci 20(22):5525
Gokturk F, Erkoc-Kaya D, Arikoglu H (2021) Juglone can inhibit angiogenesis and metastasis in pancreatic cancer cells by targeting Wnt/beta-catenin signaling. Bratisl Lek Listy 122:132–137
Sterling JA et al (2011) Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone 48(1):6–15
Ben-Ghedalia-Peled N, Vago R (2022) Wnt signaling in the development of bone metastasis. Cells 11(23):3934
Khoon MC (2015) Experimental models of bone metastasis: opportunities for the study of cancer dormancy. Adv Drug Deliv Rev 94:141–150
Micalizzi DS et al (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15(2):117–134
Cui J et al (2022) Targeting the Wnt signaling pathway for breast cancer bone metastasis therapy. J Mol Med 100(3):373–384
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Giles RH, Van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653(1):1–24
Prestwich TC, MacDougald OA (2007) Wnt/β-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19(6):612–617
Ross SE et al (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950–953
Breuer E-K et al (2019) Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis 10(3):1–15
Satriyo PB et al (2019) Cadherin 11 inhibition downregulates β-catenin, deactivates the canonical WNT signalling pathway and suppresses the cancer stem cell-like phenotype of triple negative breast cancer. J Clin Med 8(2):148
Xi Y, Chen Y (2014) Wnt signaling pathway: implications for therapy in lung cancer and bone metastasis. Cancer Lett 353(1):8–16
Zhang W et al (2022) KDM1A promotes thyroid cancer progression and maintains stemness through the Wnt/β-catenin signaling pathway. Theranostics 12(4):1500–1517
Wang Y et al (2022) Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ 29(11):2190–2202
Luo Y et al (2022) Long noncoding RNA LINC01606 protects colon cancer cells from ferroptotic cell death and promotes stemness by SCD1-Wnt/β-catenin-TFE3 feedback loop signalling. Clin Transl Med 12(4):e752
Ren X et al (2022) The protein kinase activity of NME7 activates Wnt/β-catenin signaling to promote one-carbon metabolism in hepatocellular carcinoma. Cancer Res 82(1):60–74
Dong S et al (2022) ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res 41(1):15
Li Y et al (2022) circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol Ther 30(1):415–430
Chen B et al (2022) N(7)-methylguanosine tRNA modification promotes tumorigenesis and chemoresistance through WNT/β-catenin pathway in nasopharyngeal carcinoma. Oncogene 41(15):2239–2253
Xu Q et al (2022) Loss of TET reprograms Wnt signaling through impaired demethylation to promote lung cancer development. Proc Natl Acad Sci U S A 119(6):e2107599119
Yan R et al (2022) Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep 38(5):110319
Geleta B et al (2022) Targeting Wnt/tenascin C-mediated cross talk between pancreatic cancer cells and stellate cells via activation of the metastasis suppressor NDRG1. J Biol Chem 298(3):101608
Yamamoto D et al (2022) Characterization of RNF43 frameshift mutations that drive Wnt ligand- and R-spondin-dependent colon cancer. J Pathol 257(1):39–52
Yan R et al (2022) Inhibition of DCLK1 sensitizes resistant lung adenocarcinomas to EGFR-TKI through suppression of Wnt/β-catenin activity and cancer stemness. Cancer Lett 531:83–97
Lv F et al (2023) MAGP1 maintains tumorigenicity and angiogenesis of laryngeal cancer by activating Wnt/β-catenin/MMP7 pathway. Carcinogenesis 16:bgad003
Wellenstein MD et al (2019) Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 572(7770):538–542
Takeuchi Y et al (2021) Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol 6(65):eabc6424
Mohapatra P et al (2021) CMTM6 drives cisplatin resistance by regulating Wnt signaling through the ENO-1/AKT/GSK3β axis. JCI Insight 6(4):e143643
Xiong X et al (2022) Activation of Drp1 promotes fatty acids-induced metabolic reprograming to potentiate Wnt signaling in colon cancer. Cell Death Differ 29(10):1913–1927
Luan F et al (2020) TNFRSF11B activates Wnt/β-catenin signaling and promotes gastric cancer progression. Int J Biol Sci 16(11):1956–1971
Zhan T et al (2019) MEK inhibitors activate Wnt signalling and induce stem cell plasticity in colorectal cancer. Nat Commun 10(1):2197
Chen Z et al (2019) RCC2 promotes breast cancer progression through regulation of Wnt signaling and inducing EMT. J Cancer 10(27):6837–6847
Liao H et al (2020) A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov 6:35
Chen Z et al (2019) RSPO3 promotes the aggressiveness of bladder cancer via Wnt/β-catenin and Hedgehog signaling pathways. Carcinogenesis 40(2):360–369
Shao Z et al (2022) The role of long noncoding RNAs as regulators of the epithelial-mesenchymal transition process in oral squamous cell carcinoma cells. Front Mol Biosci 9:942636
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119(6):1420–1428
Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14(6):818–829
Lim J, Thiery JP (2012) Epithelial-mesenchymal transitions: insights from development. Development 139(19):3471–3486
Horejs CM (2016) Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur J Cell Biol 95(11):427–440
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20(2):69–84
Liu C et al (2022) Hypoxia promotes epithelial-mesenchymal transition in lung cancer cells via regulating the NRF2/miR-27a/BUB1 pathway. Clin Transl Oncol 25:510–522
Du Q et al (2022) Downregulation of iNOS/NO promotes epithelial-mesenchymal transition and metastasis in colorectal cancer. Mol Cancer Res 21:102–114
Liu X et al (2022) PODNL1 promotes cell migration and regulates the epithelial/mesenchymal transition process in bladder cancer. Biochem Biophys Res Commun 620:165–172
Sahoo SS et al (2022) FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity. J Clin Invest 132(12):e157574
Yun H et al (2022) NANOG regulates epithelial-mesenchymal transition via AMPK/mTOR signalling pathway in ovarian cancer SKOV-3 and A2780 cells. J Cell Mol Med 26(20):5277–5291
Deng H, Chen G, Zhang J (2022) β-elemene regulates epithelial-mesenchymal transformation and inhibits invasion and metastasis of colorectal cancer cells. J Complement Integr Med 8:e78700
Tu H et al (2022) β-Patchoulene represses hypoxia-induced proliferation and epithelial-mesenchymal transition of liver cancer cells. Bioengineered 13(5):11907–11922
Guo Z et al (2023) Molecular profile of metastasis, cell plasticity and EMT in pancreatic cancer: a pre-clinical connection to aggressiveness and drug resistance. Cancer Metastasis Rev. https://doi.org/10.1007/s10555-023-10125-y
Luan H et al (2022) Fucoxanthin induces apoptosis and reverses epithelial-mesenchymal transition via inhibiting Wnt/β-catenin pathway in lung adenocarcinoma. Discov Oncol 13(1):98
Ghosh D et al (2023) Ets1 facilitates EMT/invasion through Drp1-mediated mitochondrial fragmentation in ovarian cancer. iScience 26(9):107537
Cassier PA et al (2023) Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 620(7973):409–416
Meng X et al (2023) CircPTK2/PABPC1/SETDB1 axis promotes EMT-mediated tumor metastasis and gemcitabine resistance in bladder cancer. Cancer Lett 554:216023
Debaugnies M et al (2023) RHOJ controls EMT-associated resistance to chemotherapy. Nature 616(7955):168–175
Gao H et al (2023) Phase separation of DDX21 promotes colorectal cancer metastasis via MCM5-dependent EMT pathway. Oncogene 42(21):1704–1715
Ji Q et al (2023) PYGL-mediated glucose metabolism reprogramming promotes EMT phenotype and metastasis of pancreatic cancer. Int J Biol Sci 19(6):1894–1909
Zheng Y et al (2023) Silencing TRAIP suppresses cell proliferation and migration/invasion of triple negative breast cancer via RB-E2F signaling and EMT. Cancer Gene Ther 30(1):74–84
Shao M et al (2023) STMN2 overexpression promotes cell proliferation and EMT in pancreatic cancer mediated by WNT/β-catenin signaling. Cancer Gene Ther 30(3):472–480
Long L et al (2023) Regulating lactate-related immunometabolism and EMT reversal for colorectal cancer liver metastases using shikonin targeted delivery. J Exp Clin Cancer Res 42(1):117
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109(1):93–108
Dolecek TA et al (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(suppl_5):v1–v49
Van Meir EG et al (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60(3):166–193
Carlsson SK, Brothers SP, Wahlestedt C (2014) Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med 6(11):1359–1370
Auffinger B et al (2015) The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother 15(7):741–752
Zhao L et al (2020) STAT1 determines aggressiveness of glioblastoma both in vivo and in vitro through wnt/β-catenin signalling pathway. Cell Biochem Funct 38(5):630–641
Zhu H et al (2020) Long noncoding RNA LINC-PINT suppresses cell proliferation, invasion, and EMT by blocking Wnt/β-catenin signaling in glioblastoma. Front Pharmacol 11:586653
Zhuang XH, Liu Y, Li JL (2019) Overexpression of long noncoding RNA HOXB-AS3 indicates an unfavorable prognosis and promotes tumorigenesis in epithelial ovarian cancer via Wnt/β-catenin signaling pathway. Biosci Rep 39(8):BSR20190906
Ding X et al (2019) GOLM1 silencing inhibits the proliferation and motility of human glioblastoma cells via the Wnt/β-catenin signaling pathway. Brain Res 1717:117–126
Wu S et al (2019) Silencing expression of PHF14 in glioblastoma promotes apoptosis, mitigates proliferation and invasiveness via Wnt signal pathway. Cancer Cell Int 19:314
Han L et al (2019) SNHG29 regulates miR-223-3p/CTNND1 axis to promote glioblastoma progression via Wnt/β-catenin signaling pathway. Cancer Cell Int 19:345
Zhang J et al (2018) LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res 37(1):225
Nawaz Z et al (2016) Cbx7 is epigenetically silenced in glioblastoma and inhibits cell migration by targeting YAP/TAZ-dependent transcription. Sci Rep 6:27753
Yu T et al (2017) CBX7 is a glioma prognostic marker and induces G1/S arrest via the silencing of CCNE1. Oncotarget 8(16):26637–26647
Chen L et al (2020) Knockdown of TRIM47 inhibits glioma cell proliferation, migration and invasion through the inactivation of Wnt/β-catenin pathway. Mol Cell Probes 53:101623
Huang G et al (2022) Methyl-CpG binding protein 2 (MeCP2) as a potential diagnostic and prognostic marker facilitates glioma progression through activation of the Wnt/β-Catenin pathway. World Neurosurg 171:e560–e571
Ni H et al (2021) The nuclear transporter importin-11 regulates the Wnt/β-catenin pathway and acts as a tumor promoter in glioma. Int J Biol Macromol 176:145–156
Fitzmaurice C et al (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 5(12):1749–1768
Chen W et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132
Ferlay J et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Biagioni A et al (2019) Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev 38(3):537–548
Guo Q et al (2021) ADMA mediates gastric cancer cell migration and invasion via Wnt/β-catenin signaling pathway. Clin Transl Oncol 23(2):325–334
Ge Q et al (2020) Zic1 suppresses gastric cancer metastasis by regulating Wnt/β-catenin signaling and epithelial-mesenchymal transition. Faseb j 34(2):2161–2172
Fayngerts SA et al (2014) TIPE3 is the transfer protein of lipid second messengers that promote cancer. Cancer Cell 26(4):465–478
Kumar D et al (2004) Expression of SCC-S2, an antiapoptotic molecule, correlates with enhanced proliferation and tumorigenicity of MDA-MB 435 cells. Oncogene 23(2):612–616
Sun H et al (2008) TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133(3):415–426
Zhang Y et al (2012) TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem 287(39):32546–32555
Zhang Z et al (2015) TIPE1 induces apoptosis by negatively regulating Rac1 activation in hepatocellular carcinoma cells. Oncogene 34(20):2566–2574
Liu W et al (2018) TIPE1 suppresses invasion and migration through down-regulating Wnt/β-catenin pathway in gastric cancer. J Cell Mol Med 22(2):1103–1117
Li S et al (2020) Wnt/β-catenin signaling axis is required for TFEB-mediated gastric cancer metastasis and epithelial-mesenchymal transition. Mol Cancer Res 18(11):1650–1659
Peng Y et al (2021) MiRNA-20b/SUFU/Wnt axis accelerates gastric cancer cell proliferation, migration and EMT. Heliyon 7(4):e06695
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Mauri G et al (2019) Early-onset colorectal cancer in young individuals. Mol Oncol 13(2):109–131
Miller KD et al (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69(5):363–385
Shi Y et al (2022) NCAPG facilitates colorectal cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition by activating the Wnt/β-catenin signaling pathway. Cancer Cell Int 22(1):119
Zhang M et al (2018) RHBDD1 promotes colorectal cancer metastasis through the Wnt signaling pathway and its downstream target ZEB1. J Exp Clin Cancer Res 37(1):22
Liang G et al (2018) Silencing of CEMIP suppresses Wnt/β-catenin/Snail signaling transduction and inhibits EMT program of colorectal cancer cells. Acta Histochem 120(1):56–63
Xing S et al (2019) The prognostic value of major facilitator superfamily domain-containing protein 2A in patients with hepatocellular carcinoma. Aging (Albany NY) 11(19):8474–8483
Zheng R et al (2017) Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 36(1):66
Bhaumik P (2015) Epidemiology of viral hepatitis and liver diseases in India. Euroasian J Hepatogastroenterol 5(1):34–36
Younossi ZM et al (2015) Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62(6):1723–1730
Liao S et al (2020) Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging (Albany NY) 12(2):1527–1544
Chen B et al (2022) Borealin promotes tumor growth and metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. J Hepatocell Carcinoma 9:171–188
Sun L et al (2017) Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol Lett 13(4):2599–2606
Center MM et al (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61(6):1079–1092
Taichman RS et al (2007) The evolving biology and treatment of prostate cancer. J Clin Investig 117(9):2351–2361
Liu H et al (2015) FOXO3a modulates WNT/β-catenin signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cell Signal 27(3):510–518
Yang M et al (2022) GPX2 predicts recurrence-free survival and triggers the Wnt/β-catenin/EMT pathway in prostate cancer. PeerJ 10:e14263
Sha J et al (2018) PRKAR2B promotes prostate cancer metastasis by activating Wnt/β-catenin and inducing epithelial-mesenchymal transition. J Cell Biochem 119(9):7319–7327
Lee MS et al (2019) The metastasis suppressor CD82/KAI1 represses the TGF-β (1) and Wnt signalings inducing epithelial-to-mesenchymal transition linked to invasiveness of prostate cancer cells. Prostate 79(12):1400–1411
Zhou Q et al (2019) EFEMP2 suppresses epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway in human bladder cancer. Int J Biol Sci 15(10):2139–2155
Zhang P et al (2022) ARL4C Regulates the Progression of Clear Cell Renal Cell Carcinoma by Affecting the Wnt/β-Catenin Signaling Pathway. J Oncol 2022:2724515
Jin W, Wang X (2022) PLAGL2 promotes the proliferation and migration of diffuse large B-cell lymphoma cells via Wnt/β-catenin pathway. Ann Clin Lab Sci 52(3):359–366
Guo B et al (2021) miR-744-5p inhibits multiple myeloma proliferation, epithelial mesenchymal transformation and glycolysis by targeting SOX12/Wnt/β-catenin signaling. Onco Targets Ther 14:1161–1172
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Vaughan S et al (2011) Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11(10):719–725
Hong J et al (2021) Inactivation of Wnt-LRP5 signaling suppresses the proliferation and migration of ovarian cancer cells. Transl Cancer Res 10(5):2277–2285
Liu X et al (2022) Benzophenone-1 induced aberrant proliferation and metastasis of ovarian cancer cells via activated ERα and Wnt/β-catenin signaling pathways. Environ Pollut 292(Pt B):118370
Wen J et al (2020) IL-8 promotes cell migration through regulating EMT by activating the Wnt/β-catenin pathway in ovarian cancer. J Cell Mol Med 24(2):1588–1598
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Zhang J et al (2022) DYNLT3 overexpression induces apoptosis and inhibits cell growth and migration via inhibition of the Wnt pathway and EMT in cervical cancer. Front Oncol 12:889238
Zhu X et al (2021) APMAP promotes epithelial-mesenchymal transition and metastasis of cervical cancer cells by activating the Wnt/β-catenin pathway. J Cancer 12(20):6265–6273
Lan C et al (2021) FAM83A promotes the proliferative and invasive abilities of cervical cancer cells via epithelial-mesenchymal transition and the Wnt signaling pathway. J Cancer 12(21):6320–6329
Duan H et al (2017) TET1 inhibits EMT of ovarian cancer cells through activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2. Gynecol Oncol 147(2):408–417
Liu Y et al (2021) Cysteine-rich intestinal protein 1 served as an epithelial ovarian cancer marker via promoting Wnt/β-catenin-mediated EMT and tumour metastasis. Dis Markers 2021:3566749
Zhang LM et al (2021) CCAAT enhancer binding protein α suppresses proliferation, metastasis, and epithelial-mesenchymal transition of ovarian cancer cells via suppressing the Wnt/β-catenin signaling. Neoplasma 68(3):602–612
Wang T et al (2021) FTO-stabilized lncRNA HOXC13-AS epigenetically upregulated FZD6 and activated Wnt/β-catenin signaling to drive cervical cancer proliferation, invasion, and EMT. J buon 26(4):1279–1291
Zhang LZ et al (2018) CRIP1 promotes cell migration, invasion and epithelial-mesenchymal transition of cervical cancer by activating the Wnt/β-catenin signaling pathway. Life Sci 207:420–427
Gao X et al (2020) Aberrantly enhanced melanoma-associated antigen (MAGE)-A3 expression facilitates cervical cancer cell proliferation and metastasis via actuating Wnt signaling pathway. Biomed Pharmacother 122:109710
Chung MT et al (2009) SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol Oncol 112(3):646–653
Meng F et al (2020) SMYD2 suppresses APC2 expression to activate the Wnt/β-catenin pathway and promotes epithelial-mesenchymal transition in colorectal cancer. Am J Cancer Res 10(3):997–1011
Xiao B et al (2020) Oncolytic adenovirus CD55-Smad4 suppresses cell proliferation, metastasis, and tumor stemness in colorectal cancer by regulating Wnt/β-catenin signaling pathway. Biomedicines 8(12):593
Tian W et al (2021) HYD-PEP06 suppresses hepatocellular carcinoma metastasis, epithelial-mesenchymal transition and cancer stem cell-like properties by inhibiting PI3K/AKT and WNT/β-catenin signaling activation. Acta Pharm Sin B 11(6):1592–1606
Chen Y et al (2019) TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res 378(1):41–50
Yang Y et al (2017) Downregulated connexin32 promotes EMT through the Wnt/β-catenin pathway by targeting Snail expression in hepatocellular carcinoma. Int J Oncol 50(6):1977–1988
Jiang J et al (2015) Over-expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Biochem Biophys Res Commun 464(4):1120–1127
Mitra T et al (2018) Stemness and chemoresistance are imparted to the OC cells through TGFβ1 driven EMT. J Cell Biochem 119(7):5775–5787
Ahmad SM et al (2020) Par-4 activation restrains EMT-induced chemoresistance in PDAC by attenuating MDM-2. Pancreatology 20(8):1698–1710
Zhao C et al (2020) MicroRNA-128-3p enhances the chemosensitivity of temozolomide in glioblastoma by targeting c-Met and EMT. Sci Rep 10(1):9471
Rosanò L et al (2014) Endothelin A receptor/β-arrestin signaling to the Wnt pathway renders ovarian cancer cells resistant to chemotherapy. Cancer Res 74(24):7453–7464
Li Y et al (2019) Oncolytic Ad co-expressing decorin and Wnt decoy receptor overcomes chemoresistance of desmoplastic tumor through degradation of ECM and inhibition of EMT. Cancer Lett 459:15–29
Su HY et al (2010) Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer 127(3):555–567
Kwon M et al (2016) Reduced expression of FILIP1L, a novel WNT pathway inhibitor, is associated with poor survival, progression and chemoresistance in ovarian cancer. Oncotarget 7(47):77052–77070
Zhang W et al (2022) Sanguisorba officinalis L. suppresses 5-fluorouracil-sensitive and-resistant colorectal cancer growth and metastasis via inhibition of the Wnt/β-catenin pathway. Phytomedicine 94:153844
Xing Y et al (2022) A novel O(2)- (2,4-dinitrophenyl) diazeniumdiolate inhibits hepatocellular carcinoma migration, invasion, and EMT through the Wnt/β-catenin pathway. Toxicol In Vitro 84:105456
Shalev N et al (2022) Phytocannabinoid compositions from cannabis act synergistically with PARP1 inhibitor against ovarian cancer cells in vitro and affect the Wnt signaling pathway. Molecules 27(21):7523
Pisanti S et al (2017) Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther 175:133–150
Jo MJ et al (2021) Cannabidiol suppresses angiogenesis and stemness of breast cancer cells by downregulation of hypoxia-inducible factors-1α. Cancers 13(22):5667
Feng P et al (2022) Cannabidiol inhibits invasion and metastasis in colorectal cancer cells by reversing epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway. J Cancer Res Clin Oncol 149:3587–3598
Chen T et al (2019) Paris saponin H suppresses human hepatocellular carcinoma (HCC) by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Int J Clin Exp Pathol 12(8):2875–2886
Wei C et al (2019) Wogonoside inhibits prostate cancer cell growth and metastasis via regulating Wnt/β-catenin pathway and epithelial-mesenchymal transition. Pharmacology 104(5–6):312–319
Li K et al (2018) Oldhamianoside II inhibits prostate cancer progression via regulation of EMT and the Wnt/β-catenin signaling pathway. Oncol Lett 15(6):9457–9463
Tsao CW et al (2021) Regulation of carcinogenesis and mediation through Wnt/β-catenin signaling by 3,3’-diindolylmethane in an enzalutamide-resistant prostate cancer cell line. Sci Rep 11(1):1239
Wu S et al (2018) 2’-Hydroxyflavanone inhibits epithelial-mesenchymal transition, and cell migration and invasion via suppression of the Wnt/β-catenin signaling pathway in prostate cancer. Oncol Rep 40(5):2836–2843
Effenberger-Neidnicht K, Schobert R (2011) Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol 67(4):867–874
Kruk I et al (2000) The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 41(7):1059–1064
Ahmad A et al (2013) A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed 3(5):337–352
Salem ML (2005) Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 5(13–14):1749–1770
Zhang M et al (2020) Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem Biol Interact 320:109022
Liang Z et al (2015) Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin. Int J Clin Exp Pathol 8(5):4503
Liang Z et al (2017) Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Dis 8(10):e3066
Cilibrasi C et al (2017) Resveratrol Impairs Glioma Stem Cells Proliferation and Motility by Modulating the Wnt Signaling Pathway. PLoS One 12(1):e0169854
Fang Q et al (2022) β-ionone inhibits epithelial-mesenchymal transition (EMT) in prostate cancer cells by negatively regulating the Wnt/β-catenin pathway. Front Biosci (Landmark Ed) 27(12):335
Zhao M et al (2020) Gigantol attenuates the metastasis of human bladder cancer cells, possibly through Wnt/EMT signaling. Onco Targets Ther 13:11337–11346
Fang RH, Gao W, Zhang L (2023) Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol 20(1):33–48
Zeng Y et al (2022) Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm Sin B 12(8):3233–3254
Gavas S, Quazi S, Karpiński TM (2021) Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett 16(1):173
Liang X et al (2023) cRGD-targeted heparin nanoparticles for effective dual drug treatment of cisplatin-resistant ovarian cancer. J Control Release 356:691–701
Huang S et al (2021) Anticancer activity of sweroside nanoparticles in prostate cancer bone metastasis in PC-3 cells involved in Wnt/β-catenin signaling pathway. J Biomed Nanotechnol 17(10):1960–1971
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16(3):203–222
Anastasiadou E, Jacob LS, Slack FJ (2018) Non-coding RNA networks in cancer. Nat Rev Cancer 18(1):5–18
Yang F et al (2019) MiR-1246 promotes metastasis and invasion of A549 cells by targeting GSK-3β-mediated Wnt/β-catenin pathway. Cancer Res Treat 51(4):1420–1429
Du F et al (2020) KRAS mutation-responsive miR-139-5p inhibits colorectal cancer progression and is repressed by Wnt signaling. Theranostics 10(16):7335–7350
Xi Y et al (2022) CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR-27a-3p. Mol Cancer 21(1):145
Liu J, Yuan W, Gong D (2022) Hsa_circ_0000994 inhibits pancreatic cancer progression by clearing immune-related miR-27a and miR-27b. J Oncol 2022:7274794
Yang W et al (2020) MiR-135-5p inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by targeting SMAD3 in breast cancer. J Cancer 11(21):6402–6412
Yang Y et al (2019) MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun 10(1):809
Yue H et al (2017) MIR-519d suppresses the gastric cancer epithelial-mesenchymal transition via Twist1 and inhibits Wnt/β-catenin signaling pathway. Am J Transl Res 9(8):3654–3664
Han X et al (2022) Knockdown of mediator complex subunit 27 suppresses gastric cancer cell metastasis and angiogenesis via Wnt/β-catenin pathway. Tissue Cell 79:101973
Sun Y et al (2022) Overexpression of FOXC1 promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in gastric cancer. Dig Dis Sci 67(8):3742–3752
Ma B et al (2020) Effects of miR-330-3p on invasion, migration and emt of gastric cancer cells by targeting PRRX1-mediated Wnt/β-catenin signaling pathway. Onco Targets Ther 13:3411–3423
Lin L et al (2022) SOX17 and PAX8 constitute an actionable lineage-survival transcriptional complex in ovarian cancer. Oncogene 41(12):1767–1779
Wu X et al (2022) miR-200a-3p promoted cell proliferation and metastasis by downregulating SOX17 in non-small cell lung cancer cells. J Biochem Mol Toxicol 36(6):e23037
Gallo A et al (2012) The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 7(3):e30679
Zomer A et al (2010) Exosomes: Fit to deliver small RNA. Commun Integr Biol 3(5):447–450
Paskeh MDA et al (2022) Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol 15(1):83
Mirzaei S et al (2023) Exosome-mediated miR-200a delivery into TGF-β-treated AGS cells abolished epithelial-mesenchymal transition with normalization of ZEB1, vimentin and Snail1 expression. Environ Res 231:116115
Record M et al (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841(1):108–120
Tyagi A et al (2022) Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene 41(22):3079–3092
Liang Z et al (2022) Downregulation of exosomal miR-7-5p promotes breast cancer migration and invasion by targeting RYK and participating in the atypical WNT signalling pathway. Cell Mol Biol Lett 27(1):88
Yang SS et al (2020) Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res 391(2):111983
Chen M et al (2018) Decreased expression of lncRNA VPS9D1-AS1 in gastric cancer and its clinical significance. Cancer Biomark 21(1):23–28
Bracken CP, Scott HS, Goodall GJ (2016) A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 17(12):719–732
Xue X et al (2016) LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 35(21):2746–2755
Yang XZ et al (2018) LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer 17(1):126
Li X et al (2020) LINC01089 inhibits tumorigenesis and epithelial-mesenchymal transition of non-small cell lung cancer via the miR-27a/SFRP1/Wnt/β-catenin axis. Front Oncol 10:532581
Zhang Y, Li Y (2020) Long non-coding RNA NORAD contributes to the proliferation, invasion and EMT progression of prostate cancer via the miR-30a-5p/RAB11A/WNT/β-catenin pathway. Cancer Cell Int 20(1):571
Zhang Y et al (2017) LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/β-catenin signaling pathway. Biomed Pharmacother 96:1216–1221
Meng S et al (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16(1):1–8
Ebbesen KK, Hansen TB, Kjems J (2017) Insights into circular RNA biology. RNA Biol 14(8):1035–1045
Yang M et al (2022) EIF4A3-regulated circ_0087429 can reverse EMT and inhibit the progression of cervical cancer via miR-5003-3p-dependent upregulation of OGN expression. J Exp Clin Cancer Res 41(1):165
Luo YY et al (2022) Hsa_Circ_0098181 suppresses hepatocellular carcinoma by sponging miR-18a-3p and targeting PPARA. Front Pharmacol 13:819735
Gao S et al (2019) Circular RNA hsa_circ_0007059 restrains proliferation and epithelial-mesenchymal transition in lung cancer cells via inhibiting microRNA-378. Life Sci 233:116692
Li XY et al (2019) Enhanced expression of circular RNA hsa_circ_000984 promotes cells proliferation and metastasis in non-small cell lung cancer by modulating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci 23(8):3366–3374
Liu W et al (2022) SP1-mediated up-regulation of lncRNA TUG1 underlines an oncogenic property in colorectal cancer. Cell Death Dis 13(5):433
Zhou J et al (2021) Hsa_circ_0001666 suppresses the progression of colorectal cancer through the miR-576-5p/PCDH10 axis. Clin Transl Med 11(11):e565
Wang HH, Ma JN, Zhan XR (2021) Circular RNA Circ_0067934 attenuates ferroptosis of thyroid cancer cells by miR-545-3p/SLC7A11 signaling. Front Endocrinol (Lausanne) 12:670031
Yin Y et al (2022) Circ_0067934 reduces JNK phosphorylation through a microRNA-545-3p/PPA1 axis to enhance tumorigenesis and cisplatin resistance in ovarian cancer. Immunopharmacol Immunotoxicol 44(2):261–274
Zhao M, Ma W, Ma C (2020) Circ_0067934 promotes non-small cell lung cancer development by regulating miR-1182/KLF8 axis and activating Wnt/β-catenin pathway. Biomed Pharmacother 129:110461
Liu R et al (2021) Circ_0082182 promotes oncogenesis and metastasis of colorectal cancer in vitro and in vivo by sponging miR-411 and miR-1205 to activate the Wnt/β-catenin pathway. World J Surg Oncol 19(1):51
Chen X et al (2022) MicroRNA-621 functions as a metastasis suppressor in colorectal cancer by directly targeting LEF1 and suppressing Wnt/β-catenin signaling. Life Sci 308:120941
Liu Y, Zhou WL (2022) LINC01315 accelerates the growth and epithelial-mesenchymal transition of colorectal cancer cells via activating the Wnt/β-catenin signal. Bioengineered 13(4):8396–8406
Tan A, Li Q, Chen L (2019) CircZFR promotes hepatocellular carcinoma progression through regulating miR-3619-5p/CTNNB1 axis and activating Wnt/β-catenin pathway. Arch Biochem Biophys 661:196–202
Tang H et al (2019) MicroRNA-194 inhibits cell invasion and migration in hepatocellular carcinoma through PRC1-mediated inhibition of Wnt/β-catenin signaling pathway. Dig Liver Dis 51(9):1314–1322
Huang H et al (2020) LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways. Transl Androl Urol 9(5):2251–2261
Guo J et al (2019) The lncRNA DLX6-AS1 promoted cell proliferation, invasion, migration and epithelial-to-mesenchymal transition in bladder cancer via modulating Wnt/β-catenin signaling pathway. Cancer Cell Int 19:312
Zhu X et al (2018) LSINCT5 activates Wnt/β-catenin signaling by interacting with NCYM to promote bladder cancer progression. Biochem Biophys Res Commun 502(3):299–306
Jia GQ et al (2018) Long non-coding RNA PlncRNA-1 promotes cell proliferation and hepatic metastasis in colorectal cancer. J Cell Biochem 119(8):7091–7104
Li N et al (2020) Long non-coding RNA ADAMTS9-AS1 suppresses colorectal cancer by inhibiting the Wnt/β-catenin signalling pathway and is a potential diagnostic biomarker. J Cell Mol Med 24(19):11318–11329
Park SA et al (2020) Long non-coding RNA steroid receptor activator promotes the progression of endometrial cancer via Wnt/ β-catenin signaling pathway. Int J Biol Sci 16(1):99–115
Xu Y et al (2019) Long non-coding RNA LINC01225 promotes proliferation, invasion and migration of gastric cancer via Wnt/β-catenin signalling pathway. J Cell Mol Med 23(11):7581–7591
Shao Z et al (2020) Circ_0003789 facilitates gastric cancer progression by inducing the epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm 38:102–110
Pan J et al (2020) lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer 19(1):9
Chi Y et al (2019) miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J Cell Mol Med 23(9):6295–6307
Cui Y et al (2019) miR-15a-3p suppresses prostate cancer cell proliferation and invasion by targeting SLC39A7 via downregulating Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm 34(7):472–479
Yuan G et al (2019) LncRNA-MIR17HG mediated upregulation of miR-17 and miR-18a promotes colon cancer progression via activating Wnt/β-catenin signaling. Transl Cancer Res 8(4):1097–1108
Huang X et al (2018) Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem 293(18):6693–6706
Acknowledgements
The authors would like to appreciate the professional service of Biorender.com.
Funding
Young and Middle-aged Health Science and Technology Innovation Talents in 2020 (Excellent Young): YXKC2020055, Medical Science and Technology Project of Henan Province: SBGJ202002077, Natural Science Foundation of Henan Province of China (202300410460), General Program of China Postdoctoral Science Foundation.
Author information
Authors and Affiliations
Contributions
WX, LY, CC and MA: wrote the first draft of paper. MA, YT and RS: developed the concept, outline, revised the paper critically and collected the papers.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, W., Yang, L., Chen, C. et al. Wnt/β-catenin-driven EMT regulation in human cancers. Cell. Mol. Life Sci. 81, 79 (2024). https://doi.org/10.1007/s00018-023-05099-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05099-7