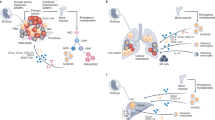
Abstract
Macrophages play an important role in tissue development and homeostasis. They serve as a nexus between adaptive and innate immunity, and employ considerable plasticity. In cancer, they play a pivotal role in chronic inflammation and tumor growth either by directly stimulating the proliferation of cancer cells or by producing angiogenic and lymphangiogenic factors. Although numerous immune cells play an important role in the tumor microenvironment, tumor-associated macrophages (TAMs) are by far the most extensively studied. A better understanding of the role of TAMs in mediating chemo- and radiotherapy resistance and suppressing immunosurveillance has led to numerous strategies targeting TAMs as an anticancer therapy either by targeting them directly or by polarizing TAMs toward a tumoricidal phenotype.


Similar content being viewed by others
References
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. https://doi.org/10.1038/nri2448
Tang D, Kang R, Coyne CB et al (2012) PAMPs and DAMPs: signal 0 s that spur autophagy and immunity. Immunol Rev 249:158–175. https://doi.org/10.1111/j.1600-065X.2012.01146.x
Mass E, Ballesteros I, Farlik M et al (2016) Specification of tissue-resident macrophages during organogenesis. Science 353:aaf4238. https://doi.org/10.1126/science.aaf4238
O’Neill LAJ, Golenbock D, Bowie AG (2013) The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13:453–460. https://doi.org/10.1038/nri3446
Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. https://doi.org/10.1038/nri3671
Cignarella A, Tedesco S, Cappellari R, Fadini GP (2018) The continuum of monocyte phenotypes: experimental evidence and prognostic utility in assessing cardiovascular risk. J Leukoc Biol. https://doi.org/10.1002/jlb.5ru1217-477rr
Hamm A, Prenen H, Van Delm W et al (2016) Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut 65:990–1000. https://doi.org/10.1136/gutjnl-2014-308988
Feng A-L, Zhu J-K, Sun J-T et al (2011) CD16+ monocytes in breast cancer patients: expanded by monocyte chemoattractant protein-1 and may be useful for early diagnosis. Clin Exp Immunol 164:57–65. https://doi.org/10.1111/j.1365-2249.2011.04321.x
Takeda Y, Costa S, Delamarre E et al (2011) Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479:122–126. https://doi.org/10.1038/nature10507
Madsen DH, Leonard D, Masedunskas A et al (2013) M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol 202:951–966. https://doi.org/10.1083/jcb.201301081
Mantovani A, Sozzani S, Locati M et al (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Duluc D, Corvaisier M, Blanchard S et al (2009) Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 125:367–373. https://doi.org/10.1002/ijc.24401
Junttila MR, de Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501:346–354. https://doi.org/10.1038/nature12626
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. https://doi.org/10.1172/JCI59643
Biswas SK, Sica A, Lewis CE (2008) Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 180:2011–2017
Zarif JC, Taichman RS, Pienta KJ (2014) TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology 3:e941734. https://doi.org/10.4161/21624011.2014.941734
Qian B-Z, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51. https://doi.org/10.1016/j.cell.2010.03.014
Elinav E, Nowarski R, Thaiss CA et al (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13:759–771. https://doi.org/10.1038/nrc3611
Franklin RA, Liao W, Sarkar A et al (2014) The cellular and molecular origin of tumor-associated macrophages. Science (80-) 344:921–925. https://doi.org/10.1126/science.1252510
Henze A-T, Mazzone M (2016) The impact of hypoxia on tumor-associated macrophages. J Clin Invest 126:3672–3679. https://doi.org/10.1172/JCI84427
Xiao M, Zhang J, Chen W, Chen W (2018) M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res 37:143. https://doi.org/10.1186/s13046-018-0815-2
Chen X, Ying X, Wang X et al (2017) Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep 38:522–528. https://doi.org/10.3892/or.2017.5697
Hsieh C-H, Tai S-K, Yang M-H (2018) Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia 20:775–788. https://doi.org/10.1016/j.neo.2018.06.004
Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol 27:16–25. https://doi.org/10.1016/j.coi.2014.01.004
Qian B-Z, Pollard JW (2012) New tricks for metastasis-associated macrophages. Breast Cancer Res 14:316. https://doi.org/10.1186/bcr3143
Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462–472. https://doi.org/10.1016/j.ccell.2015.02.015
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444. https://doi.org/10.1038/nature07205
Mantovani A, Allavena P (2015) The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 212:435–445. https://doi.org/10.1084/jem.20150295
Koong AC, Chen EY, Mivechi NF et al (1994) Hypoxic activation of nuclear factor-kappa B is mediated by a Ras and Raf signaling pathway and does not involve MAP kinase (ERK1 or ERK2). Cancer Res 54:5273–5279
Minet E, Arnould T, Michel G et al (2000) ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 468:53–58
Keith B, Johnson RS, Simon MC (2011) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9–22. https://doi.org/10.1038/nrc3183
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410. https://doi.org/10.1038/nrc1093
Leite de Oliveira R, Hamm A, Mazzone M (2011) Growing tumor vessels: more than one way to skin a cat—implications for angiogenesis targeted cancer therapies. Mol Aspects Med 32:71–87. https://doi.org/10.1016/j.mam.2011.04.001
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887. https://doi.org/10.1016/j.cell.2011.08.039
Varricchi G, Loffredo S, Galdiero MR et al (2018) Innate effector cells in angiogenesis and lymphangiogenesis. Curr Opin Immunol 53:152–160. https://doi.org/10.1016/j.coi.2018.05.002
Sherwood LM, Parris EE, Folkman J (1971) Tumor Angiogenesis: therapeutic Implications. N Engl J Med 285:1182–1186. https://doi.org/10.1056/NEJM197111182852108
De Palma M, Biziato D, Petrova TV (2017) Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17:457–474. https://doi.org/10.1038/nrc.2017.51
Eubank TD, Galloway M, Montague CM et al (2003) M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J Immunol 171:2637–2643
Hill LM, Gavala ML, Lenertz LY, Bertics PJ (2010) Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol 185:3028–3034. https://doi.org/10.4049/jimmunol.1001298
Poulin S, Thompson C, Thivierge M et al (2011) Cysteinyl-leukotrienes induce vascular endothelial growth factor production in human monocytes and bronchial smooth muscle cells. Clin Exp Allergy 41:204–217. https://doi.org/10.1111/j.1365-2222.2010.03653.x
Czepluch FS, Olieslagers S, van Hulten R et al (2011) VEGF-A-induced chemotaxis of CD16+ monocytes is decreased secondary to lower VEGFR-1 expression. Atherosclerosis 215:331–338. https://doi.org/10.1016/j.atherosclerosis.2011.01.004
De Palma M, Venneri MA, Galli R et al (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8:211–226. https://doi.org/10.1016/j.ccr.2005.08.002
Venneri MA, Palma MD, Ponzoni M et al (2007) Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109:5276–5285. https://doi.org/10.1182/blood-2006-10-053504
Matsubara T, Kanto T, Kuroda S et al (2013) TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology 57:1416–1425. https://doi.org/10.1002/hep.25965
Turrini R, Pabois A, Xenarios I et al (2017) TIE-2 expressing monocytes in human cancers. Oncoimmunology 6:e1303585. https://doi.org/10.1080/2162402X.2017.1303585
Coffelt SB, Chen Y-Y, Muthana M et al (2011) Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol 186:4183–4190. https://doi.org/10.4049/jimmunol.1002802
Mazzieri R, Pucci F, Moi D et al (2011) Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19:512–526. https://doi.org/10.1016/j.ccr.2011.02.005
Lin EY, Li J-F, Gnatovskiy L et al (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–11246. https://doi.org/10.1158/0008-5472.CAN-06-1278
Stockmann C, Doedens A, Weidemann A et al (2008) Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456:814–818. https://doi.org/10.1038/nature07445
Priceman SJ, Sung JL, Shaposhnik Z et al (2010) Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115:1461–1471. https://doi.org/10.1182/blood-2009-08-237412
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67. https://doi.org/10.1016/j.cell.2010.03.015
Fischer C, Jonckx B, Mazzone M et al (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475. https://doi.org/10.1016/j.cell.2007.08.038
Rolny C, Mazzone M, Tugues S et al (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19:31–44. https://doi.org/10.1016/j.ccr.2010.11.009
Nozawa H, Chiu C, Hanahan D (2006) Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA 103:12493–12498. https://doi.org/10.1073/pnas.0601807103
Mantovani A (2010) Molecular pathways linking inflammation and cancer. Curr Mol Med 10:369–373
DeNardo DG, Barreto JB, Andreu P et al (2009) CD4+ T Cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16:91–102. https://doi.org/10.1016/j.ccr.2009.06.018
Facciabene A, Peng X, Hagemann IS et al (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475:226–230. https://doi.org/10.1038/nature10169
Tammela T, Alitalo K (2010) Lymphangiogenesis: molecular mechanisms and future promise. Cell 140:460–476. https://doi.org/10.1016/j.cell.2010.01.045
Wang Y, Oliver G (2010) Current views on the function of the lymphatic vasculature in health and disease. Genes Dev 24:2115–2126. https://doi.org/10.1101/gad.1955910
Joukov V, Pajusola K, Kaipainen A et al (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15:290–298
Achen MG, Jeltsch M, Kukk E et al (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A 95:548–553
Alitalo A, Detmar M (2012) Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 31:4499–4508. https://doi.org/10.1038/onc.2011.602
Hong Y-K (2004) VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the 1 1 and 2 1 integrins. FASEB J 18:1111–1113. https://doi.org/10.1096/fj.03-1179fje
Yuan L, Moyon D, Pardanaud L et al (2002) Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129:4797–4806
Christiansen A, Detmar M (2011) Lymphangiogenesis and cancer. Genes Cancer 2:1146–1158. https://doi.org/10.1177/1947601911423028
Tammela T, Petrova TV, Alitalo K (2005) Molecular lymphangiogenesis: new players. Trends Cell Biol 15:434–441. https://doi.org/10.1016/j.tcb.2005.06.004
Watari K, Shibata T, Kawahara A et al (2014) Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS One 9:e99568. https://doi.org/10.1371/journal.pone.0099568
Casazza A, Laoui D, Wenes M et al (2013) Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24:695–709. https://doi.org/10.1016/j.ccr.2013.11.007
Cursiefen C, Chen L, Borges LP et al (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113:1040–1050. https://doi.org/10.1172/JCI20465
Shree T, Olson OC, Elie BT et al (2011) Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev 25:2465–2479. https://doi.org/10.1101/gad.180331.111
Alishekevitz D, Gingis-Velitski S, Kaidar-Person O et al (2016) Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep 17:1344–1356. https://doi.org/10.1016/j.celrep.2016.09.083
Thomas L (1982) On immunosurveillance in human cancer. Yale J Biol Med 55:329–333
Prehn RT, Main JM (1957) Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst 18:769–778
Dunn GP, Bruce AT, Ikeda H et al (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998. https://doi.org/10.1038/ni1102-991
Pagès F, Mlecnik B, Marliot F et al (2018) International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391:2128–2139. https://doi.org/10.1016/S0140-6736(18)30789-X
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (80-) 313:1960–1964. https://doi.org/10.1126/science.1129139
Burke B, Giannoudis A, Corke KP et al (2003) Hypoxia-induced gene expression in human macrophages. Am J Pathol 163:1233–1243. https://doi.org/10.1016/S0002-9440(10)63483-9
Doedens AL, Stockmann C, Rubinstein MP et al (2010) macrophage expression of hypoxia-inducible factor-1 suppresses T-cell function and promotes tumor progression. Cancer Res 70:7465–7475. https://doi.org/10.1158/0008-5472.CAN-10-1439
Movahedi K, Laoui D, Gysemans C et al (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70:5728–5739. https://doi.org/10.1158/0008-5472.CAN-09-4672
Cortesi F, Delfanti G, Grilli A et al (2018) Bimodal CD40/Fas-dependent crosstalk between iNKT cells and tumor-associated macrophages impairs prostate cancer progression. Cell Rep 22:3006–3020. https://doi.org/10.1016/j.celrep.2018.02.058
Gordon SR, Maute RL, Dulken BW et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545:495–499. https://doi.org/10.1038/nature22396
Neubert NJ, Schmittnaegel M, Bordry N et al (2018) T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med 10:eaan3311. https://doi.org/10.1126/scitranslmed.aan3311
Arlauckas SP, Garris CS, Kohler RH et al (2017) In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 9:eaal3604. https://doi.org/10.1126/scitranslmed.aal3604
Binnewies M, Roberts EW, Kersten K et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24:541–550. https://doi.org/10.1038/s41591-018-0014-x
DeNardo DG, Brennan DJ, Rexhepaj E et al (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67. https://doi.org/10.1158/2159-8274.CD-10-0028
Dijkgraaf EM, Heusinkveld M, Tummers B et al (2013) Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res 73:2480–2492. https://doi.org/10.1158/0008-5472.CAN-12-3542
Jinushi M, Chiba S, Yoshiyama H et al (2011) Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA 108:12425–12430. https://doi.org/10.1073/pnas.1106645108
Mitchem JB, Brennan DJ, Knolhoff BL et al (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73:1128–1141. https://doi.org/10.1158/0008-5472.CAN-12-2731
Tsai C-S, Chen F-H, Wang C-C et al (2007) Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys 68:499–507. https://doi.org/10.1016/j.ijrobp.2007.01.041
Stafford JH, Hirai T, Deng L et al (2016) Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol 18:797–806. https://doi.org/10.1093/neuonc/nov272
Kalbasi A, Komar C, Tooker GM et al (2017) Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res 23:137–148. https://doi.org/10.1158/1078-0432.CCR-16-0870
Meng Y, Beckett MA, Liang H et al (2010) Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res 70:1534–1543. https://doi.org/10.1158/0008-5472.CAN-09-2995
Kioi M, Vogel H, Schultz G et al (2010) Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705. https://doi.org/10.1172/JCI40283
Kozin SV, Kamoun WS, Huang Y et al (2010) Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res 70:5679–5685. https://doi.org/10.1158/0008-5472.CAN-09-4446
Kaplan RN, Riba RD, Zacharoulis S et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827. https://doi.org/10.1038/nature04186
Erler JT, Bennewith KL, Cox TR et al (2009) Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15:35–44. https://doi.org/10.1016/j.ccr.2008.11.012
Hiratsuka S, Duda DG, Huang Y et al (2011) C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci 108:302–307. https://doi.org/10.1073/pnas.1016917108
Kitamura T, Doughty-Shenton D, Cassetta L et al (2017) Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Front Immunol 8:2004. https://doi.org/10.3389/fimmu.2017.02004
Celus W, Di Conza G, Oliveira AI et al (2017) Loss of caveolin-1 in metastasis-associated macrophages drives lung metastatic growth through increased angiogenesis. Cell Rep 21:2842–2854. https://doi.org/10.1016/j.celrep.2017.11.034
Qian B-Z, Zhang H, Li J et al (2015) FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med 212:1433–1448. https://doi.org/10.1084/jem.20141555
Qian B, Deng Y, Im JH et al (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4:e6562. https://doi.org/10.1371/journal.pone.0006562
Qian B-Z, Li J, Zhang H et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225. https://doi.org/10.1038/nature10138
Kitamura T, Qian B-Z, Soong D et al (2015) CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212:1043–1059. https://doi.org/10.1084/jem.20141836
Headley MB, Bins A, Nip A et al (2016) Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531:513–517. https://doi.org/10.1038/nature16985
Quail DF, Joyce JA (2017) Molecular pathways: deciphering mechanisms of resistance to macrophage-targeted therapies. Clin Cancer Res 23:876–884. https://doi.org/10.1158/1078-0432.CCR-16-0133
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61. https://doi.org/10.1016/j.immuni.2014.06.010
Zhu Y, Knolhoff BL, Meyer MA et al (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74:5057–5069. https://doi.org/10.1158/0008-5472.CAN-13-3723
Pyonteck SM, Akkari L, Schuhmacher AJ et al (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19:1264–1272. https://doi.org/10.1038/nm.3337
Peyraud F, Cousin S, Italiano A (2017) CSF-1R inhibitor development: current clinical status. Curr Oncol Rep 19:70. https://doi.org/10.1007/s11912-017-0634-1
Quail DF, Bowman RL, Akkari L et al (2016) The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science (80-) 352:aad3018. https://doi.org/10.1126/science.aad3018
Kumar V, Donthireddy L, Marvel D et al (2017) Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC Infiltration of tumors. Cancer Cell 32:654.e5–668.e5. https://doi.org/10.1016/j.ccell.2017.10.005
Loberg RD, Ying C, Craig M et al (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67:9417–9424. https://doi.org/10.1158/0008-5472.CAN-07-1286
Brana I, Calles A, LoRusso PM et al (2015) Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol 10:111–123. https://doi.org/10.1007/s11523-014-0320-2
Sandhu SK, Papadopoulos K, Fong PC et al (2013) A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol 71:1041–1050. https://doi.org/10.1007/s00280-013-2099-8
Bonapace L, Coissieux M-M, Wyckoff J et al (2014) Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515:130–133. https://doi.org/10.1038/nature13862
D’Incalci M, Badri N, Galmarini CM, Allavena P (2014) Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer 111:646–650. https://doi.org/10.1038/bjc.2014.149
Guerriero JL (2018) Macrophages: the road less traveled, changing anticancer therapy. Trends Mol Med 24:472–489. https://doi.org/10.1016/j.molmed.2018.03.006
Beatty GL, Chiorean EG, Fishman MP et al (2011) CD40 Agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science (80-) 331:1612–1616. https://doi.org/10.1126/science.1198443
Beatty GL, Li Y, Long KB (2017) Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther 17:175–186. https://doi.org/10.1080/14737140.2017.1270208
Folkes AS, Feng M, Zain JM et al (2018) Targeting CD47 as a cancer therapeutic strategy. Curr Opin Oncol. https://doi.org/10.1097/cco.0000000000000468
Willingham SB, Volkmer J-P, Gentles AJ et al (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci 109:6662–6667. https://doi.org/10.1073/pnas.1121623109
Guerriero JL, Sotayo A, Ponichtera HE et al (2017) Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543:428–432. https://doi.org/10.1038/nature21409
Poh AR, Ernst M (2018) Targeting macrophages in cancer: from bench to bedside. Front Oncol 8:49. https://doi.org/10.3389/fonc.2018.00049
Le RQ, Li L, Yuan W et al (2018) FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T Cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23:943–947. https://doi.org/10.1634/theoncologist.2018-0028
Daei Farshchi Adli A, Jahanban-Esfahlan R, Seidi K et al (2018) An overview on Vadimezan (DMXAA): the vascular disrupting agent. Chem Biol Drug Des 91:996–1006. https://doi.org/10.1111/cbdd.13166
Cheng B, Yuan W-E, Su J et al (2018) Recent advances in small molecule based cancer immunotherapy. Eur J Med Chem 157:582–598. https://doi.org/10.1016/j.ejmech.2018.08.028
Peterson TE, Kirkpatrick ND, Huang Y et al (2016) Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci USA 113:4470–4475. https://doi.org/10.1073/pnas.1525349113
Acknowledgements
Hans Prenen is a Senior Clinical investigator of the Belgian Foundation against Cancer. This study was funded by H2020 European Research Council (Grant nos. OxyMO, 308459).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prenen, H., Mazzone, M. Tumor-associated macrophages: a short compendium. Cell. Mol. Life Sci. 76, 1447–1458 (2019). https://doi.org/10.1007/s00018-018-2997-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2997-3