Abstract
Amphiregulin (AREG) is an epidermal growth factor (EGF)-like growth factor that binds exclusively to the EGF receptor (EGFR). Treatment with luteinizing hormone (LH) and/or human chorionic gonadotropin dramatically induces the expression of AREG in the granulosa cells of the preovulatory follicle. In addition, AREG is the most abundant EGFR ligand in human follicular fluid. Therefore, AREG is considered a predominant propagator that mediates LH surge-regulated ovarian functions in an autocrine and/or paracrine manner. In addition to the well-characterized stimulatory effect of LH on AREG expression, recent studies discovered that several local factors and epigenetic modifications participate in the regulation of ovarian AREG expression. Moreover, aberrant expression of AREG has recently been reported to contribute to the pathogenesis of several ovarian diseases, such as ovarian hyperstimulation syndrome, polycystic ovary syndrome, and epithelial ovarian cancer. Furthermore, increasing evidence has elucidated new applications of AREG in assisted reproductive technology. Collectively, these studies highlight the importance of AREG in female reproductive health and disease. Understanding the normal and pathological roles of AREG and elucidating the molecular and cellular mechanisms of AREG regulation of ovarian functions will inform innovative approaches for fertility regulation and the prevention and treatment of ovarian diseases. Therefore, this review summarizes the functional roles of AREG in ovarian function and disease.
Graphical abstract







Similar content being viewed by others
Availability of data and material
This is a narrative review based on published data.
References
Chang HM, Qiao J, Leung PC (2016) Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update 23:1–18
Conti M, Hsieh M, Park JY, Su YQ (2006) Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723
Richani D, Gilchrist RB (2018) The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update 24:1–14
Shoyab M, McDonald VL, Bradley JG, Todaro GJ (1988) Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA 85:6528–6532
Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M (1990) The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol 10:1969–1981
Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ (1989) Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 243:1074–1076
Johnson GR, Kannan B, Shoyab M, Stromberg K (1993) Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem 268:2924–2931
Berasain C, Avila MA (2014) Amphiregulin. Semin Cell Dev Biol 28:31–41
Sebio A et al (2014) Intergenic polymorphisms in the amphiregulin gene region as biomarkers in metastatic colorectal cancer patients treated with anti-EGFR plus irinotecan. Pharmacogenom J 14:256–262
Sanderson MP, Dempsey PJ, Dunbar AJ (2006) Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24:121–136
Levano KS, Kenny PA (2012) Clarification of the C-terminal proteolytic processing site of human amphiregulin. FEBS Lett 586:3500–3502
Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE, Lee DC (2004) Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem 279:24179–24188
Sahin U et al (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164:769–779
Sunnarborg SW et al (2002) Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem 277:12838–12845
Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ (1998) Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem 273:17258–17268
Gephart JD, Singh B, Higginbotham JN, Franklin JL, Gonzalez A, Folsch H, Coffey RJ (2011) Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 12:1793–1804
Fukuda S, Nishida-Fukuda H, Nakayama H, Inoue H, Higashiyama S (2012) Monoubiquitination of pro-amphiregulin regulates its endocytosis and ectodomain shedding. Biochem Biophys Res Commun 420:315–320
Inui S, Higashiyama S, Hashimoto K, Higashiyama M, Yoshikawa K, Taniguchi N (1997) Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J Cell Physiol 171:291–298
Willmarth NE, Ethier SP (2006) Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem 281:37728–37737
Higginbotham JN et al (2011) Amphiregulin exosomes increase cancer cell invasion. Curr Biol 21:779–786
Kowalczyk A, Wrzecinska M, Czerniawska-Piatkowska E, Kupczynski R (2022) Exosomes—spectacular role in reproduction. Biomed Pharmacother 148:112752
Di Pietro C (2016) Exosome-mediated communication in the ovarian follicle. J Assist Reprod Genet 33:303–311
Peterson EA, Shabbeer S, Kenny PA (2012) Normal range of serum Amphiregulin in healthy adult human females. Clin Biochem 45:460–463
Singh SS, Chauhan SB, Kumar A, Kumar S, Engwerda CR, Sundar S, Kumar R (2022) Amphiregulin in cellular physiology, health, and disease: potential use as a biomarker and therapeutic target. J Cell Physiol 237:1143–1156
Modrell B, McDonald VL, Shoyab M (1992) The interaction of amphiregulin with nuclei and putative nuclear localization sequence binding proteins. Growth Factors 7:305–314
Isokane M, Hieda M, Hirakawa S, Shudou M, Nakashiro K, Hashimoto K, Hamakawa H, Higashiyama S (2008) Plasma-membrane-anchored growth factor pro-amphiregulin binds A-type lamin and regulates global transcription. J Cell Sci 121:3608–3618
Taira N, Yamaguchi T, Kimura J, Lu ZG, Fukuda S, Higashiyama S, Ono M, Yoshida K (2014) Induction of amphiregulin by p53 promotes apoptosis via control of microRNA biogenesis in response to DNA damage. Proc Natl Acad Sci USA 111:717–722
Johnson GR, Saeki T, Auersperg N, Gordon AW, Shoyab M, Salomon DS, Stromberg K (1991) Response to and expression of amphiregulin by ovarian carcinoma and normal ovarian surface epithelial cells: nuclear localization of endogenous amphiregulin. Biochem Biophys Res Commun 180:481–488
Lysiak JJ, Johnson GR, Lala PK (1995) Localization of amphiregulin in the human placenta and decidua throughout gestation: role in trophoblast growth. Placenta 16:359–366
Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9:52
Singh B, Carpenter G, Coffey RJ (2016) EGF receptor ligands: recent advances. F1000Res 5:2270
Macdonald-Obermann JL, Pike LJ (2014) Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J Biol Chem 289:26178–26188
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137
Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A (2011) The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta 1816:119–131
Threadgill DW et al (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230–234
Sibilia M, Wagner EF (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269:234–238
Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A (2005) Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84
Prochazka R, Srsen V, Nagyova E, Miyano T, Flechon JE (2000) Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: nuclear maturation, expansion, and F-actin remodeling. Mol Reprod Dev 56:63–73
Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M (1998) In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod 13:1638–1644
Das K, Phipps WR, Hensleigh HC, Tagatz GE (1992) Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril 57:895–901
Dekel N, Sherizly I (1985) Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology 116:406–409
Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T (2009) Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril 91:1035–1041
Reeka N, Berg FD, Brucker C (1998) Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod 13:2199–2205
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684
Hsieh M, Zamah AM, Conti M (2009) Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 27:52–61
Arroyo A, Kim B, Yeh J (2020) Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod Sci 27:1223–1252
Richani D, Ritter LJ, Thompson JG, Gilchrist RB (2013) Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod 19:500–509
Chen J et al (2013) Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol 15:1415–1423
Hsieh M et al (2007) Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924
Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126:2739–2750
Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K (2004) Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol 33:281–291
Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M (2007) Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology 148:6164–6175
Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ (2016) Protein kinase A: a master kinase of granulosa cell differentiation. Sci Rep 6:28132
Freimann S, Ben-Ami I, Dantes A, Armon L, Ben Ya’cov-Klein A, Ron-El R, Amsterdam A (2005) Differential expression of genes coding for EGF-like factors and ADAMTS1 following gonadotropin stimulation in normal and transformed human granulosa cells. Biochem Biophys Res Commun 333:935–943
Duffy DM (2015) Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update 21:652–670
Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS (2006) Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365
Ben-Ami I et al (2006) PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod 12:593–599
Shrestha K, Lukasik K, Baufeld A, Vanselow J, Moallem U, Meidan R (2015) Regulation of ovulatory genes in bovine granulosa cells: lessons from siRNA silencing of PTGS2. Reproduction 149:21–29
Wayne CM, Fan HY, Cheng X, Richards JS (2007) Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957
Liu Z, Fan HY, Wang Y, Richards JS (2010) Targeted disruption of Mapk14 (p38MAPKalpha) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue COC expansion and maintain fertility. Mol Endocrinol 24:1794–1804
Dunning KR, Watson LN, Zhang VJ, Brown HM, Kaczmarek AK, Robker RL, Russell DL (2015) Activation of mouse cumulus-oocyte complex maturation in vitro through EGF-like activity of versican. Biol Reprod 92:116
Heldring N et al (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931
Olde B, Leeb-Lundberg LM (2009) GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab 20:409–416
Zhang H et al (2020) Mechanisms of estradiol-induced EGF-like factor expression and oocyte maturation via G protein-coupled estrogen receptor. Endocrinology 161:bqaa190
Ma Y et al (2021) Lysophosphatidic acid improves oocyte quality during IVM by activating the ERK1/2 pathway in cumulus cells and oocytes. Mol Hum Reprod 27:gaab032
Ruohonen ST, Poutanen M, Tena-Sempere M (2020) Role of kisspeptins in the control of the hypothalamic-pituitary-ovarian axis: old dogmas and new challenges. Fertil Steril 114:465–474
Fabova Z, Loncova B, Mlyn Ek M, Sirotkin AV (2022) Interrelationships between amphiregulin, kisspeptin, FSH and FSH receptor in promotion of human ovarian cell functions. Reprod Fertil Dev 34:362–377
Puttabyatappa M, Brogan RS, Vandevoort CA, Chaffin CL (2013) EGF-like ligands mediate progesterone’s anti-apoptotic action on macaque granulosa cells. Biol Reprod 88:18
Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS (2009) MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941
Yu C, Zhang YL, Fan HY (2013) Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol Endocrinol 27:966–978
Kim K, Lee H, Threadgill DW, Lee D (2011) Epiregulin-dependent amphiregulin expression and ERBB2 signaling are involved in luteinizing hormone-induced paracrine signaling pathways in mouse ovary. Biochem Biophys Res Commun 405:319–324
Nautiyal J et al (2010) The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary. Endocrinology 151:2923–2932
Li F, Liu J, Jo M, Curry TE Jr (2011) A role for nuclear factor interleukin-3 (NFIL3), a critical transcriptional repressor, in down-regulation of periovulatory gene expression. Mol Endocrinol 25:445–459
Bertolin K, Gossen J, Schoonjans K, Murphy BD (2014) The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology 155:1931–1943
Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K (2008) Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876
Bertolin K, Meinsohn MC, Suzuki J, Gossen J, Schoonjans K, Duggavathi R, Murphy BD (2017) Ovary-specific depletion of the nuclear receptor Nr5a2 compromises expansion of the cumulus oophorus but not fertilization by intracytoplasmic sperm injection. Biol Reprod 96:1231–1243
Godin P, Tsoi MF, Morin M, Gevry N, Boerboom D (2022) The granulosa cell response to luteinizing hormone is partly mediated by YAP1-dependent induction of amphiregulin. Cell Commun Signal 20:72
Jeppesen JV et al (2012) LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab 97:E1524–E1531
Wang H et al (2019) HDAC3 maintains oocyte meiosis arrest by repressing amphiregulin expression before the LH surge. Nat Commun 10:5719
Ji SY et al (2017) The polycystic ovary syndrome-associated gene Yap1 is regulated by gonadotropins and sex steroid hormones in hyperandrogenism-induced oligo-ovulation in mouse. Mol Hum Reprod 23:698–707
Stocco DM, Clark BJ (1996) Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244
Jamnongjit M, Gill A, Hammes SR (2005) Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102:16257–16262
Noma N et al (2011) LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol 25:104–116
Kitasaka H, Kawai T, Hoque SAM, Umehara T, Fujita Y, Shimada M (2018) Inductions of granulosa cell luteinization and cumulus expansion are dependent on the fibronectin-integrin pathway during ovulation process in mice. PLoS ONE 13:e0192458
Prochazka R, Petlach M, Nagyova E, Nemcova L (2011) Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction 141:425–435
Ben-Ami I, Armon L, Freimann S, Strassburger D, Ron-El R, Amsterdam A (2009) EGF-like growth factors as LH mediators in the human corpus luteum. Hum Reprod 24:176–184
Negishi H, Ikeda C, Nagai Y, Satoh A, Kumasako Y, Makinoda S, Ustunomiya T (2007) Regulation of amphiregulin, EGFR-like factor expression by hCG in cultured human granulosa cells. Acta Obstet Gynecol Scand 86:706–710
Fang L, Yu Y, Zhang R, He J, Sun YP (2016) Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci Rep 6:24917
Fang L et al (2019) Human chorionic gonadotropin-induced amphiregulin stimulates aromatase expression in human granulosa-lutein cells: a mechanism for estradiol production in the luteal phase. Hum Reprod 34:2018–2026
Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A (2002) Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 187:153–159
Jones KT (2004) Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol Hum Reprod 10:1–5
Conti M, Hsieh M, Zamah AM, Oh JS (2012) Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 356:65–73
Prochazka R, Blaha M, Nemcova L (2012) Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig. Reproduction 144:535–546
Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M (2009) Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604
Norris RP, Freudzon M, Nikolaev VO, Jaffe LA (2010) Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 140:655–662
Liu X, Xie F, Zamah AM, Cao B, Conti M (2014) Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod 91:9
Tsuji T, Kiyosu C, Akiyama K, Kunieda T (2012) CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol Reprod Dev 79:795–802
Hsieh M, Thao K, Conti M (2011) Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE 6:e21574
Richards JS, Ascoli M (2018) Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab 29:313–325
Fulop C, Salustri A, Hascall VC (1997) Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys 337:261–266
Richards JS (2005) Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79
Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS (2003) Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 278:42330–42339
Reizel Y, Elbaz J, Dekel N (2010) Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol 24:402–411
Fang L, Cheng JC, Chang HM, Sun YP, Leung PC (2013) EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab 98:4932–4941
Tamura M, Sasano H, Suzuki T, Fukaya T, Funayama Y, Takayama K, Takaya R, Yajima A (1995) Expression of epidermal growth factors and epidermal growth factor receptor in normal cycling human ovaries. Hum Reprod 10:1891–1896
Stocco C, Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149
Devoto L, Henriquez S, Kohen P, Strauss JF 3rd (2017) The significance of estradiol metabolites in human corpus luteum physiology. Steroids 123:50–54
Practice Committee of the American Society for Reproductive Medicine. Electronic address, A.a.o., Practice Committee of the American Society for Reproductive, M (2016) Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 106:1634–1647
McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV Jr, Connolly DT, Robertson DM (1994) Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 344:235–236
Nishi Y et al (2001) Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142:437–445
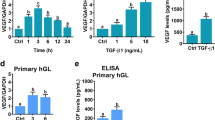
Karakida S, Kawano Y, Utsunomiya Y, Furukawa Y, Sasaki T, Narahara H (2011) Effect of heparin-binding EGF-like growth factor and amphiregulin on the MAP kinase-induced production of vascular endothelial growth factor by human granulosa cells. Growth Factors 29:271–277
Fang L, Yu Y, Li Y, Wang S, He J, Zhang R, Sun YP (2019) Upregulation of AREG, EGFR, and HER2 contributes to increased VEGF expression in granulosa cells of patients with OHSSdagger. Biol Reprod 101:426–432
Cabrita MA, Christofori G (2008) Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 11:53–62
Haimov-Kochman R et al (2005) Expression and regulation of Sprouty-2 in the granulosa-lutein cells of the corpus luteum. Mol Hum Reprod 11:537–542
Cheng JC, Fang L, Chang HM, Sun YP, Leung PC (2016) hCG-induced Sprouty2 mediates amphiregulin-stimulated COX-2/PGE2 up-regulation in human granulosa cells: a potential mechanism for the OHSS. Sci Rep 6:31675
Wang B, Li J, Yang Q, Zhang F, Hao M, Guo Y (2017) Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction 153:285–292
Wang B, Wang J, Liu Y, Wang L, Du M, Zhang Z, Guan Y (2021) sRAGE downregulates the VEGF expression in OHSS ovarian granulosa cells. Gynecol Endocrinol 37:836–840
McCartney CR, Marshall JC (2016) CLINICAL PRACTICE. polycystic ovary syndrome. N Engl J Med 375:54–64
Haouzi D, Assou S, Monzo C, Vincens C, Dechaud H, Hamamah S (2012) Altered gene expression profile in cumulus cells of mature MII oocytes from patients with polycystic ovary syndrome. Hum Reprod 27:3523–3530
Ouandaogo ZG, Frydman N, Hesters L, Assou S, Haouzi D, Dechaud H, Frydman R, Hamamah S (2012) Differences in transcriptomic profiles of human cumulus cells isolated from oocytes at GV, MI and MII stages after in vivo and in vitro oocyte maturation. Hum Reprod 27:2438–2447
Patil K, Shinde G, Hinduja I, Mukherjee S (2022) Compromised cumulus-oocyte complex matrix organization and expansion in women with PCOS. Reprod Sci 29:836–848
Ambekar AS et al (2015) Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab 100:744–753
Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M (2014) Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod 20:49–58
Doherty JA, Peres LC, Wang C, Way GP, Greene CS, Schildkraut JM (2017) Challenges and opportunities in studying the epidemiology of ovarian cancer subtypes. Curr Epidemiol Rep 4:211–220
Chen VW, Ruiz B, Killeen JL, Cote TR, Wu XC, Correa CN (2003) Pathology and classification of ovarian tumors. Cancer 97:2631–2642
Romero I, Bast RC Jr (2012) Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology 153:1593–1602
Gurung A, Hung T, Morin J, Gilks CB (2013) Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology 62:59–70
Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, Butzow R (2006) Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med (Berl) 84:671–681
Yagi H et al (2005) Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer 92:1737–1745
Carvalho S et al (2016) An antibody to amphiregulin, an abundant growth factor in patients’ fluids, inhibits ovarian tumors. Oncogene 35:438–447
Yotsumoto F et al (2008) Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun 365:555–561
Tanaka Y et al (2005) Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res 11:4783–4792
Tung SL et al (2017) miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis 6:e326
Bolitho C, Moscova M, Baxter RC, Marsh DJ (2021) Amphiregulin increases migration and proliferation of epithelial ovarian cancer cells by inducing its own expression via PI3-kinase signaling. Mol Cell Endocrinol 533:111338
So WK, Fan Q, Lau MT, Qiu X, Cheng JC, Leung PC (2014) Amphiregulin induces human ovarian cancer cell invasion by down-regulating E-cadherin expression. FEBS Lett 588:3998–4007
Cheng JC, Chang HM, Xiong S, So WK, Leung PC (2016) Sprouty2 inhibits amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Oncotarget 7:81645–81660
So WK, Cheng JC, Liu Y, Xu C, Zhao J, Chang VT, Leung PC (2016) Sprouty4 mediates amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Tumour Biol 37:9197–9207
Qiu X, Cheng JC, Klausen C, Fan Q, Chang HM, So WK, Leung PC (2015) Transforming growth factor-alpha induces human ovarian cancer cell invasion by down-regulating E-cadherin in a Snail-independent manner. Biochem Biophys Res Commun 461:128–135
Cai H, Xu Y (2013) The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal 11:31
Panupinthu N et al (2014) Self-reinforcing loop of amphiregulin and Y-box binding protein-1 contributes to poor outcomes in ovarian cancer. Oncogene 33:2846–2856
Lindzen M et al (2021) Targeting autocrine amphiregulin robustly and reproducibly inhibits ovarian cancer in a syngeneic model: roles for wildtype p53. Oncogene 40:3665–3679
Casamassimi A, De Luca A, Agrawal S, Stromberg K, Salomon DS, Normanno N (2000) EGF-related antisense oligonucleotides inhibit the proliferation of human ovarian carcinoma cells. Ann Oncol 11:319–325
Fauser BC (2019) Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online 38:133–137
Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M (2010) Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod 25:2569–2578
Liu N, Ma Y, Li R, Jin H, Li M, Huang X, Feng HL, Qiao J (2012) Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine 42:708–716
Huang Y, Zhao Y, Yu Y, Li R, Lin S, Zhang C, Liu P, Qiao J (2015) Altered amphiregulin expression induced by diverse luteinizing hormone receptor reactivity in granulosa cells affects IVF outcomes. Reprod Biomed Online 30:593–601
Alyasin A, Mehdinejadiani S, Ghasemi M (2016) GnRH agonist trigger versus hCG trigger in GnRH antagonist in IVF/ICSI cycles: a review article. Int J Reprod Biomed 14:557–566
Humaidan P, Westergaard LG, Mikkelsen AL, Fukuda M, Yding Andersen C (2011) Levels of the epidermal growth factor-like peptide amphiregulin in follicular fluid reflect the mode of triggering ovulation: a comparison between gonadotrophin-releasing hormone agonist and urinary human chorionic gonadotrophin. Fertil Steril 95:2034–2038
Haas J, Ophir L, Barzilay E, Yerushalmi GM, Yung Y, Kedem A, Maman E, Hourvitz A (2014) GnRH agonist vs. hCG for triggering of ovulation–differential effects on gene expression in human granulosa cells. PLoS ONE 9:e90359
Lin MH, Wu FS, Lee RK, Li SH, Lin SY, Hwu YM (2013) Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 100:1296–1302
Haas J, Ophir L, Barzilay E, Machtinger R, Yung Y, Orvieto R, Hourvitz A (2016) Standard human chorionic gonadotropin versus double trigger for final oocyte maturation results in different granulosa cells gene expressions: a pilot study. Fertil Steril 106:653-659 e1
Practice Committees of the American Society for Reproductive Medicine, t.S.o.R.B., Technologists and the Society for Assisted Reproductive Technology. Electronic address, j.a.o (2021) In vitro maturation: a committee opinion. Fertil Steril 115:298–304
Krisher RL (2022) Present state and future outlook for the application of in vitro oocyte maturation in human infertility treatment. Biol Reprod 106:235–242
Vuong LN et al (2020) In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod 35:2537–2547
Yang H et al (2021) Factors influencing the in vitro maturation (IVM) of human oocyte. Biomedicines 9:1904
Kind KL, Banwell KM, Gebhardt KM, Macpherson A, Gauld A, Russell DL, Thompson JG (2013) Microarray analysis of mRNA from cumulus cells following in vivo or in vitro maturation of mouse cumulus-oocyte complexes. Reprod Fertil Dev 25:426–438
Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA (2009) Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab 296:E1049–E1058
Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, De Vos M, Smitz J (2013) Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod 19:7–16
Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG (2014) Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod 90:49
Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD (2012) Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod 27:2430–2437
Ben-Ami I, Komsky A, Bern O, Kasterstein E, Komarovsky D, Ron-El R (2011) In vitro maturation of human germinal vesicle-stage oocytes: role of epidermal growth factor-like growth factors in the culture medium. Hum Reprod 26:76–81
Acknowledgements
This work was supported by operating grants from the National Natural Science Foundation of China to Jung-Chien Cheng (32170868) and Lanlan Fang (32070848). This work was also supported by the International (Regional) Cooperation and Exchange Projects (81820108016) from the National Natural Science Foundation of China to Ying-Pu Sun. All figures were created with BioRender.com. We have been granted a license to use all figures in journal publications.
Funding
Funding was provided by National Natural Science Foundation of China (Grant nos. 32170868, 32070848, and 81820108016).
Author information
Authors and Affiliations
Contributions
JCC and LF wrote the manuscript and prepared the figures and tables. YPS reviewed and provided suggestions for the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, L., Sun, YP. & Cheng, JC. The role of amphiregulin in ovarian function and disease. Cell. Mol. Life Sci. 80, 60 (2023). https://doi.org/10.1007/s00018-023-04709-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04709-8