Key summary points
Is IgA/IgG reactivity against neural tissue epitopes associated with delirium severity and activation of the immune response system?
AbstractSection FindingsIgA antibody levels against neurofilament protein, glial fibrillary acidic protein, myelin basic protein, metabotropic glutamate receptors 1 and 5, N-Methyl-D-Aspartate receptor GLU1 (NR1) and GLU2 (NR2), and heat shock protein 60 strongly predict severity of delirium and are associated with activation of the cytokine network.
AbstractSection MessagePolyreactive antibody-associated breakdown of immune tolerance, cytokine network activation and neuronal injuries are involved in delirium.
Abstract
Objectives
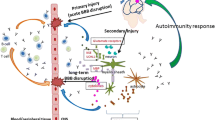
Activation of the immune–inflammatory response system (IRS) and a deficiency in the compensatory immunoregulatory system (CIRS), neuronal injuries, and alterations in the glutamate receptor (GlutaR), aquaporin-4 (AQP4) and heat shock protein 60 (HSP60) are involved in delirium. Increased serum levels of neurofilament protein (NFP), glial fibrillary acidic protein (GFAP) and myelin basic protein (MBP) are biomarkers of neuronal injury. This investigation delineates whether elevated IgA/IgG reactivity against those self-antigens is associated with delirium severity and IRS activation.
Methods
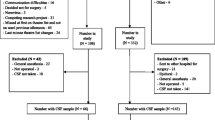
We measured peak Delirium Rating Scale (DRS) scores on days 2 and 3 following surgery in 59 hip fractured older adults, and IgA and IgG antibody levels against MBP, NFP, GFAP and myelin oligodendrocyte glycoprotein (MOG), metabotropic glutamate receptors mGluRs 1 and 5, N-Methyl-D-Aspartate receptor (NMDAR) GLU1 (NR1) and GLU2 (NR2), APQ4 and HSP60.
Results
The IgA antibody levels against those self-antigens, especially GFAP, MBP and HSP60, strongly predict peak DRS scores on days 2 and 3 post-surgery. IgA reactivity against NMDAR and baseline DRS scores explained 40.6% of the variance in peak DRS scores, while IgA against NMDAR, IgG against MBP and age explained 29.1% of the variance in the IRS/CIRS ratio. There was no correlation between DRS scores and IgG directed against other self-antigens.
Conclusions
Increased IgA levels against neuronal self-antigens, AQP4 and HSP60 are risk factors for delirium. Polyreactive antibody-associated breakdown of immune tolerance, IRS activation and injuries in the neuronal cytoskeleton, oligodendrocytes, astrocytes, glial cells, and myelin sheath are involved in the pathophysiology of delirium.




Similar content being viewed by others
Availability of data and materials
The dataset generated during and/or analyzed during the current study will be available from Prof. Dr. Michael Maes upon reasonable request and once the dataset has been fully exploited by the authors.
References
Maldonado JR (2018) Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 33(11):1428–1457. https://doi.org/10.1002/gps.4823
Thisayakorn P, Thipakorn Y, Tantavisut S, Sirivichayakul S, Maes M (2022) Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry 22(1):369. https://doi.org/10.1186/s12888-022-04021-y.PMID:35641947;PMCID:PMC9158285
Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y (2017) Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res 29(2):115–126
Wu J, Yin Y, Jin M, Li B (2021) The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry 36(1):3–14
Arshi A, Lai WC, Chen JB, Bukata SV, Stavrakis AI, Zeegen EN (2018) Predictors and sequelae of postoperative delirium in geriatric hip fracture patients. Geriatric Orthop Surg & Rehabilit 9:2151459318814823
Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB (2009) Incidence and mortality of hip fractures in the United States. JAMA 302(14):1573–1579
Lee HB, Oldham MA, Sieber FE, Oh ES (2017) Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: a case of effect modification. Am J Geriatr Psychiatry 25(3):308–315
Maldonado JR (2008) Delirium in the acute care setting: characteristics, diagnosis and treatment. Critical Care Clin 24(4):657–722, vii
Hshieh TT, Inouye SK, Oh ES (2020) Delirium in the elderly. Clin Geriatr Med 36(2):183–199
Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK (2006) Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci 61(12):1281–1286. https://doi.org/10.1093/gerona/61.12.1281
Thisayakorn P, Tangwongchai S, Tantavisut S, Thipakorn Y, Sukhanonsawat S, Wongwarawipat T, Sirivichayakul S, Maes M (2021) Immune, blood cell, and blood gas biomarkers of delirium in elderly individuals with hip fracture surgery. Dement Geriatr Cogn Disord 50(2):161–169. https://doi.org/10.1159/000517510
Dugan, K, Patel, BK, Pohlman, A, Hall, JB, Kress, JP, Wolfe, KS (2019) Prevalence of Delirium Correlates with Pro-Inflammatory Cytokines in Patients with Septic Shock. D50 CRITICAL CARE: THE METAMORPHOSIS—PAIN, SEDATION, DELIRIUM, ICU-ACQUIRED WEAKNESS, AND PALLIATIVE CARE. Am Thoracic Soc Int Conf Abstracts: Am Thoracic Soc. pp. A6671.
Egberts A, Mattace-Raso FU (2017) Increased neutrophil-lymphocyte ratio in delirium: a pilot study. Clin Interv Aging 12:1115–1121
Kotfis K, Ślozowska J, Safranow K, Szylińska A, Listewnik M (2019) The practical use of white cell inflammatory biomarkers in prediction of postoperative delirium after cardiac surgery. Brain Sci 9(11):308
He R, Wang F, Shen H, Zeng Y, Zhang L (2020) Association between increased neutrophil-to-lymphocyte ratio and postoperative delirium in elderly patients with total hip arthroplasty for hip fracture. BMC Psychiatry 20(1):496
Maes M (2018) Carvalho AF (2018) the compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol 55(12):8885–8903. https://doi.org/10.1007/s12035-018-1016-x
van Eden W, Spiering R, Broere F, van der Zee R (2012) A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 17(3):281–292. https://doi.org/10.1007/s12192-011-0311-5
Broere F, van der Zee R, van Eden W (2011) Heat shock proteins are no DAMPs, rather “DAMPERs.” Nat Rev Immunol 11(8):565. https://doi.org/10.1038/nri2873-c1
Restrepo-Martínez M, López-Hernández JC, Espinola-Nadurille M, Bayliss L, Medina-Rioja R, Martínez-Ángeles V, Galnares-Olalde J, Téllez-Martínez JA (2021) Ramírez-Bermúdez J (2021) Psicosis autoinmune [Autoinmune psychosis]. Rev Alerg Mex 68(4):276–290. https://doi.org/10.29262/ram.v68i4.981
Bittner D (2017) NMDA Antagonists for Treatment of Catatonia with Delirium. May 21, NMDA Antagonists for Treatment of Catatonia with Delirium (hcplive.com)
Casey CP, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, Miller S, Krause B, Prabhakaran V, Blennow K, Zetterberg H, Sanders RD (2020) Postoperative delirium is associated with increased plasma neurofilament light. Brain 143(1):47–54. https://doi.org/10.1093/brain/awz354. (Erratum.In:Brain.2020Mar1;143(3):e24)
Ballweg T, White M, Parker M, Casey C, Bo A, Farahbakhsh Z, Kayser A, Blair A, Lindroth H, Pearce RA, Blennow K, Zetterberg H, Lennertz R, Sanders RD (2021) Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth 126(2):458–466. https://doi.org/10.1016/j.bja.2020.08.061
Rappold T, Laflam A, Hori D, Brown C, Brandt J, Mintz CD, Sieber F, Gottschalk A, Yenokyan G, Everett A, Hogue CW (2016) Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br J Anaesth 116(1):83–89. https://doi.org/10.1093/bja/aev415.
Volkova YV, Dubivska SS, Omelchenko-Seliukova AV, Biletskyi OV (2021) Dynamics of neurospecific autoimmune disorders and cognitive functions in patients with polytrauma and alcohol withdrawal syndrome complicated by alcohol delirium. Rep Vinnytsia Nation Med Univ. https://doi.org/10.31393/reports-vnmedical-2021-25(4)-16
Vojdani A, Mukherjee PS, Berookhim J, Kharrazian D (2015) Detection of antibodies against human and plant aquaporins in patients with multiple sclerosis. Autoimmune Dis 2015:905208. https://doi.org/10.1155/2015/905208
Sfera A, Osorio C (2014) Water for thought: is there a role for aquaporin channels in delirium? Front Psychiatry 5:57. https://doi.org/10.3389/fpsyt.2014.00057
Vojdani A, Vojdani E (2019) Food-Associate Autoimmunities. When food turns your immune system against you. Chapter 12 The role of polyreactive antibodies in health and disease. A&G Wilshire LLC, Beverly Hills, pp 1–23 (ISBN-10: 0578499770)
Pipanmekaporn T, Wongpakaran N, Mueankwan S, Dendumrongkul P, Chittawatanarat K, Khongpheng N et al (2014) Validity and reliability of the Thai version of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Clin Interv Aging 9:879–885
Bangphichet A (2008) Reliability and validity of the Thai version of the Delirium Rating Scale-Revised-98 (DRS-R-98).
de Vries J, Thijssen WA, Snels SE, Menovsky T, Peer NG, Lamers KJ (2001) Intraoperative values of S-100 protein, myelin basic protein, lactate, and albumin in the CSF and serum of neurosurgical patients. J Neurol Neurosurg Psychiatry 71(5):671–674. https://doi.org/10.1136/jnnp.71.5.671.
Lamers KJ, van Engelen BG, Gabreëls FJ, Hommes OR, Borm GF, Wevers RA (1995) Cerebrospinal neuron-specific enolase, S-100 and myelin basic protein in neurological disorders. Acta Neurol Scand 92(3):247–251. https://doi.org/10.1111/j.1600-0404.1995.tb01696.x
Nakagawa H, Yamada M, Kanayama T, Tsuruzono K, Miyawaki Y, Tokiyoshi K, Hagiwara Y, Hayakawa T (1994) Myelin basic protein in the cerebrospinal fluid of patients with brain tumors. Neurosurgery 34(5):825–833. https://doi.org/10.1227/00006123-199405000-00006. (Discussion 833)
Zhang J, Sun X, Zheng S, Liu X, Jin J, Ren Y, Luo J (2014) Myelin basic protein induces neuron-specific toxicity by directly damaging the neuronal plasma membrane. PLoS ONE 19(9):e108646. https://doi.org/10.1371/journal.pone.0108646.
Yuan A, Rao MV, Veeranna Nixon RA (2012) Neurofilaments at a glance. J Cell Sci 125(14):3257–3263. https://doi.org/10.1242/jcs.104729
Hoffman PN, Lasek RJ (1975) The slow component of axonal transport Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol 66(2):351–366. https://doi.org/10.1083/jcb.66.2.351
Yuan A, Nixon RA (2021) Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front Neurosci 15:689938. https://doi.org/10.3389/fnins.2021.689938.
Didonna A, Opal P (2019) The role of neurofilament aggregation in neurodegeneration: lessons from rare inherited neurological disorders. Mol Neurodegener 14(1):19. https://doi.org/10.1186/s13024-019-0318-4.
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130. https://doi.org/10.1016/j.ceb.2015.02.004
Yang Z, Wang KK (2015) Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 38(6):364–374. https://doi.org/10.1016/j.tins.2015.04.003
Ambrosius W, Michalak S, Kozubski W, Kalinowska A (2020) Myelin oligodendrocyte glycoprotein antibody-associated disease: current insights into the disease pathophysiology, diagnosis and management. Int J Mol Sci 22(1):100. https://doi.org/10.3390/ijms22010100
Peschl P, Schanda K, Zeka B, Given K, Böhm D, Ruprecht K, Saiz A, Lutterotti A, Rostásy K, Höftberger R, Berger T, Macklin W, Lassmann H, Bradl M, Bennett JL, Reindl M (2017) Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflammation 14(1):208. https://doi.org/10.1186/s12974-017-0984-5.
Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ, Bill RM (2022) Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 145(1):64–75. https://doi.org/10.1093/brain/awab311.PMID:34499128;PMCID:PMC9088512
Vella J, Zammit C, Di Giovanni G, Muscat R, Valentino M (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci 9:108. https://doi.org/10.3389/fncel.2015.00108.
Verkman AS, Rossi A, Crane JM (2012) Live-cell imaging of aquaporin-4 supramolecular assembly and diffusion. Methods Enzymol 504:341–354. https://doi.org/10.1016/B978-0-12-391857-4.00017-3.
Redenbaugh V, Flanagan EP (2022) Monoclonal antibody therapies beyond complement for NMOSD and MOGAD. Neurotherapeutics. https://doi.org/10.1007/s13311-022-01206-x
Burns MJ (2022) What is the role of alcohol withdrawal in the pathogenesis of delirium tremens (DTs)? MedScape June 23, 2022. What is the role of alcohol withdrawal in the pathogenesis of delirium tremens (DTs)? (medscape.com)
Caplan J (2012) Delirium, sigma-1 receptors, dopamine, and glutamate. How does haloperidol keep the genie in the bottle? Crit Care Med 40(3):982–983
Flacker JM, Lipsitz LA (1999) Neural mechanisms of delirium: current hypotheses and evolving concepts. J Gerontol A Biol Sci Med Sci 54(6):B239–B246. https://doi.org/10.1093/gerona/54.6.b239. (Erratum in: J Gerontol A Biol Sci Med Sci 1999;54(7):B275)
Preuss UW, Zill P, Koller G, Bondy B, Hesselbrock V, Soyka M (2006) Ionotropic glutamate receptor gene GRIK3 SER310ALA functional polymorphism is related to delirium tremens in alcoholics. Pharmacogenomics J 6(1):34–41. https://doi.org/10.1038/sj.tpj.6500343
Levite M (2014) Glutamate receptor antibodies in neurological diseases. J Neural Transm (Vienna) 121(8):1029–1075. https://doi.org/10.1007/s00702-014-1193-3
Hanly JG, Robichaud J, Fisk JD (2006) Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol 33(8):1553–1558
Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, van Alphen AM, Luo C, van der Geest JN, Kros JM, Gaillard CA, Frens MA, de Zeeuw CI (2003) Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol 53(3):325–336. https://doi.org/10.1002/ana.10451
Delogu G, Signore M, Mechelli A, Famularo G (2002) Heat shock proteins and their role in heart injury. Curr Opin Crit Care 8(5):411–416. https://doi.org/10.1097/00075198-200210000-00007
Niizeki T, Takeishi Y, Watanabe T, Nitobe J, Miyashita T, Miyamoto T, Kitahara T, Suzuki S, Sasaki T, Bilim O, Ishino M, Kubota I (2008) Relation of serum heat shock protein 60 level to severity and prognosis in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 102(5):606–610. https://doi.org/10.1016/j.amjcard.2008.04.030
Martine P, Rébé C (2019) Heat shock proteins and inflammasomes. Int J Mol Sci 20(18):4508. https://doi.org/10.3390/ijms20184508
de Lima Filho JB, Freire L, Nahas EAP, Orsatti FL, Orsatti CL (2020) Heat shock protein 60 antibodies are associated with a risk factor for cardiovascular disease in bedridden elderly patients. Front Mol Biosci 7:103. https://doi.org/10.3389/fmolb.2020.00103.
Shamaei-Tousi A, Stephens JW, Bin R, Cooper JA, Steptoe A, Coates AR, Henderson B, Humphries SE (2006) Association between plasma levels of heat shock protein 60 and cardiovascular disease in patients with diabetes mellitus. Eur Heart J 27(13):1565–1570. https://doi.org/10.1093/eurheartj/ehl081
Canul-Euan AA, Zúñiga-González G, Palacios-Luna JE, Maida-Claros R, Díaz NF, Saltigeral-Tigeral P, Karina García-May P, Díaz-Ruiz O, Flores-Herrera H (2021) Increased levels of plasma extracellular heat-shock proteins 60 and 70 kDa characterized early-onset neonatal sepsis. Front Pediatr 9:740274. https://doi.org/10.3389/fped.2021.740274
Ponomarenko NA, Durova OM, Vorobiev II, Belogurov AA, Telegin GB, Suchkov SV, Misikov VK, Morse HC 3rd, Gabibov AG (2006) Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol Lett 103(1):45–50. https://doi.org/10.1016/j.imlet.2005.10.006
Warren KG, Catz I (1999) An extensive search for autoantibodies to myelin basic protein in cerebrospinal fluid of non-multiple-sclerosis patients: implications for the pathogenesis of multiple sclerosis. Eur Neurol 42(2):95–104. https://doi.org/10.1159/000069418
Hansen IS, Baeten DLP, den Dunnen J (2019) The inflammatory function of human IgA. Cell Mol Life Sci 76(6):1041–1055. https://doi.org/10.1007/s00018-018-2976-8
van Gool MMJ, van Egmond M (2021) IgA and FcαRI versatile players in homeostasis, infection, and autoimmunity. Immunotargets Ther 9:351–372. https://doi.org/10.2147/ITT.S266242
Dimitrov JD, Planchais C, Roumenina LT, Vassilev TL, Kaveri SV, Lacroix-Desmazes S (2013) Antibody polyreactivity in health and disease: statu variabilis. J Immunol 191(3):993–999. https://doi.org/10.4049/jimmunol.1300880
Reyneveld GI, Savelkoul HFJ, Parmentier HK (2020) Current understanding of natural antibodies and exploring the possibilities of modulation using veterinary models. A review. Front Immunol 11:2139. https://doi.org/10.3389/fimmu.2020.02139.
Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT, Diamond B (2009) Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun 33(3–4):270–274. https://doi.org/10.1016/j.jaut.2009.03.011
Zhou ZH, Tzioufas AG, Notkins AL (2007) Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun 29(4):219–228. https://doi.org/10.1016/j.jaut.2007.07.015
Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB (2010) The neuroinflammatory hypothesis of delirium. Acta Neuropathol 119(6):737–754. https://doi.org/10.1007/s00401-010-0674-1
Hughes CG, Pandharipande PP, Thompson JL, Chandrasekhar R, Ware LB, Ely EW, Girard TD (2016) Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med 44(9):e809–e817. https://doi.org/10.1097/CCM.0000000000001739.
Taylor J, Parker M, Casey CP, Tanabe S, Kunkel D, Rivera C, Zetterberg H, Blennow K, Pearce RA, Lennertz RC, Sanders RD (2022) Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth 129(2):219–230. https://doi.org/10.1016/j.bja.2022.01.005
Zameer A, Hoffman SA (2001) Immunoglobulin binding to brain in autoimmune mice. J Neuroimmunol 120(1–2):10–18. https://doi.org/10.1016/s0165-5728(01)00412-x
Platt MP, Agalliu D, Cutforth T (2017) Hello from the other side: how autoantibodies circumvent the blood-brain barrier in autoimmune encephalitis. Front Immunol 8:442. https://doi.org/10.3389/fimmu.2017.00442.
Kapadia M, Sakic B (2011) Autoimmune and inflammatory mechanisms of CNS damage. Prog Neurobiol 95(3):301–333. https://doi.org/10.1016/j.pneurobio.2011.08.008
Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A (2019) Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res 36(2):306–322. https://doi.org/10.1007/s12640-019-00054-6
Morris G, Puri BK, Olive L, Carvalho AF, Berk M, Maes M (2019) Emerging role of innate B1 cells in the pathophysiology of autoimmune and neuroimmune diseases: association with inflammation, oxidative and nitrosative stress and autoimmune responses. Pharmacol Res 148:104408. https://doi.org/10.1016/j.phrs.2019.104408
Acknowledgements
We gratefully acknowledge the help of all psychiatry/orthopedic/anesthesiology nursing staff and residents involved in the execution of this study.
Funding
This study was supported by a Ratchadapisek Sompoch Endowment Fund of the Faculty of Medicine, Chulalongkorn University (RA62/014).
Author information
Authors and Affiliations
Contributions
PT: designed the conception, planned, and collected the data, provided a delirium care, interpreted the results, drafted and revised the manuscript. YT: planned and collected the data, provided a delirium care, revised the manuscript. ST: planned and collected the data, provided an orthopedic care, revised the manuscript. SS: prepared, analyzed, and interpreted the laboratory specimens, revised the manuscript. MM: designed the conception, analyzed, and interpreted the data, drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Approval for the study was obtained from the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (registration number 528/61), in compliance with the International Guideline for Human Research protection, as required by the Declaration of Helsinki, conducted according to Thai and international ethics and privacy laws. Written informed consent was obtained before the study from the patients or their guardians (first-degree family members).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maes, M., Thisayakorn, P., Thipakorn, Y. et al. Reactivity to neural tissue epitopes, aquaporin 4 and heat shock protein 60 is associated with activated immune–inflammatory pathways and the onset of delirium following hip fracture surgery. Eur Geriatr Med 14, 99–112 (2023). https://doi.org/10.1007/s41999-022-00729-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00729-y