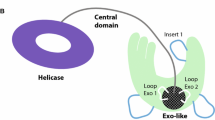
Abstract
Since its discovery in 1981, the Ku complex has been extensively studied under multiple cellular contexts, with most work focusing on Ku in terms of its essential role in non-homologous end-joining (NHEJ). In this process, Ku is well-known as the DNA-binding subunit for DNA-PK, which is central to the NHEJ repair process. However, in addition to the extensive study of Ku’s role in DNA repair, Ku has also been implicated in various other cellular processes including transcription, the DNA damage response, DNA replication, telomere maintenance, and has since been studied in multiple contexts, growing into a multidisciplinary point of research across various fields. Some advances have been driven by clarification of Ku’s structure, including the original Ku crystal structure and the more recent Ku–DNA-PKcs crystallography, cryogenic electron microscopy (cryoEM) studies, and the identification of various post-translational modifications. Here, we focus on the advances made in understanding the Ku heterodimer outside of non-homologous end-joining, and across a variety of model organisms. We explore unique structural and functional aspects, detail Ku expression, conservation, and essentiality in different species, discuss the evidence for its involvement in a diverse range of cellular functions, highlight Ku protein interactions and recent work concerning Ku-binding motifs, and finally, we summarize the clinical Ku-related research to date.




Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Fell VL, Schild-Poulter C (2015) The Ku heterodimer: function in DNA repair and beyond. Mutat Res Rev Mutat Res 763:15–29. https://doi.org/10.1016/j.mrrev.2014.06.002
Shadrina O, Garanina I, Korolev S et al (2020) Analysis of RNA binding properties of human Ku protein reveals its interactions with 7SK snRNA and protein components of 7SK snRNP complex. Biochimie 171–172:110–123. https://doi.org/10.1016/j.biochi.2020.02.016
Cohen HY, Lavu S, Bitterman KJ et al (2004) Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 13:627–638. https://doi.org/10.1016/s1097-2765(04)00094-2
Zhang X, Brann TW, Zhou M et al (2011) Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J Immunol 186:4541–4545. https://doi.org/10.4049/jimmunol.1003389
Mimori T, Akizuki M, Yamagata H et al (1981) Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest 68:611–620. https://doi.org/10.1172/jci110295
Nussenzweig A, Chen C, da Costa SV et al (1996) Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature 382:551–555. https://doi.org/10.1038/382551a0
Li G, Nelsen C, Hendrickson EA (2002) Ku86 is essential in human somatic cells. Proc Natl Acad Sci USA 99:832–837. https://doi.org/10.1073/pnas.022649699
Blomen VA, Májek P, Jae LT et al (2015) Gene essentiality and synthetic lethality in haploid human cells. Science 350:1092–1096. https://doi.org/10.1126/science.aac7557
Walker JR, Corpina RA, Goldberg J (2001) Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412:607–614. https://doi.org/10.1038/35088000
Grundy GJ, Rulten SL, Arribas-Bosacoma R et al (2016) The Ku-binding motif is a conserved module for recruitment and stimulation of non-homologous end-joining proteins. Nat Commun 7:11242. https://doi.org/10.1038/ncomms11242
Nemoz C, Ropars V, Frit P et al (2018) XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat Struct Mol Biol 25:971–980. https://doi.org/10.1038/s41594-018-0133-6
Roux KJ, Kim DI, Raida M, Burke B (2012) A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 196:801–810. https://doi.org/10.1083/jcb.201112098
Abbasi S, Schild-Poulter C (2019) Mapping the Ku interactome using proximity-dependent biotin identification in human cells. J Proteome Res 18:1064–1077. https://doi.org/10.1021/acs.jproteome.8b00771
Wang J, Satoh M, Pierani A et al (1994) Assembly and DNA binding of recombinant Ku (p70/p80) autoantigen defined by a novel monoclonal antibody specific for p70/p80 heterodimers. J Cell Sci 107(Pt 11):3223–3233
Schild-Poulter C, Pope L, Giffin W et al (2001) The binding of Ku antigen to homeodomain proteins promotes their phosphorylation by DNA-dependent protein kinase. J Biol Chem 276:16848–16856. https://doi.org/10.1074/jbc.M100768200
Gu Y, Jin S, Gao Y et al (1997) Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA 94:8076–8081
Hanakahi LA (2007) 2-Step purification of the Ku DNA repair protein expressed in Escherichia coli. Protein Expr Purif 52:139–145. https://doi.org/10.1016/j.pep.2006.10.002
Tadi SK, Tellier-Lebègue C, Nemoz C et al (2016) PAXX is an accessory c-NHEJ factor that associates with Ku70 and has overlapping functions with XLF. Cell Rep 17:541–555. https://doi.org/10.1016/j.celrep.2016.09.026
Mimori T, Hardin JA (1986) Mechanism of interaction between Ku protein and DNA. J Biol Chem 261:10375–10379
Scully R, Panday A, Elango R, Willis NA (2019) DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol 20:698–714. https://doi.org/10.1038/s41580-019-0152-0
Doherty AJ, Jackson SP (2001) DNA repair: Topological analysis of chromatin-associated protein complexes using single affinity purification. Curr Biol 11:R920–R924. https://doi.org/10.1016/S0960-9822(01)00555-3
Ribes-Zamora A, Mihalek I, Lichtarge O, Bertuch AA (2007) Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nat Struct Mol Biol 14:301–307. https://doi.org/10.1038/nsmb1214
Grundy GJ, Rulten SL, Zeng Z et al (2013) APLF promotes the assembly and activity of non-homologous end joining protein complexes. EMBO J 32:112–125. https://doi.org/10.1038/emboj.2012.304
Yoo S, Kimzey A, Dynan WS (1999) Photocross-linking of an oriented DNA repair complex. Ku bound at a single DNA end. J Biol Chem 274:20034–20039. https://doi.org/10.1074/jbc.274.28.20034
Zhang Z, Zhu L, Lin D et al (2001) The three-dimensional structure of the C-terminal DNA-binding domain of human Ku70. J Biol Chem 276:38231–38236
Göhring F, Schwab BL, Nicotera P et al (1997) The novel SAR-binding domain of scaffold attachment factor A (SAF-A) is a target in apoptotic nuclear breakdown. EMBO J 16:7361–7371. https://doi.org/10.1093/emboj/16.24.7361
Suzuki R, Shindo H, Tase A et al (2009) Solution structures and DNA binding properties of the N-terminal SAP domains of SUMO E3 ligases from Saccharomyces cerevisiae and Oryza sativa. Proteins 75:336–347. https://doi.org/10.1002/prot.22243
Lehman JA, Hoelz DJ, Turchi JJ (2008) DNA-dependant conformational changes in the Ku heterodimer. Biochemistry 47:4359–4368. https://doi.org/10.1021/bi702284c
Hu S, Pluth JM, Cucinotta FA (2012) Putative binding modes of Ku70-SAP domain with double strand DNA: a molecular modeling study. J Mol Model 18:2163–2174. https://doi.org/10.1007/s00894-011-1234-x
Rivera-Calzada A, Spagnolo L, Pearl LH, Llorca O (2007) Structural model of full-length human Ku70–Ku80 heterodimer and its recognition of DNA and DNA-PKcs. EMBO Rep 8:56–62. https://doi.org/10.1038/sj.embor.7400847
Makowski MM, Willems E, Jansen PWTC, Vermeulen M (2016) Cross-linking immunoprecipitation-MS (xIP-MS): topological analysis of chromatin-associated protein complexes using single affinity purification. Mol Cell Proteomics 15:854–865. https://doi.org/10.1074/mcp.M115.053082
Harris R, Esposito D, Sankar A et al (2004) The 3D solution structure of the C-terminal region of Ku86 (Ku86CTR). J Mol Biol 335:573–582. https://doi.org/10.1016/j.jmb.2003.10.047
Zhang Z, Hu W, Cano L et al (2004) Solution structure of the C-terminal domain of Ku80 suggests important sites for protein-protein interactions. Structure 12:495–502. https://doi.org/10.1016/j.str.2004.02.007
Hammel M, Yu Y, Mahaney BL et al (2010) Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. J Biol Chem 285:1414–1423. https://doi.org/10.1074/jbc.M109.065615
Sibanda BL, Chirgadze DY, Ascher DB, Blundell TL (2017) DNA-PKcs structure suggests an allosteric mechanism modulating DNA double-strand break repair. Science 355:520–524. https://doi.org/10.1126/science.aak9654
Sharif H, Li Y, Dong Y et al (2017) Cryo-EM structure of the DNA-PK holoenzyme. Proc Natl Acad Sci USA 114:7367–7372. https://doi.org/10.1073/pnas.1707386114
Yin X, Liu M, Tian Y et al (2017) Cryo-EM structure of human DNA-PK holoenzyme. Cell Res 27:1341–1350. https://doi.org/10.1038/cr.2017.110
Spagnolo L, Rivera-Calzada A, Pearl LH, Llorca O (2006) Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol Cell 22:511–519. https://doi.org/10.1016/j.molcel.2006.04.013
Chaplin AK, Hardwick SW, Liang S et al (2020) Dimers of DNA-PK create a stage for DNA double-strand break repair. Nat Struct Mol Biol. https://doi.org/10.1038/s41594-020-00517-x
Bowater R, Doherty AJ (2006) Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet 2:e8. https://doi.org/10.1371/journal.pgen.0020008
Dynan WS, Yoo S (1998) Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res 26:1551–1559. https://doi.org/10.1093/nar/26.7.1551
Aravind L, Koonin EV (2001) Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res 11:1365–1374. https://doi.org/10.1101/gr.181001
Gell D, Jackson SP (1999) Mapping of protein–protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Res 27:3494–3502
Lees-Miller JP, Cobban A, Katsonis P et al (2020) Uncovering DNA-PKcs ancient phylogeny, unique sequence motifs and insights for human disease. Prog Biophys Mol Biol. https://doi.org/10.1016/j.pbiomolbio.2020.09.010
Smith GCM, Jackson SP (1999) The DNA-dependent protein kinase. Genes Dev 13:916–934
Lemmens BBLG, Tijsterman M (2011) DNA double-strand break repair in Caenorhabditis elegans. Chromosoma 120:1–21. https://doi.org/10.1007/s00412-010-0296-3
Clejan I, Boerckel J, Ahmed S (2006) Developmental modulation of nonhomologous end joining in Caenorhabditis elegans. Genetics 173:1301–1317. https://doi.org/10.1534/genetics.106.058628
Emerson CH, Bertuch AA (2016) Consider the workhorse: nonhomologous end joining in budding yeast. Biochem Cell Biol 94:396–406. https://doi.org/10.1139/bcb-2016-0001
Shuman S, Glickman MS (2007) Bacterial DNA repair by non-homologous end joining. Nat Rev Microbiol 5:852–861. https://doi.org/10.1038/nrmicro1768
Pitcher RS, Brissett NC, Doherty AJ (2007) Nonhomologous end-joining in bacteria: a microbial perspective. Annu Rev Microbiol 61:259–282. https://doi.org/10.1146/annurev.micro.61.080706.093354
White MF, Allers T (2018) DNA repair in the archaea—an emerging picture. FEMS Microbiol Rev 42:514–526. https://doi.org/10.1093/femsre/fuy020
Bartlett EJ, Brissett NC, Doherty AJ (2013) Ribonucleolytic resection is required for repair of strand displaced nonhomologous end-joining intermediates. Proc Natl Acad Sci USA 110:E1984–E1991. https://doi.org/10.1073/pnas.1302616110
di Fagagna F, d’Adda WGR, Doherty AJ, Jackson SP (2003) The Gam protein of bacteriophage Mu is an orthologue of eukaryotic Ku. EMBO Rep 4:47–52. https://doi.org/10.1038/sj.embor.embor709
Bhattacharyya S, Soniat MM, Walker D et al (2018) Phage Mu Gam protein promotes NHEJ in concert with Escherichia coli ligase. Proc Natl Acad Sci USA 115:E11614–E11622. https://doi.org/10.1073/pnas.1816606115
Nenarokova A, Záhonová K, Krasilnikova M et al (2019) Causes and effects of loss of classical nonhomologous end joining pathway in parasitic eukaryotes. MBio 10:e01541-e1619. https://doi.org/10.1128/mBio.01541-19
Indiviglio SM, Bertuch AA (2009) Ku’s essential role in keeping telomeres intact. Proc Natl Acad Sci USA 106:12217–12218. https://doi.org/10.1073/pnas.0906427106
de Sena-Tomás C, Yu EY, Calzada A et al (2015) Fungal Ku prevents permanent cell cycle arrest by suppressing DNA damage signaling at telomeres. Nucleic Acids Res 43:2138–2151. https://doi.org/10.1093/nar/gkv082
Omori S, Takiguchi Y, Suda A et al (2002) Suppression of a DNA double-strand break repair gene, Ku70, increases radio- and chemosensitivity in a human lung carcinoma cell line. DNA Repair 1:299–310. https://doi.org/10.1016/s1568-7864(02)00006-x
Uegaki K, Adachi N, So S et al (2006) Heterozygous inactivation of human Ku70/Ku86 heterodimer does not affect cell growth, double-strand break repair, or genome integrity. DNA Repair (Amst) 5:303–311. https://doi.org/10.1016/j.dnarep.2005.10.008
Fattah FJ, Lichter NF, Fattah KR et al (2008) Ku70, an essential gene, modulates the frequency of rAAV-mediated gene targeting in human somatic cells. PNAS 105:8703–8708. https://doi.org/10.1073/pnas.0712060105
Gama V, Gomez JA, Mayo LD et al (2009) Hdm2 is a ubiquitin ligase of Ku70-Akt promotes cell survival by inhibiting Hdm2-dependent Ku70 destabilization. Cell Death Differ 16:758–769. https://doi.org/10.1038/cdd.2009.6
Li GC, Ouyang H, Li X et al (1998) Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol Cell 2:1–8. https://doi.org/10.1016/s1097-2765(00)80108-2
Ouyang H, Nussenzweig A, Kurimasa A et al (1997) Ku70 is required for DNA repair but not for T cell antigen receptor gene recombination in vivo. J Exp Med 186:921–929. https://doi.org/10.1084/jem.186.6.921
Lee SE, Pulaski CR, He DM et al (1995) Isolation of mammalian cell mutants that are X-ray sensitive, impaired in DNA double-strand break repair and defective for V(D)J recombination. Mutat Res/DNA Repair 336:279–291. https://doi.org/10.1016/0921-8777(95)00002-2
Finnie NJ, Gottlieb TM, Blunt T et al (1995) DNA-dependent protein kinase activity is absent in xrs-6 cells: implications for site-specific recombination and DNA double-strand break repair. PNAS 92:320–324. https://doi.org/10.1073/pnas.92.1.320
Singleton BK, Priestley A, Steingrimsdottir H et al (1997) Molecular and biochemical characterization of xrs mutants defective in Ku80. Mol Cell Biol 17:1264–1273. https://doi.org/10.1128/MCB.17.3.1264
Zdzienicka MZ, Tran Q, van der Schans GP, Simons JWIM (1988) Characterization of an X-ray-hypersensitive mutant of V79 Chinese hamster cells. Mutat Res/DNA Repair Rep 194:239–249. https://doi.org/10.1016/0167-8817(88)90025-9
Zdzienicka MZ (1995) Mammalian mutants defective in the response to ionizing radiation-induced DNA damage. Mutat Res/DNA Repair 336:203–213. https://doi.org/10.1016/0921-8777(95)00003-3
Marangoni E, Le Romancer M, Foray N et al (2000) Transfer of Ku86 RNA antisense decreases the radioresistance of human fibroblasts. Cancer Gene Ther 7:339–346. https://doi.org/10.1038/sj.cgt.7700111
Jaco I, Muñoz P, Blasco MA (2004) Role of human Ku86 in telomere length maintenance and telomere capping. Cancer Res 64:7271–7278. https://doi.org/10.1158/0008-5472.CAN-04-1381
Zhu C, Bogue MA, Lim D-S et al (1996) Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell 86:379–389. https://doi.org/10.1016/S0092-8674(00)80111-7
Fan Y, Li J, Wei W et al (2019) Ku80 gene knockdown by the CRISPR/Cas9 technique affects the biological functions of human thyroid carcinoma cells. Oncol Rep 42:2486–2498. https://doi.org/10.3892/or.2019.7348
Fattah KR, Ruis BL, Hendrickson EA (2008) Mutations to Ku reveal differences in human somatic cell lines. DNA Repair 7:762–774. https://doi.org/10.1016/j.dnarep.2008.02.008
Ghosh G, Li G, Myung K, Hendrickson EA (2007) The lethality of Ku86 (XRCC5) loss-of-function mutations in human cells is independent of p53 (TP53). Radiat Res 167:66–79. https://doi.org/10.1667/RR0692.1
Gu Y, Seidl KJ, Rathbun GA et al (1997) Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity 7:653–665. https://doi.org/10.1016/S1074-7613(00)80386-6
Nussenzweig A, Sokol K, Burgman P et al (1997) Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc Natl Acad Sci U S A 94:13588–13593. https://doi.org/10.1073/pnas.94.25.13588
Karanjawala ZE, Grawunder U, Hsieh CL, Lieber MR (1999) The nonhomologous DNA end joining pathway is important for chromosome stability in primary fibroblasts. Curr Biol 9:1501–1504. https://doi.org/10.1016/s0960-9822(00)80123-2
Hasty P, Vijg J (2004) Accelerating aging by mouse reverse genetics: a rational approach to understanding longevity. Aging Cell 3:55–65. https://doi.org/10.1111/j.1474-9728.2004.00082.x
Vogel H, Lim D-S, Karsenty G et al (1999) Deletion of Ku86 causes early onset of senescence in mice. PNAS 96:10770–10775. https://doi.org/10.1073/pnas.96.19.10770
Difilippantonio MJ, Zhu J, Chen HT et al (2000) DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature 404:510–514. https://doi.org/10.1038/35006670
Ferguson DO, Sekiguchi JM, Chang S et al (2000) The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci U S A 97:6630–6633. https://doi.org/10.1073/pnas.110152897
Ma S, Chang J, Wang X et al (2014) CRISPR/Cas9 mediated multiplex genome editing and heritable mutagenesis of BmKu70 in Bombyx mori. Sci Rep 4:4489. https://doi.org/10.1038/srep04489
C. elegans Deletion Mutant Consortium (2012) Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 (Bethesda) 2:1415–1425. https://doi.org/10.1534/g3.112.003830
Fukushima T, Takata M, Morrison C et al (2001) Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J Biol Chem 276:44413–44418. https://doi.org/10.1074/jbc.M106295200
Jeggo PA, Kemp LM (1983) X-ray-sensitive mutants of Chinese hamster ovary cell line isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res/DNA Repair Rep 112:313–327. https://doi.org/10.1016/0167-8817(83)90026-3
Qiao Y-M, Yu R-L, Zhu P (2019) Advances in targeting and heterologous expression of genes involved in the synthesis of fungal secondary metabolites. RSC Adv 9:35124–35134. https://doi.org/10.1039/C9RA06908A
Baumann P, Cech TR (2000) Protection of telomeres by the Ku protein in fission yeast. Mol Biol Cell 11:3265–3275. https://doi.org/10.1091/mbc.11.10.3265
West CE, Waterworth WM, Story GW et al (2002) Disruption of the Arabidopsis AtKu80 gene demonstrates an essential role for AtKu80 protein in efficient repair of DNA double-strand breaks in vivo. Plant J 31:517–528. https://doi.org/10.1046/j.1365-313X.2002.01370.x
Fox BA, Ristuccia JG, Gigley JP, Bzik DJ (2009) Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell 8:520–529. https://doi.org/10.1128/EC.00357-08
Koike M (2002) Dimerization, translocation and localization of Ku70 and Ku80 proteins. J Radiat Res 43:223–236. https://doi.org/10.1269/jrr.43.223
Koike M, Shiomi T, Koike A (2000) Ku70 can translocate to the nucleus independent of Ku80 translocation and DNA-PK autophosphorylation. Biochem Biophys Res Commun 276:1105–1111. https://doi.org/10.1006/bbrc.2000.3567
Bertinato J, Schild-Poulter C, Haché RJG (2001) Nuclear localization of Ku antigen is promoted independently by basic motifs in the Ku70 and Ku80 subunits. J Cell Sci 114:89–99
Miyoshi T, Sadaie M, Kanoh J, Ishikawa F (2003) Telomeric DNA ends are essential for the localization of Ku at telomeres in fission yeast. J Biol Chem 278:1924–1931. https://doi.org/10.1074/jbc.M208813200
Mukherjee S, Chakraborty P, Saha P (2016) Phosphorylation of Ku70 subunit by cell cycle kinases modulates the replication related function of Ku heterodimer. Nucleic Acids Res 44:7755–7765. https://doi.org/10.1093/nar/gkw622
Schild-Poulter C, Matheos D, Novac O et al (2003) Differential DNA binding of Ku antigen determines its involvement in DNA replication. DNA Cell Biol 22:65–78. https://doi.org/10.1089/104454903321515887
Bertinato J, Tomlinson JJ, Schild-Poulter C, Haché RJG (2003) Evidence implicating Ku antigen as a structural factor in RNA polymerase II-mediated transcription. Gene 302:53–64. https://doi.org/10.1016/s0378111902010892
Mo X, Dynan WS (2002) Subnuclear localization of Ku protein: functional association with RNA polymerase II elongation sites. Mol Cell Biol 22:8088–8099. https://doi.org/10.1128/mcb.22.22.8088-8099.2002
Adelmant G, Calkins AS, Garg BK et al (2012) DNA ends alter the molecular composition and localization of Ku multicomponent complexes. Mol Cell Proteomics 11:411–421. https://doi.org/10.1074/mcp.M111.013581
Moore HM, Bai B, Boisvert F-M et al (2011) Quantitative proteomics and dynamic imaging of the nucleolus reveal distinct responses to UV and ionizing radiation. Mol Cell Proteomics 10:009241. https://doi.org/10.1074/mcp.M111.009241
Shao Z, Flynn RA, Crowe JL et al (2020) DNA-PKcs has KU-dependent function in rRNA processing and haematopoiesis. Nature 579:291–296. https://doi.org/10.1038/s41586-020-2041-2
Hanakahi LA, West SC (2002) Specific interaction of IP6 with human Ku70/80, the DNA-binding subunit of DNA-PK. EMBO J 21:2038–2044. https://doi.org/10.1093/emboj/21.8.2038
Byrum J, Jordan S, Safrany ST, Rodgers W (2004) Visualization of inositol phosphate-dependent mobility of Ku: depletion of the DNA-PK cofactor InsP6 inhibits Ku mobility. Nucleic Acids Res 32:2776–2784. https://doi.org/10.1093/nar/gkh592
Cheung JCY, Salerno B, Hanakahi LA (2008) Evidence for an inositol hexakisphosphate-dependent role for Ku in mammalian nonhomologous end joining that is independent of its role in the DNA-dependent protein kinase. Nucleic Acids Res 36:5713–5726. https://doi.org/10.1093/nar/gkn572
Dejmek J, Iglehart JD, Lazaro J-B (2009) DNA-dependent protein kinase (DNA-PK)-dependent cisplatin-induced loss of nucleolar facilitator of chromatin transcription (FACT) and regulation of cisplatin sensitivity by DNA-PK and FACT. Mol Cancer Res 7:581–591. https://doi.org/10.1158/1541-7786.MCR-08-0049
Higashiura M, Shimizu Y, Tanimoto M et al (1992) Immunolocalization of Ku-proteins (p80/p70): localization of p70 to nucleoli and periphery of both interphase nuclei and metaphase chromosomes. Exp Cell Res 201:444–451. https://doi.org/10.1016/0014-4827(92)90293-h
Coffey G, Lakshmipathy U, Campbell C (1999) Mammalian mitochondrial extracts possess DNA end-binding activity. Nucleic Acids Res 27:3348–3354. https://doi.org/10.1093/nar/27.16.3348
Tadi SK, Sebastian R, Dahal S et al (2016) Microhomology-mediated end joining is the principal mediator of double-strand break repair during mitochondrial DNA lesions. Mol Biol Cell 27:223–235. https://doi.org/10.1091/mbc.E15-05-0260
Mimori T, Hardin JA, Steitz JA (1986) Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem 261:2274–2278
Choi EK, Lee YH, Choi YS et al (2002) Heterogeneous expression of Ku70 in human tissues is associated with morphological and functional alterations of the nucleus. J Pathol 198:121–130. https://doi.org/10.1002/path.1164
Yagura T, Sumi K (1999) Molecular cloning and sequencing of cDNAs encoding homologues of human Ku70 and Ku80 autoantigen from Xenopus and their expression in various Xenopus tissues. Biochim Biophys Acta Gene Struct Expr 1445:160–164. https://doi.org/10.1016/S0167-4781(99)00028-7
Jacoby DB, Wensink PC (1994) Yolk protein factor 1 is a Drosophila homolog of Ku, the DNA-binding subunit of a DNA-dependent protein kinase from humans. J Biol Chem 269:11484–11491
Tamura K, Adachi Y, Chiba K et al (2002) Identification of Ku70 and Ku80 homologues in Arabidopsis thaliana: evidence for a role in the repair of DNA double-strand breaks. Plant J 29:771–781. https://doi.org/10.1046/j.1365-313x.2002.01258.x
Pink RC, Carter DRF (2013) Pseudogenes as regulators of biological function. Essays Biochem 54:103–112. https://doi.org/10.1042/bse0540103
Myung K, He DM, Lee SE, Hendrickson EA (1997) KARP-1: a novel leucine zipper protein expressed from the Ku86 autoantigen locus is implicated in the control of DNA-dependent protein kinase activity. EMBO J 16:3172–3184. https://doi.org/10.1093/emboj/16.11.3172
Koike M, Yutoku Y, Koike A (2011) KARP-1 works as a heterodimer with Ku70, but the function of KARP-1 cannot perfectly replace that of Ku80 in DSB repair. Exp Cell Res 317:2267–2275. https://doi.org/10.1016/j.yexcr.2011.06.015
Myung K, Braastad C, He DM, Hendrickson EA (1998) KARP-1 is induced by DNA damage in a p53- and ataxia telangiectasia mutated-dependent fashion. Proc Natl Acad Sci USA 95:7664–7669. https://doi.org/10.1073/pnas.95.13.7664
Do E, Taira E, Irie Y et al (2003) Molecular cloning and characterization of rKAB1, which interacts with KARP-1, localizes in the nucleus and protects cells against oxidative death. Mol Cell Biochem 248:77–83. https://doi.org/10.1023/a:1024157515342
Davis AJ, Lee K-J, Chen DJ (2013) The N-terminal region of the DNA-dependent protein kinase catalytic subunit is required for its DNA double-stranded break-mediated activation. J Biol Chem 288:7037–7046. https://doi.org/10.1074/jbc.M112.434498
Singleton BK, Torres-Arzayus MI, Rottinghaus ST et al (1999) The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Mol Cell Biol 19:3267–3277. https://doi.org/10.1128/mcb.19.5.3267
Weterings E, Verkaik NS, Keijzers G et al (2009) The Ku80 carboxy terminus stimulates joining and artemis-mediated processing of DNA ends. Mol Cell Biol 29:1134–1142. https://doi.org/10.1128/MCB.00971-08
Radhakrishnan SK, Lees-Miller SP (2017) DNA requirements for interaction of the C-terminal region of Ku80 with the DNA-dependent protein kinase catalytic subunit (DNA-PKcs). DNA Repair 57:17–28. https://doi.org/10.1016/j.dnarep.2017.06.001
Jette N, Lees-Miller SP (2015) The DNA-dependent protein kinase: a multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol 117:194–205. https://doi.org/10.1016/j.pbiomolbio.2014.12.003
Douglas P, Ye R, Radhamani S et al (2020) Nocodazole-induced expression and phosphorylation of Anillin and other mitotic proteins are decreased in DNA-dependent protein kinase catalytic subunit-deficient cells and rescued by inhibition of the anaphase-promoting complex/cyclosome with proTAME but not Apcin. Mol Cell Biol 40:e00191-e219. https://doi.org/10.1128/MCB.00191-19
Bürckstümmer T, Bennett KL, Preradovic A et al (2006) An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat Methods 3:1013–1019. https://doi.org/10.1038/nmeth968
Shirodkar P, Fenton AL, Meng L, Koch CA (2013) Identification and functional characterization of a Ku-binding motif in aprataxin polynucleotide kinase/phosphatase-like factor (APLF). J Biol Chem 288:19604–19613. https://doi.org/10.1074/jbc.M112.440388
Chung JH (2018) The role of DNA-PK in aging and energy metabolism. FEBS J 285:1959–1972. https://doi.org/10.1111/febs.14410
Mari P-O, Florea BI, Persengiev SP et al (2006) Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA 103:18597–18602. https://doi.org/10.1073/pnas.0609061103
Song K, Jung D, Jung Y et al (2000) Interaction of human Ku70 with TRF2. FEBS Lett 481:81–85. https://doi.org/10.1016/s0014-5793(00)01958-x
Hsu H-L, Gilley D, Blackburn EH, Chen DJ (1999) Ku is associated with the telomere in mammals. Proc Natl Acad Sci USA 96:12454–12458. https://doi.org/10.1073/pnas.96.22.12454
O’Connor MS, Safari A, Liu D et al (2004) The human Rap1 protein complex and modulation of telomere length. J Biol Chem 279:28585–28591. https://doi.org/10.1074/jbc.M312913200
Chai W, Ford LP, Lenertz L et al (2002) Human Ku70/80 associates physically with telomerase through interaction with hTERT. J Biol Chem 277:47242–47247. https://doi.org/10.1074/jbc.M208542200
Fell VL, Walden EA, Hoffer SM et al (2016) Ku70 serine 155 mediates Aurora B inhibition and activation of the DNA damage response. Sci Rep 6:37194. https://doi.org/10.1038/srep37194
Feki A, Jefford CE, Berardi P et al (2005) BARD1 induces apoptosis by catalysing phosphorylation of p53 by DNA-damage response kinase. Oncogene 24:3726–3736. https://doi.org/10.1038/sj.onc.1208491
Goudelock DM, Jiang K, Pereira E et al (2003) Regulatory interactions between the checkpoint kinase Chk1 and the proteins of the DNA-dependent protein kinase complex. J Biol Chem 278:29940–29947. https://doi.org/10.1074/jbc.M301765200
Thapar R, Wang JL, Hammel M et al (2020) Mechanism of efficient double-strand break repair by a long non-coding RNA. Nucleic Acids Res 48:10953–10972. https://doi.org/10.1093/nar/gkaa784
Gallardo F, Olivier C, Dandjinou AT et al (2008) TLC1 RNA nucleo-cytoplasmic trafficking links telomerase biogenesis to its recruitment to telomeres. EMBO J 27:748–757. https://doi.org/10.1038/emboj.2008.21
Yuan M, Eberhart CG, Kai M (2014) RNA binding protein RBM14 promotes radio-resistance in glioblastoma by regulating DNA repair and cell differentiation. Oncotarget 5:2820–2826. https://doi.org/10.18632/oncotarget.1924
Maldonado E, Shiekhattar R, Sheldon M et al (1996) A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature 381:86–89. https://doi.org/10.1038/381086a0
Willis DM, Loewy AP, Charlton-Kachigian N et al (2002) Regulation of osteocalcin gene expression by a novel Ku antigen transcription factor complex. J Biol Chem 277:37280–37291. https://doi.org/10.1074/jbc.M206482200
Shao RG, Cao CX, Zhang H et al (1999) Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J 18:1397–1406. https://doi.org/10.1093/emboj/18.5.1397
Matheos D, Ruiz MT, Price GB, Zannis-Hadjopoulos M (2002) Ku antigen, an origin-specific binding protein that associates with replication proteins, is required for mammalian DNA replication. Biochim Biophys Acta 1578:59–72. https://doi.org/10.1016/s0167-4781(02)00497-9
Raval P, Kriatchko AN, Kumar S, Swanson PC (2008) Evidence for Ku70/Ku80 association with full-length RAG1. Nucleic Acids Res 36:2060–2072. https://doi.org/10.1093/nar/gkn049
Purugganan MM, Shah S, Kearney JF, Roth DB (2001) Ku80 is required for addition of N nucleotides to V(D)J recombination junctions by terminal deoxynucleotidyl transferase. Nucleic Acids Res 29:1638–1646. https://doi.org/10.1093/nar/29.7.1638
Kim K-B, Kim D-W, Park JW et al (2014) Inhibition of Ku70 acetylation by INHAT subunit SET/TAF-Iβ regulates Ku70-mediated DNA damage response. Cell Mol Life Sci 71:2731–2745. https://doi.org/10.1007/s00018-013-1525-8
Gomez JA, Gama V, Yoshida T et al (2007) Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans 35:797–801. https://doi.org/10.1042/BST0350797
Mazumder S, Plesca D, Kinter M, Almasan A (2007) Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis. Mol Cell Biol 27:3511–3520. https://doi.org/10.1128/MCB.01448-06
Downs JA, Jackson SP (2004) A means to a DNA end: the many roles of Ku. Nat Rev Mol Cell Biol 5:367–378. https://doi.org/10.1038/nrm1367
Song K, Jung Y, Jung D, Lee I (2001) Human Ku70 interacts with heterochromatin protein 1alpha. J Biol Chem 276:8321–8327. https://doi.org/10.1074/jbc.M008779200
Yang CR, Yeh S, Leskov K et al (1999) Isolation of Ku70-binding proteins (KUBs). Nucleic Acids Res 27:2165–2174. https://doi.org/10.1093/nar/27.10.2165
Costes SV, Daelemans D, Cho EH et al (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86:3993–4003. https://doi.org/10.1529/biophysj.103.038422
Fredriksson S, Gullberg M, Jarvius J et al (2002) Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 20:473–477. https://doi.org/10.1038/nbt0502-473
Frit P, Ropars V, Modesti M et al (2019) Plugged into the Ku-DNA hub: the NHEJ network. Prog Biophys Mol Biol 147:62–76. https://doi.org/10.1016/j.pbiomolbio.2019.03.001
Hung PJ, Johnson B, Chen B-R et al (2018) MRI is a DNA damage response adaptor during classical non-homologous end joining. Mol Cell 71:332-342.e8. https://doi.org/10.1016/j.molcel.2018.06.018
Kim K, Min J, Kirby TW et al (2020) Ligand binding characteristics of the Ku80 von Willebrand domain. DNA Repair (Amst) 85:102739. https://doi.org/10.1016/j.dnarep.2019.102739
Bouley J, Saad L, Grall R et al (2015) A new phosphorylated form of Ku70 identified in resistant leukemic cells confers fast but unfaithful DNA repair in cancer cell lines. Oncotarget 6:27980–28000. https://doi.org/10.18632/oncotarget.4735
Lee K-J, Saha J, Sun J et al (2016) Phosphorylation of Ku dictates DNA double-strand break (DSB) repair pathway choice in S phase. Nucleic Acids Res 44:1732–1745. https://doi.org/10.1093/nar/gkv1499
Brown JS, Lukashchuk N, Sczaniecka-Clift M et al (2015) Neddylation promotes ubiquitylation and release of Ku from DNA-damage sites. Cell Rep 11:704–714. https://doi.org/10.1016/j.celrep.2015.03.058
Chan DW, Ye R, Veillette CJ, Lees-Miller SP (1999) DNA-dependent protein kinase phosphorylation sites in Ku 70/80 heterodimer. Biochemistry 38:1819–1828. https://doi.org/10.1021/bi982584b
Jin S, Weaver DT (1997) Double-strand break repair by Ku70 requires heterodimerization with Ku80 and DNA binding functions. EMBO J 16:6874–6885. https://doi.org/10.1093/emboj/16.22.6874
Douglas P, Gupta S, Morrice N et al (2005) DNA-PK-dependent phosphorylation of Ku70/80 is not required for non-homologous end joining. DNA Repair 4:1006–1018. https://doi.org/10.1016/j.dnarep.2005.05.003
Koike M, Koike A (2008) Accumulation of Ku80 proteins at DNA double-strand breaks in living cells. Exp Cell Res 314:1061–1070. https://doi.org/10.1016/j.yexcr.2007.11.014
Chi Y, Welcker M, Hizli AA et al (2008) Identification of CDK2 substrates in human cell lysates. Genome Biol 9:R149. https://doi.org/10.1186/gb-2008-9-10-r149
Müller-Tidow C, Ji P, Diederichs S et al (2004) The cyclin A1-CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol 24:8917–8928. https://doi.org/10.1128/MCB.24.20.8917-8928.2004
Ji P, Bäumer N, Yin T et al (2007) DNA damage response involves modulation of Ku70 and Rb functions by cyclin A1 in leukemia cells. Int J Cancer 121:706–713. https://doi.org/10.1002/ijc.22634
Fell VL, Schild-Poulter C (2012) Ku regulates signaling to DNA damage response pathways through the Ku70 von Willebrand A domain. Mol Cell Biol 32:76–87. https://doi.org/10.1128/MCB.05661-11
Blasius M, Forment JV, Thakkar N et al (2011) A phospho-proteomic screen identifies substrates of the checkpoint kinase Chk1. Genome Biol 12:R78. https://doi.org/10.1186/gb-2011-12-8-r78
Kettenbach AN, Wang T, Faherty BK et al (2012) Rapid determination of multiple linear kinase substrate motifs by mass spectrometry. Chem Biol 19:608–618. https://doi.org/10.1016/j.chembiol.2012.04.011
Pucci S, Mazzarelli P, Sesti F et al (2009) Interleukin-6 affects cell death escaping mechanisms acting on Bax–Ku70-Clusterin interactions in human colon cancer progression. Cell Cycle 8:473–481. https://doi.org/10.4161/cc.8.3.7652
Koike M, Yutoku Y, Koike A (2017) Cloning of canine Ku80 and its localization and accumulation at DNA damage sites. FEBS Open Bio 7:1854–1863. https://doi.org/10.1002/2211-5463.12311
Al-Emam A, Arbon D, Kysela B (2014) Deacetylation of Ku70 regulates ionizing-radiation induced DNA damage responses in human cells. BMC Genomics 15:P24. https://doi.org/10.1186/1471-2164-15-S2-P24
Subramanian C, Hada M, Opipari AW et al (2013) CREB-binding protein regulates Ku70 acetylation in response to ionization radiation in neuroblastoma. Mol Cancer Res 11:173–181. https://doi.org/10.1158/1541-7786.MCR-12-0065
Subramanian C, Opipari AW, Bian X et al (2005) Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 102:4842–4847. https://doi.org/10.1073/pnas.0408351102
Xiao Y, Wang J, Qin Y et al (2015) Ku80 cooperates with CBP to promote COX-2 expression and tumor growth. Oncotarget 6:8046–8061. https://doi.org/10.18632/oncotarget.3508
Li L, Ye S, Yang M et al (2015) SIRT1 downregulation enhances chemosensitivity and survival of adult T-cell leukemia-lymphoma cells by reducing DNA double-strand repair. Oncol Rep 34:2935–2942. https://doi.org/10.3892/or.2015.4287
Tao N-N, Ren J-H, Tang H et al (2017) Deacetylation of Ku70 by SIRT6 attenuates Bax-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun 485:713–719. https://doi.org/10.1016/j.bbrc.2017.02.111
Chaudhary N, Nakka KK, Chavali PL et al (2014) SMAR1 coordinates HDAC6-induced deacetylation of Ku70 and dictates cell fate upon irradiation. Cell Death Dis 5:e1447. https://doi.org/10.1038/cddis.2014.397
Schwertman P, Bekker-Jensen S, Mailand N (2016) Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nat Rev Mol Cell Biol 17:379–394. https://doi.org/10.1038/nrm.2016.58
Postow L, Ghenoiu C, Woo EM et al (2008) Ku80 removal from DNA through double strand break–induced ubiquitylation. J Cell Biol 182:467–479. https://doi.org/10.1083/jcb.200802146
Hang LE, Lopez CR, Liu X et al (2014) Regulation of Ku-DNA association by Yku70 C-terminal tail and SUMO modification. J Biol Chem 289:10308–10317. https://doi.org/10.1074/jbc.M113.526178
Williams ES, Stap J, Essers J et al (2007) DNA double-strand breaks are not sufficient to initiate recruitment of TRF2. Nat Genet 39:696–699. https://doi.org/10.1038/ng0607-696
Weterings E, Verkaik NS, Brüggenwirth HT et al (2003) The role of DNA dependent protein kinase in synapsis of DNA ends. Nucleic Acids Res 31:7238–7246. https://doi.org/10.1093/nar/gkg889
Dobbs TA, Tainer JA, Lees-Miller SP (2010) A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair (Amst) 9:1307–1314. https://doi.org/10.1016/j.dnarep.2010.09.019
Radhakrishnan SK, Jette N, Lees-Miller SP (2014) Non-homologous end joining: emerging themes and unanswered questions. DNA Repair (Amst) 17:2–8. https://doi.org/10.1016/j.dnarep.2014.01.009
Brouwer I, Sitters G, Candelli A et al (2016) Sliding sleeves of XRCC4-XLF bridge DNA and connect fragments of broken DNA. Nature 535:566–569. https://doi.org/10.1038/nature18643
Menon V, Povirk LF (2016) End-processing nucleases and phosphodiesterases: an elite supporting cast for the non-homologous end joining pathway of DNA double-strand break repair. DNA Repair (Amst) 43:57–68. https://doi.org/10.1016/j.dnarep.2016.05.011
Critchlow SE, Bowater RP, Jackson SP (1997) Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Curr Biol 7:588–598. https://doi.org/10.1016/s0960-9822(06)00258-2
Deshpande RA, Myler LR, Soniat MM et al (2020) DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci Adv 6:eaay0922. https://doi.org/10.1126/sciadv.aay0922
Deriano L, Roth DB (2013) Modernizing the nonhomologous end-joining repertoire: alternative and classical NHEJ share the stage. Annu Rev Genet 47:433–455. https://doi.org/10.1146/annurev-genet-110711-155540
Postow L (2011) Destroying the ring: freeing DNA from Ku with ubiquitin. FEBS Lett 585:2876–2882. https://doi.org/10.1016/j.febslet.2011.05.046
Fugmann SD, Lee AI, Shockett PE et al (2000) The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol 18:495–527. https://doi.org/10.1146/annurev.immunol.18.1.495
Jackson SP, Jeggo PA (1995) DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem Sci 20:412–415. https://doi.org/10.1016/s0968-0004(00)89090-8
Bassing CH, Swat W, Alt FW (2002) The mechanism and regulation of chromosomal V(D)J recombination. Cell 109:S45–S55. https://doi.org/10.1016/s0092-8674(02)00675-x
Soulas-Sprauel P, Rivera-Munoz P, Malivert L et al (2007) V(D)J and immunoglobulin class switch recombinations: a paradigm to study the regulation of DNA end-joining. Oncogene 26:7780–7791. https://doi.org/10.1038/sj.onc.1210875
Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408:433–439. https://doi.org/10.1038/35044005
Nowsheen S, Yang ES (2012) The intersection between DNA damage response and cell death pathways. Exp Oncol 34:243–254
Harper JW, Elledge SJ (2007) The DNA damage response: ten years after. Mol Cell 28:739–745. https://doi.org/10.1016/j.molcel.2007.11.015
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461:1071–1078. https://doi.org/10.1038/nature08467
Wang Y, Cortez D, Yazdi P et al (2000) BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev 14:927–939
Rouse J, Jackson SP (2002) Interfaces between the detection, signaling, and repair of DNA damage. Science 297:547–551. https://doi.org/10.1126/science.1074740
Lamarche BJ, Orazio NI, Weitzman MD (2010) The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett 584:3682–3695. https://doi.org/10.1016/j.febslet.2010.07.029
Wang M, Wu W, Wu W et al (2006) PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res 34:6170–6182. https://doi.org/10.1093/nar/gkl840
Blackford AN, Jackson SP (2017) ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell 66:801–817. https://doi.org/10.1016/j.molcel.2017.05.015
Burma S, Chen BP, Murphy M et al (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276:42462–42467. https://doi.org/10.1074/jbc.C100466200
Tomimatsu N, Tahimic CGT, Otsuki A et al (2007) Ku70/80 modulates ATM and ATR signaling pathways in response to DNA double strand breaks. J Biol Chem 282:10138–10145. https://doi.org/10.1074/jbc.M611880200
Barnes G, Rio D (1997) DNA double-strand-break sensitivity, DNA replication, and cell cycle arrest phenotypes of Ku-deficient Saccharomyces cerevisiae. Proc Natl Acad Sci USA 94:867–872. https://doi.org/10.1073/pnas.94.3.867
Cosgrove AJ, Nieduszynski CA, Donaldson AD (2002) Ku complex controls the replication time of DNA in telomere regions. Genes Dev 16:2485–2490. https://doi.org/10.1101/gad.231602
Miyoshi T, Kanoh J, Ishikawa F (2009) Fission yeast Ku protein is required for recovery from DNA replication stress. Genes Cells 14:1091–1103. https://doi.org/10.1111/j.1365-2443.2009.01337.x
Sánchez A, Russell P (2015) Ku stabilizes replication forks in the absence of Brc1. PLoS ONE 10:e0126598. https://doi.org/10.1371/journal.pone.0126598
Foster SS, Balestrini A, Petrini JHJ (2011) Functional interplay of the Mre11 nuclease and Ku in the response to replication-associated DNA damage. Mol Cell Biol 31:4379–4389. https://doi.org/10.1128/MCB.05854-11
Teixeira-Silva A, Ait Saada A, Hardy J et al (2017) The end-joining factor Ku acts in the end-resection of double strand break-free arrested replication forks. Nat Commun 8:1982. https://doi.org/10.1038/s41467-017-02144-5
Abdelbaqi K, Di Paola D, Rampakakis E, Zannis-Hadjopoulos M (2013) Ku protein levels, localization and association to replication origins in different stages of breast tumor progression. J Cancer 4:358–370. https://doi.org/10.7150/jca.6289
Novac O, Matheos D, Araujo FD et al (2001) In vivo association of Ku with mammalian origins of DNA replication. Mol Biol Cell 12:3386–3401. https://doi.org/10.1091/mbc.12.11.3386
Park S-J, Ciccone SLM, Freie B et al (2004) A positive role for the Ku complex in DNA replication following strand break damage in mammals. J Biol Chem 279:6046–6055. https://doi.org/10.1074/jbc.M311054200
Rampakakis E, Di Paola D, Zannis-Hadjopoulos M (2008) Ku is involved in cell growth, DNA replication and G1-S transition. J Cell Sci 121:590–600. https://doi.org/10.1242/jcs.021352
Galande S, Kohwi-Shigematsu T (1999) Poly(ADP-ribose) polymerase and Ku autoantigen form a complex and synergistically bind to matrix attachment sequences. J Biol Chem 274:20521–20528. https://doi.org/10.1074/jbc.274.29.20521
Giffin W, Torrance H, Rodda DJ et al (1996) Sequence-specific DNA binding by Ku autoantigen and its effects on transcription. Nature 380:265–268. https://doi.org/10.1038/380265a0
Giffin W, Kwast-Welfeld J, Rodda DJ et al (1997) Sequence-specific DNA binding and transcription factor phosphorylation by Ku autoantigen/DNA-dependent protein kinase. J Biol Chem 272:5647–5658. https://doi.org/10.1074/jbc.272.9.5647
Ludwig DL, Chen F, Peterson SR et al (1997) Ku80 gene expression is Sp1-dependent and sensitive to CpG methylation within a novel cis element. Gene 199:181–194. https://doi.org/10.1016/s0378-1119(97)00366-1
Li GC, Yang SH, Kim D et al (1995) Suppression of heat-induced hsp70 expression by the 70-kDa subunit of the human Ku autoantigen. Proc Natl Acad Sci USA 92:4512–4516. https://doi.org/10.1073/pnas.92.10.4512
Ono M, Tucker PW, Capra JD (1996) Ku is a general inhibitor of DNA-protein complex formation and transcription. Mol Immunol 33:787–796. https://doi.org/10.1016/0161-5890(96)00030-2
Bunch H, Lawney BP, Lin Y-F et al (2015) Transcriptional elongation requires DNA break-induced signalling. Nat Commun 6:10191. https://doi.org/10.1038/ncomms10191
Dvir A, Stein LY, Calore BL, Dynan WS (1993) Purification and characterization of a template-associated protein kinase that phosphorylates RNA polymerase II. J Biol Chem 268:10440–10447
Anderson CW (1993) DNA damage and the DNA-activated protein kinase. Trends Biochem Sci 18:433–437. https://doi.org/10.1016/0968-0004(93)90144-c
Trotter KW, King HA, Archer TK (2015) Glucocorticoid receptor transcriptional activation via the BRG1-dependent recruitment of TOP2β and Ku70/86. Mol Cell Biol 35:2799–2817. https://doi.org/10.1128/MCB.00230-15
Nolens G, Pignon J-C, Koopmansch B et al (2009) Ku proteins interact with activator protein-2 transcription factors and contribute to ERBB2 overexpression in breast cancer cell lines. Breast Cancer Res 11:R83. https://doi.org/10.1186/bcr2450
Oh WJ, Kim EK, Ko JH et al (2002) Nuclear proteins that bind to metal response element a (MREa) in the Wilson disease gene promoter are Ku autoantigens and the Ku-80 subunit is necessary for basal transcription of the WD gene. Eur J Biochem 269:2151–2161. https://doi.org/10.1046/j.1432-1033.2002.02865.x
Manic G, Maurin-Marlin A, Laurent F et al (2013) Impact of the Ku complex on HIV-1 expression and latency. PLoS ONE 8:e69691. https://doi.org/10.1371/journal.pone.0069691
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110. https://doi.org/10.1101/gad.1346005
Shay JW, Wright WE (2019) Telomeres and telomerase: three decades of progress. Nat Rev Genet 20:299–309. https://doi.org/10.1038/s41576-019-0099-1
Wood AM, Laster K, Rice EL, Kosak ST (2015) A beginning of the end: new insights into the functional organization of telomeres. Nucleus 6:172–178. https://doi.org/10.1080/19491034.2015.1048407
Shay JW (2018) Telomeres and aging. Curr Opin Cell Biol 52:1–7. https://doi.org/10.1016/j.ceb.2017.12.001
Boulton SJ, Jackson SP (1998) Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J 17:1819–1828. https://doi.org/10.1093/emboj/17.6.1819
Gravel S, Larrivée M, Labrecque P, Wellinger RJ (1998) Yeast Ku as a regulator of chromosomal DNA end structure. Science 280:741–744. https://doi.org/10.1126/science.280.5364.741
Martin SG, Laroche T, Suka N et al (1999) Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97:621–633. https://doi.org/10.1016/s0092-8674(00)80773-4
Porter SE, Greenwell PW, Ritchie KB, Petes TD (1996) The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res 24:582–585. https://doi.org/10.1093/nar/24.4.582
Boulton SJ, Jackson SP (1996) Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res 24:4639–4648. https://doi.org/10.1093/nar/24.23.4639
Melnikova L, Biessmann H, Georgiev P (2005) The Ku protein complex is involved in length regulation of Drosophila telomeres. Genetics 170:221–235. https://doi.org/10.1534/genetics.104.034538
Hsu HL, Gilley D, Galande SA et al (2000) Ku acts in a unique way at the mammalian telomere to prevent end joining. Genes Dev 14:2807–2812. https://doi.org/10.1101/gad.844000
Samper E, Goytisolo FA, Slijepcevic P et al (2000) Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep 1:244–252. https://doi.org/10.1093/embo-reports/kvd051
Espejel S, Franco S, Rodríguez-Perales S et al (2002) Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J 21:2207–2219. https://doi.org/10.1093/emboj/21.9.2207
Fisher TS, Zakian VA (2005) Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amst) 4:1215–1226. https://doi.org/10.1016/j.dnarep.2005.04.021
Bertuch AA, Lundblad V (2003) The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol Cell Biol 23:8202–8215. https://doi.org/10.1128/mcb.23.22.8202-8215.2003
Sui J, Zhang S, Chen BPC (2020) DNA-dependent protein kinase in telomere maintenance and protection. Cell Mol Biol Lett 25:2. https://doi.org/10.1186/s11658-020-0199-0
Wang Y, Ghosh G, Hendrickson EA (2009) Ku86 represses lethal telomere deletion events in human somatic cells. PNAS 106:12430–12435. https://doi.org/10.1073/pnas.0903362106
Matsui M, Corey DR (2017) Non-coding RNAs as drug targets. Nat Rev Drug Discov 16:167–179. https://doi.org/10.1038/nrd.2016.117
Donlic A, Hargrove AE (2018) Targeting RNA in mammalian systems with small molecules. Wiley Interdiscip Rev: RNA 9:e1477. https://doi.org/10.1002/wrna.1477
Dutertre M, Vagner S (2017) DNA-damage response RNA-binding proteins (DDRBPs): perspectives from a new class of proteins and their RNA targets. J Mol Biol 429:3139–3145. https://doi.org/10.1016/j.jmb.2016.09.019
Kaczmarski W, Khan SA (1993) Lupus autoantigen Ku protein binds HIV-1 TAR RNA in vitro. Biochem Biophys Res Commun 196:935–942. https://doi.org/10.1006/bbrc.1993.2339
Yoo S, Dynan WS (1998) Characterization of the RNA binding properties of Ku protein. Biochemistry 37:1336–1343. https://doi.org/10.1021/bi972100w
Peterson SE, Stellwagen AE, Diede SJ et al (2001) The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nat Genet 27:64–67. https://doi.org/10.1038/83778
Stellwagen AE, Haimberger ZW, Veatch JR, Gottschling DE (2003) Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev 17:2384–2395. https://doi.org/10.1101/gad.1125903
Chen H, Xue J, Churikov D et al (2018) Structural insights into yeast telomerase recruitment to telomeres. Cell 172:331–343. https://doi.org/10.1016/j.cell.2017.12.008
Pfingsten JS, Goodrich KJ, Taabazuing C et al (2012) Mutually exclusive binding of telomerase RNA and DNA by Ku alters telomerase recruitment model. Cell 148:922–932. https://doi.org/10.1016/j.cell.2012.01.033
Ting NSY, Yu Y, Pohorelic B et al (2005) Human Ku70/80 interacts directly with hTR, the RNA component of human telomerase. Nucleic Acids Res 33:2090–2098. https://doi.org/10.1093/nar/gki342
Zhang Y, He Q, Hu Z et al (2016) Long noncoding RNA LINP1 regulates repair of DNA double-strand breaks in triple-negative breast cancer. Nat Struct Mol Biol 23:522–530. https://doi.org/10.1038/nsmb.3211
Santos-Pereira JM, Aguilera A (2015) R loops: new modulators of genome dynamics and function. Nat Rev Genet 16:583–597. https://doi.org/10.1038/nrg3961
Crossley MP, Bocek M, Cimprich KA (2019) R-loops as cellular regulators and genomic threats. Mol Cell 73:398–411. https://doi.org/10.1016/j.molcel.2019.01.024
Bader AS, Hawley BR, Wilczynska A, Bushell M (2020) The roles of RNA in DNA double-strand break repair. Br J Cancer 122:613–623. https://doi.org/10.1038/s41416-019-0624-1
Ohle C, Tesorero R, Schermann G et al (2016) Transient RNA-DNA hybrids are required for efficient double-strand break repair. Cell 167:1001-1013.e7. https://doi.org/10.1016/j.cell.2016.10.001
Lu W-T, Hawley BR, Skalka GL et al (2018) Drosha drives the formation of DNA:RNA hybrids around DNA break sites to facilitate DNA repair. Nat Commun 9:532. https://doi.org/10.1038/s41467-018-02893-x
Britton S, Dernoncourt E, Delteil C et al (2014) DNA damage triggers SAF-A and RNA biogenesis factors exclusion from chromatin coupled to R-loops removal. Nucleic Acids Res 42:9047–9062. https://doi.org/10.1093/nar/gku601
Wang IX, Grunseich C, Fox J et al (2018) Human proteins that interact with RNA/DNA hybrids. Genome Res 28:1405–1414. https://doi.org/10.1101/gr.237362.118
Cristini A, Groh M, Kristiansen MS, Gromak N (2018) RNA/DNA hybrid interactome identifies DXH9 as a molecular player in transcriptional termination and R-loop-associated DNA damage. Cell Rep 23:1891–1905. https://doi.org/10.1016/j.celrep.2018.04.025
Lamaa A, Le Bras M, Skuli N et al (2016) A novel cytoprotective function for the DNA repair protein Ku in regulating p53 mRNA translation and function. EMBO Rep 17:508–518. https://doi.org/10.15252/embr.201541181
Lombard DB, Chua KF, Mostoslavsky R et al (2005) DNA repair, genome stability, and aging. Cell 120:497–512. https://doi.org/10.1016/j.cell.2005.01.028
Li H, Vogel H, Holcomb VB et al (2007) Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol 27:8205–8214. https://doi.org/10.1128/MCB.00785-07
Park S-J, Gavrilova O, Brown AL et al (2017) DNA-PK promotes the mitochondrial, metabolic, and physical decline that occurs during aging. Cell Metab 25:1135-1146.e7. https://doi.org/10.1016/j.cmet.2017.04.008
Armanios M (2013) Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 123:996–1002. https://doi.org/10.1172/JCI66370
Liu G-H, Barkho BZ, Ruiz S et al (2011) Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472:221–225. https://doi.org/10.1038/nature09879
Karmakar P, Snowden CM, Ramsden DA, Bohr VA (2002) Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus. Nucleic Acids Res 30:3583–3591. https://doi.org/10.1093/nar/gkf482
Shamanna RA, Croteau DL, Lee J-H, Bohr VA (2017) Recent advances in understanding Werner syndrome. F1000Research 6:1779. https://doi.org/10.12688/f1000research.12110.1
Opresko PL, Cheng W-H, von Kobbe C et al (2003) Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis 24:791–802. https://doi.org/10.1093/carcin/bgg034
Belizna C, Henrion D, Beucher A et al (2010) Anti-Ku antibodies: clinical, genetic and diagnostic insights. Autoimmun Rev 9:691–694. https://doi.org/10.1016/j.autrev.2010.05.020
Cavazzana I, Ceribelli A, Quinzanini M et al (2008) Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus 17:727–732. https://doi.org/10.1177/0961203308089442
Chang WSJ, Schollum J, White DHN, Solanki KK (2015) A cross-sectional study of autoantibody profiles in the Waikato systemic sclerosis cohort, New Zealand. Clin Rheumatol 34:1921–1927. https://doi.org/10.1007/s10067-015-2981-3
Rozman B, Cucnik S, Sodin-Semrl S et al (2008) Prevalence and clinical associations of anti-Ku antibodies in patients with systemic sclerosis: a European EUSTAR-initiated multi-centre case-control study. Ann Rheum Dis 67:1282–1286. https://doi.org/10.1136/ard.2007.073981
Hoa S, Hudson M, Troyanov Y et al (2016) Single-specificity anti-Ku antibodies in an international cohort of 2140 systemic sclerosis subjects: clinical associations. Medicine 95:e4713. https://doi.org/10.1097/MD.0000000000004713
Low AHL, Wong S, Thumboo J et al (2012) Evaluation of a new multi-parallel line immunoassay for systemic sclerosis-associated antibodies in an Asian population. Rheumatology (Oxford) 51:1465–1470. https://doi.org/10.1093/rheumatology/kes055
Patterson KA, Roberts-Thomson PJ, Lester S et al (2015) Interpretation of an extended autoantibody profile in a well-characterized Australian Systemic Sclerosis (Scleroderma) cohort using principal components analysis. Arthritis Rheumatol 67:3234–3244. https://doi.org/10.1002/art.39316
Rodriguez-Reyna TS, Hinojosa-Azaola A, Martinez-Reyes C et al (2011) Distinctive autoantibody profile in Mexican Mestizo systemic sclerosis patients. Autoimmunity 44:576–584. https://doi.org/10.3109/08916934.2011.592886
Ogawa-Momohara M, Muro Y, Akiyama M (2019) Overlap of systemic lupus erythematosus and myositis is rare in anti-Ku antibody-positive patients. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2019-216375
Spielmann L, Nespola B, Séverac F et al (2019) Anti-Ku syndrome with elevated CK and anti-Ku syndrome with anti-dsDNA are two distinct entities with different outcomes. Ann Rheum Dis 78:1101–1106. https://doi.org/10.1136/annrheumdis-2018-214439
Schild-Poulter C, Su A, Shih A et al (2008) Association of autoantibodies with Ku and DNA repair proteins in connective tissue diseases. Rheumatology (Oxford) 47:165–171. https://doi.org/10.1093/rheumatology/kem338
Takeda Y (2001) Autoantibodies against DNA double-strand break repair proteins. Front Biosci 6:d1412. https://doi.org/10.2741/Takeda
Pichlmair A, e Sousa CR (2007) Innate recognition of viruses. Immunity 27:370–383. https://doi.org/10.1016/j.immuni.2007.08.012
Ferguson BJ, Mansur DS, Peters NE et al (2012) DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife 1:e00047. https://doi.org/10.7554/eLife.00047
Wang J, Kang L, Song D et al (2017) Ku70 senses HTLV-1 DNA and modulates HTLV-1 replication. J Immunol 199:2475–2482. https://doi.org/10.4049/jimmunol.1700111
Li Y, Wu Y, Zheng X et al (2016) Cytoplasm-translocated Ku70/80 complex sensing of HBV DNA induces hepatitis-associated chemokine secretion. Front Immunol 7:569. https://doi.org/10.3389/fimmu.2016.00569
Sui H, Zhou M, Imamichi H et al (2017) STING is an essential mediator of the Ku70-mediated production of IFN-λ1 in response to exogenous DNA. Sci Signal 10:eaah5054. https://doi.org/10.1126/scisignal.aah5054
Peters NE, Ferguson BJ, Mazzon M et al (2013) A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus. PLOS Pathog 9:e1003649. https://doi.org/10.1371/journal.ppat.1003649
Scutts SR, Ember SW, Ren H et al (2018) DNA-PK is targeted by multiple vaccinia virus proteins to inhibit DNA sensing. Cell Rep 25:1953-1965.e4. https://doi.org/10.1016/j.celrep.2018.10.034
Bonilla FA, Oettgen HC (2010) Adaptive immunity. J Allergy Clin Immunol 125:S33–S40. https://doi.org/10.1016/j.jaci.2009.09.017
Roth DB (2014) V(D)J recombination: mechanism, errors, and fidelity. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MDNA3-0041-2014
Fischer A (2000) Severe combined immunodeficiencies (SCID). Clin Exp Immunol 122:143–149. https://doi.org/10.1046/j.1365-2249.2000.01359.x
Woodbine L, Gennery AR, Jeggo PA (2014) The clinical impact of deficiency in DNA non-homologous end-joining. DNA Repair 16:84–96. https://doi.org/10.1016/j.dnarep.2014.02.011
Sishc BJ, Davis AJ (2017) The role of the core non-homologous end joining factors in carcinogenesis and cancer. Cancers 9:81. https://doi.org/10.3390/cancers9070081
Komuro Y, Watanabe T, Hosoi Y et al (2002) The expression pattern of Ku correlates with tumor radiosensitivity and disease free survival in patients with rectal carcinoma. Cancer 95:1199–1205. https://doi.org/10.1002/cncr.10807
Rigas B, Borgo S, Elhosseiny A et al (2001) Decreased expression of DNA-dependent protein kinase, a DNA repair protein, during human colon carcinogenesis. Cancer Res 61:8381–8384
Ayene IS, Ford LP, Koch CJ (2005) Ku protein targeting by Ku70 small interfering RNA enhances human cancer cell response to topoisomerase II inhibitor and gamma radiation. Mol Cancer Ther 4:529–536. https://doi.org/10.1158/1535-7163.MCT-04-0130
Beggs AD, Domingo E, McGregor M et al (2012) Loss of expression of the double strand break repair protein ATM is associated with worse prognosis in colorectal cancer and loss of Ku70 expression is associated with CIN. Oncotarget 3:1348–1355. https://doi.org/10.18632/oncotarget.694
Wilson CR, Davidson SE, Margison GP et al (2000) Expression of Ku70 correlates with survival in carcinoma of the cervix. Br J Cancer 83:1702–1706. https://doi.org/10.1054/bjoc.2000.1510
Hu H, Zhang Y, Zou M et al (2010) Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is associated with telomere shortening and may contribute to multistage carcinogenesis of gastric cancer. J Cancer Res Clin Oncol 136:1407–1414. https://doi.org/10.1007/s00432-010-0795-x
Mazzarelli P, Parrella P, Seripa D et al (2005) DNA end binding activity and Ku70/80 heterodimer expression in human colorectal tumor. World J Gastroenterol 11:6694–6700. https://doi.org/10.3748/wjg.v11.i42.6694
Ma Q, Li P, Xu M et al (2012) Ku80 is highly expressed in lung adenocarcinoma and promotes cisplatin resistance. J Exp Clin Cancer Res 31:99. https://doi.org/10.1186/1756-9966-31-99
Pucci S, Mazzarelli P, Rabitti C et al (2001) Tumor specific modulation of KU70/80 DNA binding activity in breast and bladder human tumor biopsies. Oncogene 20:739–747. https://doi.org/10.1038/sj.onc.1204148
Ai J, Pascal LE, Wei L et al (2017) EAF2 regulates DNA repair through Ku70/Ku80 in the prostate. Oncogene 36:2054–2065. https://doi.org/10.1038/onc.2016.373
Weterings E, Gallegos AC, Dominick LN et al (2016) A novel small molecule inhibitor of the DNA repair protein Ku70/80. DNA Repair (Amst) 43:98–106. https://doi.org/10.1016/j.dnarep.2016.03.014
Acknowledgements
We are grateful to Mohamed Aly for reading and providing feedback on the review.
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant and a NSERC Discovery Accelerator Supplement to C.S.P.. S.A. and G.P. were supported by Ontario Graduate Scholarships.
Author information
Authors and Affiliations
Contributions
S.A. and G.P. conceptualized the idea of the review. S.A., G.P., R.D.K., and N.B. wrote the original draft and S.A., G.P, and R.D.K. prepared the figures and tables. C.S.P. provided edits, suggestions, and critical feedback. The final version of the review was edited by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbasi, S., Parmar, G., Kelly, R.D. et al. The Ku complex: recent advances and emerging roles outside of non-homologous end-joining. Cell. Mol. Life Sci. 78, 4589–4613 (2021). https://doi.org/10.1007/s00018-021-03801-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03801-1