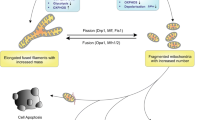
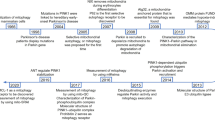
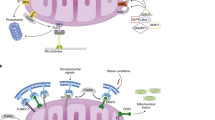
Abstract
Preservation of mitochondrial quality is paramount for cellular homeostasis. The integrity of mitochondria is guarded by the balanced interplay between anabolic and catabolic mechanisms. The removal of bio-energetically flawed mitochondria is mediated by the process of mitophagy; the impairment of which leads to the accumulation of defective mitochondria which signal the activation of compensatory mechanisms to the nucleus. This process is known as the mitochondrial retrograde response (MRR) and is enacted by Reactive Oxygen Species (ROS), Calcium (Ca2+), ATP, as well as imbalanced lipid and proteostasis. Central to this mitochondria-to-nucleus signalling are the transcription factors (e.g. the nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB) which drive the expression of genes to adapt the cell to the compromised homeostasis. An increased degree of cellular proliferation is among the consequences of the MRR and as such, engagement of mitochondrial-nuclear communication is frequently observed in cancer. Mitophagy and the MRR are therefore interlinked processes framed to, respectively, prevent or compensate for mitochondrial defects.
In this review, we discuss the available knowledge on the interdependency of these processes and their contribution to cell signalling in cancer.




Similar content being viewed by others
References
Lu H, Li G, Liu L et al (2013) Regulation and function of mitophagy in development and cancer. Autophagy 9:1720–1736. https://doi.org/10.4161/auto.26550
Palikaras K, Tavernarakis N (2014) Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 56:182–188. https://doi.org/10.1016/j.exger.2014.01.021
Herst PM, Rowe MR, Carson GM, Berridge MV (2017) Functional mitochondria in health and disease. Front Endocrinol 8:296
Quirós PM, Mottis A, Auwerx J (2016) Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol
Yang D, Kim J (2019) Mitochondrial Retrograde Signalling and Metabolic Alterations in the Tumour Microenvironment. Cells 8:. https://doi.org/https://doi.org/10.3390/cells8030275
Sanchis-Gomar F, García-Giménez JL, Gómez-Cabrera MC, Pallardó FV (2014) Mitochondrial biogenesis in health and disease. Molecular and therapeutic approaches. Curr Pharm Des 20:5619–5633. https://doi.org/10.2174/1381612820666140306095106
Johri A, Chandra A, Flint Beal M (2013) PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med 62:37–46. https://doi.org/10.1016/j.freeradbiomed.2013.04.016
Chourasia AH, Boland ML, Macleod KF (2015) Mitophagy and Cancer. Cancer Metab 3:4. https://doi.org/10.1186/s40170-015-0130-8
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9–14. https://doi.org/10.1038/nrm3028
Guha M, Srinivasan S, Ruthel G et al (2014) Mitochondrial retrograde signaling induces epithelial-mesenchymal transition and generates breast cancer stem cells. Oncogene 33:5238–5250. https://doi.org/10.1038/onc.2013.467
Butow RA, Avadhani NG (2004) Mitochondrial signaling: the retrograde response. Mol Cell 14:1–15. https://doi.org/10.1016/s1097-2765(04)00179-0
Cardamone MD, Tanasa B, Cederquist CT et al (2018) Mitochondrial retrograde signaling in mammals is mediated by the transcriptional cofactor GPS2 via direct mitochondria-to-nucleus translocation. Mol Cell 69:757-772.e7. https://doi.org/10.1016/j.molcel.2018.01.037
Kim S, Koh H (2017) Role of FOXO transcription factors in crosstalk between mitochondria and the nucleus. J Bioenerg Biomembr 49:. https://doi.org/https://doi.org/10.1007/s10863-017-9705-0
Liu Z, Butow RA (2006) Mitochondrial retrograde signaling. Annu Rev Genet 40:159–185. https://doi.org/10.1146/annurev.genet.40.110405.090613
Carden T, Singh B, Mooga V et al (2017) Epigenetic modification of miR-663 controls mitochondria-to-nucleus retrograde signaling and tumor progression. J Biol Chem 292:20694–20706. https://doi.org/10.1074/jbc.M117.797001
Feske S, Okamura H, Hogan PG, Rao A (2003) Ca2+/calcineurin signalling in cells of the immune system. Biochem Biophys Res Commun 311:1117–1132. https://doi.org/10.1016/j.bbrc.2003.09.174
Amuthan G, Biswas G, Zhang SY et al (2001) Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J 20:1910–1920. https://doi.org/10.1093/emboj/20.8.1910
Desai R, East DA, Hardy L et al (2020) Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci Adv 6(51):eabc9955: https://doi.org/10.1126/sciadv.abc9955
Palikaras K, Lionaki E, Tavernarakis N (2015) Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521:525–528. https://doi.org/10.1038/nature14300
Matic I, Strobbe D, Di Guglielmo F, Campanella M (2017) Molecular biology digest of cell mitophagy. In: International Review of Cell and Molecular Biology
Ordureau A, Sarraf SA, Duda DM et al (2014) Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 56:360–375. https://doi.org/10.1016/j.molcel.2014.09.007
Wong YC, Holzbaur ELF (2014) Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci 111:E4439 LP-E4448. https://doi.org/https://doi.org/10.1073/pnas.1405752111
Hamacher-Brady A, Brady NR (2016) Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 73:775–795. https://doi.org/10.1007/s00018-015-2087-8
Narendra D, Tanaka A, Suen D-F, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. https://doi.org/10.1083/jcb.200809125
Lazarou M, Sliter DA, Kane LA et al (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. https://doi.org/10.1038/nature14893
Liu L, Feng D, Chen G et al (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14:177–185. https://doi.org/10.1038/ncb2422
Zhu Y, Massen S, Terenzio M et al (2013) Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J Biol Chem 288:1099–1113. https://doi.org/10.1074/jbc.M112.399345
Novak I, Dikic I (2011) Autophagy receptors in developmental clearance of mitochondria. Autophagy 7:301–303. https://doi.org/10.4161/auto.7.3.14509
Murakawa T, Yamaguchi O, Hashimoto A et al (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun 6:7527. https://doi.org/10.1038/ncomms8527
Koganti P, Levy-Cohen G, Blank M (2018) Smurfs in Protein Homeostasis, Signaling, and Cancer. Front Oncol 8:295. https://doi.org/10.3389/fonc.2018.00295
Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24:24–41. https://doi.org/10.1038/cr.2013.168
Roca-Agujetas V, de Dios C, Lestón L et al (2019) Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid Med Cell Longev 2019:3809308. https://doi.org/10.1155/2019/3809308
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19:121–135. https://doi.org/10.1038/nrm.2017.95
Tang BL (2016) Sirt1 and the Mitochondria. Mol Cells 39:87–95. https://doi.org/https://doi.org/10.14348/molcells.2016.2318
Ploumi C, Daskalaki I, Tavernarakis N (2017) Mitochondrial biogenesis and clearance: a balancing act. FEBS J 284:183–195. https://doi.org/10.1111/febs.13820
Yan C, Li T-S (2018) Dual Role of Mitophagy in Cancer Drug Resistance. Anticancer Res 38:617–621. https://doi.org/10.21873/anticanres.12266
Weigl S, Paradiso A, Tommasi S (2013) Mitochondria and familial predisposition to breast cancer. Curr Genomics 14:195–203. https://doi.org/10.2174/1389202911314030005
Zong W-X, Rabinowitz JD, White E (2016) Mitochondria and Cancer. Mol Cell 61:667–676. https://doi.org/10.1016/j.molcel.2016.02.011
Sullivan LB, Chandel NS (2014) Mitochondrial reactive oxygen species and cancer. Cancer Metab 2:17. https://doi.org/10.1186/2049-3002-2-17
Hsu C-C, Tseng L-M, Lee H-C (2016) Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood) 241:1281–1295. https://doi.org/10.1177/1535370216641787
Ribas V, García-Ruiz C, Fernández-Checa JC (2016) Mitochondria, cholesterol and cancer cell metabolism. Clin Transl Med 5:22. https://doi.org/10.1186/s40169-016-0106-5
Kirkinezos IG, Moraes CT (2001) Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 12:449–457. https://doi.org/10.1006/scdb.2001.0282
Yakes FM, Van Houten B (1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94:514–519. https://doi.org/10.1073/pnas.94.2.514
Sekito T, Thornton J, Butow RA (2000) Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol Biol Cell 11:2103–2115. https://doi.org/10.1091/mbc.11.6.2103
da Cunha FM, Torelli NQ, Kowaltowski AJ (2015) Mitochondrial retrograde signaling: triggers, pathways, and outcomes. Oxid Med Cell Longev 2015:482582. https://doi.org/10.1155/2015/482582
Zhang F, Pracheil T, Thornton J, Liu Z (2013) Adenosine triphosphate (ATP) is a candidate signaling molecule in the mitochondria-to-nucleus retrograde response pathway. Genes (Basel) 4:86–100. https://doi.org/10.3390/genes4010086
Borghouts C, Benguria A, Wawryn J, Jazwinski SM (2004) Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics 166:765–777. https://doi.org/10.1534/genetics.166.2.765
Miceli MV, Jiang JC, Tiwari A et al (2011) Loss of mitochondrial membrane potential triggers the retrograde response extending yeast replicative lifespan. Front Genet 2:102. https://doi.org/10.3389/fgene.2011.00102
Arnould T, Michel S, Renard P (2015) Mitochondria retrograde signaling and the UPR mt: Where are we in mammals? Int J Mol Sci 16:18224–18251. https://doi.org/10.3390/ijms160818224
Formentini L, Sánchez-Aragó M, Sánchez-Cenizo L, Cuezva JM (2012) The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell 45:731–742. https://doi.org/10.1016/j.molcel.2012.01.008
Srinivasan S, Avadhani NG (2012) Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med 53:1252–1263. https://doi.org/10.1016/j.freeradbiomed.2012.07.021
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Yun J, Finkel T (2014) Mitohormesis. Cell Metab 19:757–766. https://doi.org/10.1016/j.cmet.2014.01.011
Bohovych I, Khalimonchuk O (2016) Sending out an SOS: mitochondria as a signaling hub. Front cell Dev Biol 4:109. https://doi.org/10.3389/fcell.2016.00109
Sullivan LB, Gui DY, Hosios AM et al (2015) Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162:552–563. https://doi.org/10.1016/j.cell.2015.07.017
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668. https://doi.org/10.1056/NEJMra022567
Cantó C, Menzies KJ, Auwerx J (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22:31–53. https://doi.org/10.1016/j.cmet.2015.05.023
Adam J, Yang M, Soga T, Pollard PJ (2014) Rare insights into cancer biology. Oncogene 33:2547–2556. https://doi.org/10.1038/onc.2013.222
Sullivan LB, Martinez-Garcia E, Nguyen H et al (2013) The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell 51:236–248. https://doi.org/10.1016/j.molcel.2013.05.003
Losman J-A, Kaelin WGJ (2013) What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev 27:836–852. https://doi.org/10.1101/gad.217406.113
Nargund AM, Pellegrino MW, Fiorese CJ et al (2012) Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337:587–590. https://doi.org/10.1126/science.1223560
Zhang Z, Tan M, Xie Z et al (2011) Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol 7:58–63. https://doi.org/10.1038/nchembio.495
Trotta AP, Chipuk JE (2017) Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci 74:1999–2017. https://doi.org/10.1007/s00018-016-2451-3
Picard M, Shirihai OS, Gentil BJ, Burelle Y (2013) Mitochondrial morphology transitions and functions: Implications for retrograde signaling? Am J Physiol—Regul Integr Comp Physiol 304:. https://doi.org/https://doi.org/10.1152/ajpregu.00584.2012
Chen H, Chan DC (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab 26(1):39–48. https://doi.org/10.1016/j.cmet.2017.05.016
Maycotte P, Marín-Hernández A, Goyri-Aguirre M et al (2017) Mitochondrial dynamics and cancer. Tumour Biol J Int Soc Oncodev Biol Med 39:1010428317698391. https://doi.org/10.1177/1010428317698391
Ferreira-da-Silva A, Valacca C, Rios E et al (2015) Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PLoS ONE 10:e0122308. https://doi.org/10.1371/journal.pone.0122308
Zhao J, Zhang J, Yu M et al (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32:4814–4824. https://doi.org/10.1038/onc.2012.494
Sánchez-Aragó M, Formentini L, García-Bermúdez J, Cuezva JM (2012) IF1 reprograms energy metabolism and signals the oncogenic phenotype in cancer. Cell Cycle 11:2963–2964. https://doi.org/10.4161/cc.21387
Faccenda D, Campanella M (2012) Molecular Regulation of the mitochondrial F(1)F(o)-ATPsynthase: physiological and pathological significance of the inhibitory factor 1 (IF(1)). Int J Cell Biol 2012:367934. https://doi.org/10.1155/2012/367934
García-Bermúdez J, Cuezva JM (2016) The ATPase inhibitory factor 1 (IF1): a master regulator of energy metabolism and of cell survival. Biochim Biophys Acta 1857:1167–1182. https://doi.org/10.1016/j.bbabio.2016.02.004
Sánchez-Cenizo L, Formentini L, Aldea M et al (2010) Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J Biol Chem 285:25308–25313. https://doi.org/10.1074/jbc.M110.146480
Faccenda D, Nakamura J, Gorini G et al (2017) Control of mitochondrial remodeling by the ATPase inhibitory factor 1 unveils a pro-survival relay via OPA1. Cell Rep 18:1869–1883. https://doi.org/10.1016/j.celrep.2017.01.070
Faccenda D, Tan CH, Seraphim A et al (2013) IF1 limits the apoptotic-signalling cascade by preventing mitochondrial remodelling. Cell Death Differ 20:686–697. https://doi.org/10.1038/cdd.2012.163
Gatliff J, Campanella M (2016) TSPO: kaleidoscopic 18-kDa amid biochemical pharmacology, control and targeting of mitochondria. Biochem J 473(2):107–121. https://doi.org/10.1042/BJ20150899
Gatliff J, East D, Crosby J et al (2014) TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy 10:2279–2296. https://doi.org/10.4161/15548627.2014.991665
Acknowledgements
We would like to express our genuine gratitude to Dr. Previdelli, Miss Jemma Gane and Miss Parmis Vadafar for carefully reading the manuscript and providing feedback. The researches activities lead by M.C. on the topics of this script are supported by: The European Research Council Consolidator Grant COG 2018—819600_FIRM; AIRC-MFAG 21903; The Petplan Charitable Trust; LAM-Bighi Grant Initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests of any nature to report. This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strobbe, D., Sharma, S. & Campanella, M. Links between mitochondrial retrograde response and mitophagy in pathogenic cell signalling. Cell. Mol. Life Sci. 78, 3767–3775 (2021). https://doi.org/10.1007/s00018-021-03770-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03770-5