Abstract
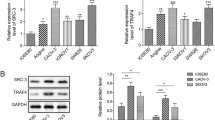
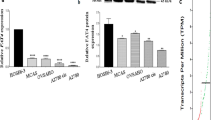
Sprouty (SPRY) proteins are well-characterized factors that inhibit receptor tyrosine kinase (RTK)-mediated activation of cellular signaling pathways. The down-regulation of SPRY4 expression has been reported in human ovarian cancer. However, the specific roles and mechanisms by which SPRY4 affects ovarian cancer progression are completely unknown. Amphiregulin (AREG) binds exclusively to the epidermal growth factor receptor (EGFR) and has been considered to be a dominant autocrine/paracrine EGFR ligand in ovarian cancer. In the present study, we first examined the effects of AREG on SPRY4 expression and the possible underlying molecular mechanisms involved in this process in two human ovarian cancer cell lines. Our results demonstrated that treatment with AREG up-regulated SPRY4 expression by activating the ERK1/2 signaling pathway. In addition, we showed that small interfering RNA (siRNA)-mediated knockdown of SPRY4 attenuated the AREG-induced down-regulation of E-cadherin by inhibiting the expression of SNAIL but not SLUG. In contrast, overexpression of SPRY4 enhanced AREG-induced down-regulation of E-cadherin by increasing the expression of SNAIL. Moreover, SPRY4 knockdown attenuated AREG-induced cell migration and invasion. Overexpression of SPRY4 enhanced AREG-induced cell invasion. This study reveals that SPRY4 is involved in EGFR-mediated human ovarian cancer progression.








Similar content being viewed by others
References
Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the drosophila airways. Cell. 1998;92:253–63.
Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, et al. Vertebrate sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75.
Zhang S, Lin Y, Itaranta P, Yagi A, Vainio S. Expression of sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev. 2001;109:367–70.
Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202.
Masoumi-Moghaddam S, Amini A, Morris DL. The developing story of sprouty and cancer. Cancer Metastasis Rev. 2014;33:695–720.
Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996;73:301–6.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, et al. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–61.
Yagi H, Miyamoto S, Tanaka Y, Sonoda K, Kobayashi H, Kishikawa T, et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer. 2005;92:1737–45.
Qiu X, Cheng JC, Klausen C, Fan Q, Chang HM, So WK, et al. Transforming growth factor-alpha induces human ovarian cancer cell invasion by down-regulating e-cadherin in a snail-independent manner. Biochem Biophys Res Commun. 2015;461:128–35.
So WK, Fan Q, Lau MT, Qiu X, Cheng JC, Leung PC. Amphiregulin induces human ovarian cancer cell invasion by down-regulating e-cadherin expression. FEBS Lett. 2014;588:3998–4007.
Cheng JC, Auersperg N, Leung PC. EGF-induced EMT and invasiveness in serous borderline ovarian tumor cells: a possible step in the transition to low-grade serous carcinoma cells? PLoS ONE. 2012;7, e34071.
Cheng JC, Chang HM, Leung PC. Egr-1 mediates epidermal growth factor-induced downregulation of e-cadherin expression via slug in human ovarian cancer cells. Oncogene. 2013;32:1041–9.
Cheng JC, Chang HM, Leung PC. Epidermal growth factor induces human oviductal epithelial cell invasion by down-regulating e-cadherin expression. J Clin Endocrinol Metab. 2012;97:E1380–1389.
Masoumi-Moghaddam S, Amini A, Wei AQ, Robertson G, Morris DL. Sprouty 2 protein, but not sprouty 4, is an independent prognostic biomarker for human epithelial ovarian cancer. Int J Cancer J Int Cancer. 2015;137:560–70.
So WK, Cheng JC, Fan Q, Wong AS, Huntsman DG, Gilks CB, et al. Loss of sprouty2 in human high-grade serous ovarian carcinomas promotes EGF-induced e-cadherin down-regulation and cell invasion. FEBS Lett. 2015;589:302–9.
Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2009;91:1035–41.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28.
Cheng JC, Klausen C, Leung PC. Hydrogen peroxide mediates EGF-induced down-regulation of e-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol Endocrinol. 2010;24:1569–80.
Mayer CE, Haigl B, Jantscher F, Siegwart G, Grusch M, Berger W, et al. Bimodal expression of sprouty2 during the cell cycle is mediated by phase-specific Ras/MAPK and c-Cbl activities. Cell Mol Life Sci: CMLS. 2010;67:3299–311.
Ozaki K, Kadomoto R, Asato K, Tanimura S, Itoh N, Kohno M. ERK pathway positively regulates the expression of sprouty genes. Biochem Biophys Res Commun. 2001;285:1084–8.
Sasaki A, Taketomi T, Wakioka T, Kato R, Yoshimura A. Identification of a dominant negative mutant of sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J Biol Chem. 2001;276:36804–8.
Doriguzzi A, Haigl B, Gsur A, Sutterluty-Fall H. The increased sprouty4 expression in response to serum is transcriptionally controlled by specific protein 1. Int J Biochem Cell Biol. 2015;64:220–8.
Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54.
Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, Minami Y, et al. Sprouty2 and sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902.
Klempner SJ, Myers AP, Mills GB, Westin SN. Clinical investigation of receptor and non-receptor tyrosine kinase inhibitors for the treatment of epithelial ovarian cancer. Expert Opin Pharmacother. 2013;14:2171–82.
Morotti M, Becker CM, Menada MV, Ferrero S. Targeting tyrosine-kinases in ovarian cancer. Expert Opin Investig Drugs. 2013;22:1265–79.
Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res: MCR. 2010;8:833–43.
Wang J, Thompson B, Ren C, Ittmann M, Kwabi-Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613–24.
Masoumi-Moghaddam S, Amini A, Ehteda A, Wei AQ, Morris DL. The expression of the sprouty 1 protein inversely correlates with growth, proliferation, migration and invasion of ovarian cancer cells. J Ovarian Res. 2014;7:61.
Zhang Q, Wei T, Shim K, Wright K, Xu K, Palka-Hamblin HL, Jurkevich A, Khare S: Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition. Oncogene 2015.
Acknowledgments
We also thank Dr. Graeme R. Guy (The Institute of Molecular and Cell Biology, Singapore) for the generous gifts of pXJ40-FLAG and FLAG-SPRY4 plasmids. This work was supported by an operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L. P.C.K.L. is a Scientist Level 3 of the Child and Family Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Grant support
This work was supported by an operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L.
Rights and permissions
About this article
Cite this article
So, WK., Cheng, JC., Liu, Y. et al. Sprouty4 mediates amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Tumor Biol. 37, 9197–9207 (2016). https://doi.org/10.1007/s13277-016-4790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4790-y