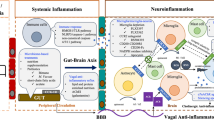
Abstract
Perioperative neurocognitive disorder (PND) is a common disorder following anesthesia and surgery, especially in the elderly. The complex cellular and molecular processes are involved in PND, but the underlying pathogenesis of which remains inconclusive due to conflicting data. A growing body of evidence has been shown that perioperative systemic inflammation plays important roles in the development of PND. We reviewed the relevant literature retrieved by a search in the PubMed database (on July 20, 2023). The search terms used were “delirium”, “post operative cognitive dysfunction”, “perioperative neurocognitive disorder”, “inflammation” and “systemic”, alone and in combination. All articles identified were English-language, full-text papers. The ones cited in the review are those that make a substantial contribution to the knowledge about systemic inflammation and PNDs. The aim of this review is to bring together the latest evidence for the understanding of how perioperative systemic inflammation mediates neuroinflammation and brain injury, how the inflammation is regulated and how we can translate these findings into prevention and/or treatment for PND.



Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- PND:
-
Perioperative neurocognitive disorders
- POCD:
-
Postoperative neurocognitive disorder
- LPS:
-
Lipopolysaccharide
- TNF-α:
-
Tumor necrosis factor α
- IL:
-
Interleukin
- IFN:
-
Interferon
- DAMP:
-
Damage-associated molecular pattern
- HMGB1:
-
High mobility group box 1
- BMDMs:
-
Bone marrow-derived monocytes
- TLR:
-
Toll-like receptor
- NF-κB:
-
Nuclear factor-kappa B
- MCP-1:
-
Monocyte chemotactic protein-1
- MIP:
-
Macrophage inflammatory protein
- NVU:
-
Neurovascular unit
- CNS:
-
Central nervous system
- BBB:
-
Blood–brain barrier
- CSF:
-
Cerebrospinal fluid
- BCB:
-
Blood-cerebrospinal fluid barrier
- CP:
-
Choroid plexus
- CVO:
-
Circumventricular organ
- ECs:
-
Endothelial cells
- CCL2:
-
C–C motif chemokine ligand 2
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- DEX:
-
Dexmedetomidine
References
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery 2018. Anesthesiology. 2018;129:872–9. https://doi.org/10.1097/ALN.0000000000002334.
Edwards ML, Bause GS. From dental to mental institutions: an american dentist and a british psychiatrist highlight insanity following nitrous-oxide administration. J Anesth Hist. 2018;4:133–4. https://doi.org/10.1016/j.janh.2018.02.002.
Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–63. https://doi.org/10.1016/s0140-6736(55)92689-1.
Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–85. https://doi.org/10.1213/ANE.0b013e318215217e.
Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–75. https://doi.org/10.1016/j.jalz.2016.03.005.
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–61. https://doi.org/10.1016/s0140-6736(97)07382-0.
Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. https://doi.org/10.3389/fnagi.2015.00112.
Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. https://doi.org/10.1097/01.anes.0000296071.19434.1e.
Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8.
Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group I. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. https://doi.org/10.1097/ALN.0b013e318195b569.
Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21:93–102. https://doi.org/10.1038/s41583-019-0255-9.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. https://doi.org/10.1016/j.pharmthera.2011.01.014.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. https://doi.org/10.1038/nrn2297.
Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–12. https://doi.org/10.1016/j.biopsych.2008.07.024.
Gisondi P, Sala F, Alessandrini F, Avesani V, Zoccatelli G, Beltramello A, et al. Mild cognitive impairment in patients with moderate to severe chronic plaque psoriasis. Dermatology. 2014;228:78–85. https://doi.org/10.1159/000357220.
Sung CE, Huang RY, Cheng WC, Kao TW, Chen WL. Association between periodontitis and cognitive impairment: Analysis of national health and nutrition examination survey (NHANES) III. J Clin Periodontol. 2019;46:790–8. https://doi.org/10.1111/jcpe.13155.
Xue L, Zou X, Yang XQ, Peng F, Yu DK, Du JR. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp Neurol. 2020;326:113176. https://doi.org/10.1016/j.expneurol.2020.113176.
Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, et al. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J Anesth. 2017;31:25–35. https://doi.org/10.1007/s00540-016-2264-4.
Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, et al. Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav Immun. 2016;54:17–26. https://doi.org/10.1016/j.bbi.2015.11.010.
Biesmans S, Bouwknecht JA, Ver Donck L, Langlois X, Acton PD, De Haes P, et al. Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice. Biomed Res Int. 2015;2015:716920. https://doi.org/10.1155/2015/716920.
Song L, Quan X, Su L, Wang K, Wang H, Wu L, et al. Inflammation and behavioral symptoms in preoperational glioma patients: Is depression, anxiety, and cognitive impairment related to markers of systemic inflammation? Brain Behav. 2020;10: e01771. https://doi.org/10.1002/brb3.1771.
Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis. 2009;24:55–68. https://doi.org/10.1007/s11011-008-9114-5.
van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15:R297. https://doi.org/10.1186/cc10598.
Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I, et al. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med. 2013;274:77–85. https://doi.org/10.1111/joim.12052.
Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, et al. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–43. https://doi.org/10.1074/jbc.M512489200.
Riera Romo M, Perez-Martinez D, Castillo Ferrer C. Innate immunity in vertebrates: an overview. Immunology. 2016;148:125–39. https://doi.org/10.1111/imm.12597.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. https://doi.org/10.1038/nature08780.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. https://doi.org/10.1146/annurev-immunol-030409-101323.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. https://doi.org/10.1038/nri1594.
Huang C, Irwin MG, Wong GTC, Chang RCC. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J Neuroinflammation. 2018;15:147. https://doi.org/10.1186/s12974-018-1163-z.
Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Nyakas C, van Leeuwen BL. Surgery-induced behavioral changes in aged rats. Exp Gerontol. 2013;48:1204–11. https://doi.org/10.1016/j.exger.2013.07.011.
Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. https://doi.org/10.1002/ana.22664.
Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA, Schoemaker RG. Prior infection exacerbates postoperative cognitive dysfunction in aged rats. Am J Physiol Regul Integr Comp Physiol. 2015;309:R148-59. https://doi.org/10.1152/ajpregu.00002.2015.
He Y, Li Z, Zuo YX. Nerve blockage attenuates postoperative inflammation in hippocampus of young rat model with surgical trauma. Mediators Inflamm. 2015;2015:460125. https://doi.org/10.1155/2015/460125.
Gong M, Wang G, Li G, Liu J, Sun P, Xu L, et al. Dysfunction of inflammation-resolving pathways is associated with postoperative cognitive decline in elderly mice. Behav Brain Res. 2020;386:112538. https://doi.org/10.1016/j.bbr.2020.112538.
Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol. 2015;10:179–89. https://doi.org/10.1007/s11481-014-9580-y.
Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13:211. https://doi.org/10.1186/s12974-016-0681-9.
Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–8. https://doi.org/10.1046/j.1365-2044.1999.00947.x.
Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21:1319–26. https://doi.org/10.1038/s41590-020-00812-1.
Thordardottir S, Vikingsdottir T, Bjarnadottir H, Jonsson H Jr, Gudbjornsson B. Activation of complement following total hip replacement. Scand J Immunol. 2016;83:219–24. https://doi.org/10.1111/sji.12411.
Hoedemaekers C, van Deuren M, Sprong T, Pickkers P, Mollnes TE, Klasen I, et al. The complement system is activated in a biphasic pattern after coronary artery bypass grafting. Ann Thorac Surg. 2010;89:710–6. https://doi.org/10.1016/j.athoracsur.2009.11.049.
Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. 2016;123:326–35. https://doi.org/10.1213/ANE.0000000000001403.
Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune modulation by volatile anesthetics. Anesthesiology. 2016;125:399–411. https://doi.org/10.1097/ALN.0000000000001195.
Whitaker EE, Christofi FL, Quinn KM, Wiemann BZ, Xia JC, Tobias JD, et al. Selective induction of IL-1beta after a brief isoflurane anesthetic in children undergoing MRI examination. J Anesth. 2017;31:219–24. https://doi.org/10.1007/s00540-016-2294-y.
Kallioinen M, Scheinin A, Maksimow M, Langsjo J, Kaisti K, Takala R, et al. The influence of dexmedetomidine and propofol on circulating cytokine levels in healthy subjects. BMC Anesthesiol. 2019;19:222. https://doi.org/10.1186/s12871-019-0895-3.
Deiner S, Baxter MG, Mincer JS, Sano M, Hall J, Mohammed I, et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br J Anaesth. 2020. https://doi.org/10.1016/j.bja.2020.04.085.
Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. https://doi.org/10.1002/ana.22082.
Terrando N, Yang T, Wang X, Fang J, Cao M, Andersson U, et al. Systemic HMGB1 neutralization prevents postoperative neurocognitive dysfunction in aged rats. Front Immunol. 2016;7:441. https://doi.org/10.3389/fimmu.2016.00441.
Li RL, Zhang ZZ, Peng M, Wu Y, Zhang JJ, Wang CY, et al. Postoperative impairment of cognitive function in old mice: a possible role for neuroinflammation mediated by HMGB1, S100B, and RAGE. J Surg Res. 2013;185:815–24. https://doi.org/10.1016/j.jss.2013.06.043.
Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–7. https://doi.org/10.2119/molmed.2012.00195.
He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, et al. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. https://doi.org/10.1111/cns.12018.
Lin GX, Wang T, Chen MH, Hu ZH, Ouyang W. Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol Scand. 2014;58:668–74. https://doi.org/10.1111/aas.12320.
Forsberg A, Cervenka S, Jonsson Fagerlund M, Rasmussen LS, Zetterberg H, Erlandsson Harris H, et al. The immune response of the human brain to abdominal surgery. Ann Neurol. 2017;81:572–82. https://doi.org/10.1002/ana.24909.
Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25:1–9. https://doi.org/10.1007/s00540-010-1042-y.
Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, Li WY, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2013;27:236–42. https://doi.org/10.1007/s00540-012-1506-3.
Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. https://doi.org/10.1186/s12871-015-0130-9.
Casey CP, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. https://doi.org/10.1093/brain/awz354.
Sun L, Xie K, Zhang C, Song R, Zhang H. Hyperbaric oxygen preconditioning attenuates postoperative cognitive impairment in aged rats. Neuroreport. 2014;25:718–24. https://doi.org/10.1097/WNR.0000000000000181.
He Z, Xu N, Qi S. Remote ischemic preconditioning improves the cognitive function of elderly patients following colon surgery: A randomized clinical trial. Medicine (Baltimore). 2017;96: e6719. https://doi.org/10.1097/MD.0000000000006719.
Hu J, Feng X, Valdearcos M, Lutrin D, Uchida Y, Koliwad SK, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–45. https://doi.org/10.1016/j.bja.2017.11.096.
Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–22. https://doi.org/10.1073/pnas.1014557107.
Xiong C, Liu J, Lin D, Zhang J, Terrando N, Wu A. Complement activation contributes to perioperative neurocognitive disorders in mice. J Neuroinflammation. 2018;15:254. https://doi.org/10.1186/s12974-018-1292-4.
Sahoo AK, Panda N, Sabharwal P, Luthra A, Balu M, Chauhan R, et al. Effect of anesthetic agents on cognitive function and peripheral inflammatory biomarkers in young patients undergoing surgery for spine disorders. Asian J Neurosurg. 2019;14:1095–105. https://doi.org/10.4103/ajns.AJNS_173_19.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. https://doi.org/10.1152/physrev.00050.2017.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. https://doi.org/10.1038/nrn1824.
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. https://doi.org/10.1038/nature09522.
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–78. https://doi.org/10.1016/j.cell.2015.10.067.
Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–6. https://doi.org/10.1038/s41591-018-0297-y.
Yang S, Gu C, Mandeville ET, Dong Y, Esposito E, Zhang Y, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. https://doi.org/10.3389/fimmu.2017.00902.
Yang T, Xu G, Newton PT, Chagin AS, Mkrtchian S, Carlstrom M, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122:350–60. https://doi.org/10.1016/j.bja.2018.10.062.
Ni P, Dong H, Wang Y, Zhou Q, Xu M, Qian Y, et al. IL-17A contributes to perioperative neurocognitive disorders through blood-brain barrier disruption in aged mice. J Neuroinflammation. 2018;15:332. https://doi.org/10.1186/s12974-018-1374-3.
Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–36. https://doi.org/10.1097/ALN.0b013e3182834d94.
Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. https://doi.org/10.1002/embj.201386609.
Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, et al. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. 2013;136:3427–40. https://doi.org/10.1093/brain/awt259.
Baruch K, Schwartz M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun. 2013;34:11–6. https://doi.org/10.1016/j.bbi.2013.04.002.
Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci USA. 2013;110:2264–9. https://doi.org/10.1073/pnas.1211270110.
Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56:1695–716. https://doi.org/10.1016/j.addr.2004.07.005.
Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. https://doi.org/10.1177/0192623310394214.
Neman J, Chen TC. The Choroid Plexus and Cerebrospinal Fluid: Emerging Roles in CNS Development, Maintenance, and Disease Progression. Academic Press.2015;1st edition:155–65.
Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75:198–213. https://doi.org/10.1093/jnen/nlv030.
Kratzer I, Ek J, Stolp H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr. 2020;1862:183430. https://doi.org/10.1016/j.bbamem.2020.183430.
Ott BR, Jones RN, Daiello LA, de la Monte SM, Stopa EG, Johanson CE, et al. Blood-cerebrospinal fluid barrier gradients in mild cognitive impairment and Alzheimer’s disease: relationship to inflammatory cytokines and chemokines. Front Aging Neurosci. 2018;10:245. https://doi.org/10.3389/fnagi.2018.00245.
Goldim MP, Danielski LG, Rodrigues JF, Joaquim L, Garbossa L, de Oliveira Junior AN, et al. Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc Res. 2019;123:19–24. https://doi.org/10.1016/j.mvr.2018.12.001.
Mesquita SD, Ferreira AC, Gao F, Coppola G, Geschwind DH, Sousa JC, et al. The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun. 2015;49:280–92. https://doi.org/10.1016/j.bbi.2015.06.008.
Zanotto C, Simao F, Gasparin MS, Biasibetti R, Tortorelli LS, Nardin P, et al. Exendin-4 reverses biochemical and functional alterations in the blood-brain and blood-CSF barriers in diabetic rats. Mol Neurobiol. 2017;54:2154–66. https://doi.org/10.1007/s12035-016-9798-1.
Hov KR, Berg JP, Frihagen F, Raeder J, Hall R, Wyller TB, et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-albumin. Dement Geriatr Cogn Disord. 2016;41:192–8. https://doi.org/10.1159/000443789.
Benarroch EE. Circumventricular organs: receptive and homeostatic functions and clinical implications. Neurology. 2011;77:1198–204. https://doi.org/10.1212/WNL.0b013e31822f04a0.
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25. https://doi.org/10.1161/HYPERTENSIONAHA.113.01404.
Korim WS, Elsaafien K, Basser JR, Setiadi A, May CN, Yao ST. In renovascular hypertension, TNF-alpha type-1 receptors in the area postrema mediate increases in cardiac and renal sympathetic nerve activity and blood pressure. Cardiovasc Res. 2019;115:1092–101. https://doi.org/10.1093/cvr/cvy268.
Okamoto A, Fujii R, Yoshimura R, Miyata S. Transcytosis of tanycytes in the circumventricular organs of adult mouse brain. Neurosci Lett. 2022;779:136633. https://doi.org/10.1016/j.neulet.2022.136633.
Jeong JK, Dow SA, Young CN. Sensory circumventricular organs, neuroendocrine control, and metabolic regulation. Metabolites. 2021. https://doi.org/10.3390/metabo11080494.
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. https://doi.org/10.1016/S1566-0702(00)00215-0.
Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. https://doi.org/10.1189/jlb.0208108.
Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. https://doi.org/10.1016/s0889-1591(03)00032-1.
Zielinski MR, Dunbrasky DL, Taishi P, Souza G, Krueger JM. Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-alpha and lipopolysaccharide in mice. Sleep. 2013;36(1227–38):38A. https://doi.org/10.5665/sleep.2892.
Luheshi GN, Bluthe RM, Rushforth D, Mulcahy N, Konsman JP, Goldbach M, et al. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton Neurosci. 2000;85:127–32. https://doi.org/10.1016/S1566-0702(00)00231-9.
Kubota T, Fang J, Guan Z, Brown RA, Krueger JM. Vagotomy attenuates tumor necrosis factor-alpha-induced sleep and EEG delta-activity in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1213-20. https://doi.org/10.1152/ajpregu.2001.280.4.R1213.
Romanovsky AA, Ivanov AI, Szekely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis. 2000;31(Suppl 5):S162-7. https://doi.org/10.1086/317515.
Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021. https://doi.org/10.1126/science.abf7844.
Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Forstera B, Zhao S, et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci. 2019;22:317–27. https://doi.org/10.1038/s41593-018-0301-3.
Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–17. https://doi.org/10.1038/s41593-018-0213-2.
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560:55–60. https://doi.org/10.1038/s41586-018-0342-5.
Galea I, Perry VH. The blood-brain interface: a culture change. Brain Behav Immun. 2018;68:11–6. https://doi.org/10.1016/j.bbi.2017.10.014.
Smyth LCD, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, et al. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation. 2018;15:138. https://doi.org/10.1186/s12974-018-1167-8.
D’Mello C, Riazi K, Le T, Stevens KM, Wang A, McKay DM, et al. P-selectin-mediated monocyte-cerebral endothelium adhesive interactions link peripheral organ inflammation to sickness behaviors. J Neurosci. 2013;33:14878–88. https://doi.org/10.1523/JNEUROSCI.1329-13.2013.
Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50:764–6. https://doi.org/10.1016/j.immuni.2019.02.012.
Erikson K, Tuominen H, Vakkala M, Liisanantti JH, Karttunen T, Syrjala H, et al. Brain tight junction protein expression in sepsis in an autopsy series. Crit Care. 2020;24:385. https://doi.org/10.1186/s13054-020-03101-3.
Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–29. https://doi.org/10.1002/cne.20052.
Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med (Berl). 2002;80:5–15. https://doi.org/10.1007/s00109-001-0289-z.
Propson NE, Roy ER, Litvinchuk A, Kohl J, Zheng H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest. 2021. https://doi.org/10.1172/JCI140966.
Bhatia K, Ahmad S, Kindelin A, Ducruet AF. Complement C3a receptor-mediated vascular dysfunction: a complex interplay between aging and neurodegeneration. J Clin Invest. 2021. https://doi.org/10.1172/JCI144348.
Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. 2017;101:1119–32. https://doi.org/10.1189/jlb.3RU0816-352RR.
Vizcaychipi MP, Watts HR, O’Dea KP, Lloyd DG, Penn JW, Wan Y, et al. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann Surg. 2014;259:1235–44. https://doi.org/10.1097/SLA.0000000000000257.
Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–405. https://doi.org/10.1038/nn.2946.
Duan L, Zhang XD, Miao WY, Sun YJ, Xiong G, Wu Q, et al. PDGFRbeta Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron. 2018. https://doi.org/10.1016/j.neuron.2018.08.030.
Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628:288–97. https://doi.org/10.1016/j.brainres.2015.08.031.
Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24:326–37. https://doi.org/10.1038/nm.4482.
Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, et al. A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11:104. https://doi.org/10.1186/1742-2094-11-104.
Greter M, Lelios I, Croxford AL. Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol. 2015;6:249. https://doi.org/10.3389/fimmu.2015.00249.
Chowen JA, Garcia-Segura LM. Microglia, neurodegeneration and loss of neuroendocrine control. Prog Neurobiol. 2020;184:101720. https://doi.org/10.1016/j.pneurobio.2019.101720.
Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–34. https://doi.org/10.1016/j.it.2008.01.010.
D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–102. https://doi.org/10.1523/JNEUROSCI.3567-08.2009.
Wang T, Zhu H, Hou Y, Gu W, Wu H, Luan Y, et al. Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. Eur J Pharmacol. 2019;846:63–72. https://doi.org/10.1016/j.ejphar.2018.12.034.
Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–10. https://doi.org/10.1097/00000542-200603000-00005.
Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. https://doi.org/10.1002/glia.10161.
Wang HL, Liu H, Xue ZG, Liao QW, Fang H. Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med. 2016;20:1632–9. https://doi.org/10.1111/jcmm.12854.
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2: e91229. https://doi.org/10.1172/jci.insight.91229.
Zhou X, Lu J, Wu T, Jiang X, Tian W, Dai W, et al. Multiple anesthesia/surgery cannot impair reference memory in adult mice. Mediators Inflamm. 2020;2020:3736912. https://doi.org/10.1155/2020/3736912.
Wang HL, Ma RH, Fang H, Xue ZG, Liao QW. Impaired spatial learning memory after isoflurane anesthesia or appendectomy in aged mice is associated with microglia activation. J Cell Death. 2015;8:9–19. https://doi.org/10.4137/JCD.S30596.
Lee HG, Wheeler MA, Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. 2022;21:339–58. https://doi.org/10.1038/s41573-022-00390-x.
Zhou Y, Wu X, Ye L, Bai Y, Zhang H, Xuan Z, et al. Edaravone at high concentrations attenuates cognitive dysfunctions induced by abdominal surgery under general anesthesia in aged mice. Metab Brain Dis. 2020;35:373–83. https://doi.org/10.1007/s11011-019-00532-y.
Quiroz-Padilla MF, Guillazo-Blanch G, Sanchez MY, Dominguez-Sanchez MA, Gomez RM. Effects of excitotoxic lesion with inhaled anesthetics on nervous system cells of rodents. Curr Pharm Des. 2018;24:4–14. https://doi.org/10.2174/1381612823666170817125015.
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. https://doi.org/10.1097/00000542-200703000-00007.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7. https://doi.org/10.1038/nature21029.
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. https://doi.org/10.1007/s00401-009-0601-5.
Maes M, Thisayakorn P, Thipakorn Y, Tantavisut S, Sirivichayakul S, Vojdani A. Reactivity to neural tissue epitopes, aquaporin 4 and heat shock protein 60 is associated with activated immune-inflammatory pathways and the onset of delirium following hip fracture surgery. Eur Geriatr Med. 2023;14:99–112. https://doi.org/10.1007/s41999-022-00729-y.
Favrais G, Bokobza C, Saliba E, Chalon S, Gressens P. Alteration of the oligodendrocyte lineage varies according to the systemic inflammatory stimulus in animal models that mimic the encephalopathy of prematurity. Front Physiol. 2022;13:881674. https://doi.org/10.3389/fphys.2022.881674.
Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–63.
Prieto GA, Snigdha S, Baglietto-Vargas D, Smith ED, Berchtold NC, Tong L, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1beta in the aged hippocampus. Proc Natl Acad Sci USA. 2015;112:E5078-87. https://doi.org/10.1073/pnas.1514486112.
Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, et al. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–96. https://doi.org/10.1016/j.celrep.2012.08.022.
Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–32. https://doi.org/10.1523/JNEUROSCI.22-08-03025.2002.
Li R, Yang L, Lindholm K, Konishi Y, Yue X, Hampel H, et al. Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. J Neurosci. 2004;24:1760–71. https://doi.org/10.1523/JNEUROSCI.4580-03.2004.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128:781–8. https://doi.org/10.1213/ANE.0000000000004053.
Kawano T, Yamanaka D, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, Nishigaki A, et al. Involvement of acute neuroinflammation in postoperative delirium-like cognitive deficits in rats. J Anesth. 2018;32:506–17. https://doi.org/10.1007/s00540-018-2504-x.
Danielson M, Wiklund A, Granath F, Blennow K, Mkrtchian S, Nellgard B, et al. Neuroinflammatory markers associate with cognitive decline after major surgery: Findings of an explorative study. Ann Neurol. 2020;87:370–82. https://doi.org/10.1002/ana.25678.
Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–52. https://doi.org/10.1523/JNEUROSCI.4485-14.2015.
Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci. 2020;21:139–52. https://doi.org/10.1038/s41583-020-0263-9.
Li D, Chen M, Meng T, Fei J. Hippocampal microglial activation triggers a neurotoxic-specific astrocyte response and mediates etomidate-induced long-term synaptic inhibition. J Neuroinflammation. 2020;17:109. https://doi.org/10.1186/s12974-020-01799-0.
Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98: e15383. https://doi.org/10.1097/MD.0000000000015383.
Lei D, Sha Y, Wen S, Xie S, Liu L, Han C. Dexmedetomidine may reduce IL-6 level and the risk of postoperative cognitive dysfunction in patients after surgery: a meta-analysis. Dose Response. 2020;18:1559325820902345. https://doi.org/10.1177/1559325820902345.
Huang JM, Lv ZT, Zhang B, Jiang WX, Nie MB. Intravenous parecoxib for early postoperative cognitive dysfunction in elderly patients: evidence from a meta-analysis. Expert Rev Clin Pharmacol. 2020;13:451–60. https://doi.org/10.1080/17512433.2020.1732815.
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A, et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5:12342. https://doi.org/10.1038/srep12342.
Mei B, Xu G, Han W, Lu X, Liu R, Cheng X, et al. The benefit of dexmedetomidine on postoperative cognitive function is unrelated to the modulation on peripheral inflammation: a single-center, prospective. Randomized Study Clin J Pain. 2020;36:88–95. https://doi.org/10.1097/AJP.0000000000000779.
Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N. Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2017;34:776–84. https://doi.org/10.1097/EJA.0000000000000647.
Ottens TH, Dieleman JM, Sauer AM, Peelen LM, Nierich AP, de Groot WJ, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121:492–500. https://doi.org/10.1097/ALN.0000000000000336.
Kluger MT, Skarin M, Collier J, Rice DA, McNair PJ, Seow MY, et al. Steroids to reduce the impact on delirium (STRIDE): a double-blind, randomised, placebo-controlled feasibility trial of pre-operative dexamethasone in people with hip fracture. Anaesthesia. 2021;76:1031–41. https://doi.org/10.1111/anae.15465.
Acknowledgements
Not applicable.
Funding
This work was supported partly by the National Natural Science Foundation of China (No. 82270997).
Author information
Authors and Affiliations
Contributions
SJ and WF conceived the idea of this review. SJ, HY and WF performed literature searching and drafted the manuscript. WF created the figures. WF and FH critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, S., Yang, H., Huang, F. et al. Systemic inflammation, neuroinflammation and perioperative neurocognitive disorders. Inflamm. Res. 72, 1895–1907 (2023). https://doi.org/10.1007/s00011-023-01792-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01792-2